Review Part 1
advertisement
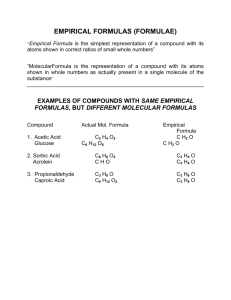
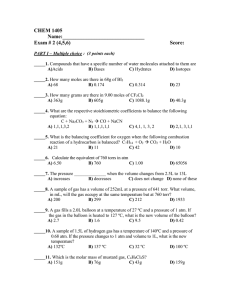
Chemistry Final Review Session 1 - May 5, 2011 Density • Density = Mass/Volume Density Graph What is the density of salt water? Precision vs accuracy • accuracy = how close a result is to the ACTUAL value • precision = how close together a set of results are to one another Mixtures, compounds • What is the difference between a mixture and a compound? Chemical vs physical changes • Physical change = no change in composition • Chemical change = change in chemical composition SI system • Be able to convert between units • milli, centi, deci, deca, kilo, etc. • meters, kilograms, liters, Ways to separate mixtures • Physical means of separating mixtures • Distillation • Filtration • Chromatography (pass mixture through a physical medium smallest molecules travel farthest, etc.) How to draw a graph • Descriptive Title • Scale for maximum coverage of graph area • Set Origins for maximum expression of data features • Axis Labels with Units • Points clearly indicated • Smooth curve, not dot-to-dot Nomenclature - IOnic • MgCl2 • NaNO3 • Fe2O3 • FeO • NaC2H3O2 Writing compounds • You have an ionic compound of Aluminum and the polyatomic ion sulfate. What is the correct formula? • What is the correct formula for Calcium Oxide? • What is the correct formula for Cu(II) Hydroxide? Covalent nomenclature • H2O • NO • SO2 • CSe2 Moles • How many moles of Oxygen atoms in 3 moles of water? • What is the molar mass of CO2? C6H12O6? • How many atoms in 3 moles of B2O3? Percent composition • What is the percent composition of Carbon in CO2? Empirical formula • Butyric acid gets its name from the Latin "butyrum", meaning butter, and is the compound in rancid butter that gives it its terrible smell. A 2.50 g sample of butyric acid was found to consist of 1.36 g carbon, 0.23 g hydrogen, and 0.91 g oxygen. Find its empirical formula. Empirical formula • Natural gas is a mixture of several hydrocarbons, but is primarily methane. Find the empirical formula of this important natural resource if its composed of 74.8% carbon and 25.2% hydrogen Molecular formula • The simplest formula for vitamin C is C3H4O3. Experimental data indicates that the molecular mass of vitamin C is about 180. What is the molecular formula of vitamin C? BAlancing equations • Balance the following equations: • ____ Na3PO4 + ____ KOH ____ NaOH + ____ K3PO4 • ____ Na3PO4 + ____ CaCl2 ____ NaCl + ____ Ca3(PO4)2 • ____ CH4 + ____ O2 ____ CO2 + ____ H2O Types of reactions • A + B --> AB • AB --> A + B • AB + C --> CB + A • AB + CD --> AD + CB • Hydrocarbon + O2 --> CO2 + H2O + heat