Supplemental Information: U S -E
advertisement

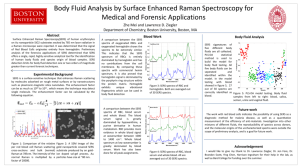
SupplementalInformation: USING SURFACE-ENHANCED RAMAN SCATTERING (SERS) AND FLUORESCENCE SPECTROSCOPY FOR SCREENING YEAST EXTRACTS, A COMPLEX COMPONENT OF CELL CULTURE MEDIA. Boyan Li, Narayana M.S. Sirimuthu, Bryan H. Ray, Alan G. Ryder.* 1 Nanoscale Biophotonics Laboratory, School of Chemistry, National University of Ireland, Galway, Galway, Ireland. * To whom all correspondence should be addressed. Tel: +353 91 49 2943 Fax: +353 91 49 4596 Email: alan.ryder@nuigalway.ie (A.G.R) Table S1: Assignment of the SERS bands for the 5 g/L yeastolate (BD-Difco-UF) solution. SERS band 1460 1396 1376 1332 1270 1130 955 795 731 650 627 Source Adenine Adenine Adenine Adenine Adenine Adenine Adenine Adenine Adenine Guanine Adenine Plane in in in in in in in out in In/out in Assignment * str N7–C8, bend C8–H, sciss NH2 str C4–N9, C4–N5, C6–N10, N7–C8, bend C2–H bend C2–H, N9–H, str C8–N9, C4–N9 bend C2–H, C8–H, N9–H, str C6–N1, O8–N9, N3–C4 bend C8–H, N9–H, str N7–C8 str C8–N9, A bend N9–H, C8–H def R5 (sqz group N7–C8–N9) def R6 (wag C4–C5–C6), wag C8–H ring breath whole molecule (distorted) breath R6, def R5 (wag N7–C8–N9), wag C8–H, N9–H def R5 (sqz group C5–N7–C8), R6 (sqz group C4–C5–C6) * str–stretching; bend–bending; sciss–scissoring; def–deformation; R5–five-membered ring; R6–sixmembered ring; wag–wagging. Page 1 of 15 Raman Intensity / Arbitr. Units 12000 Yeastolate Guanine 10000 8000 655 cm-1 6000 955 cm -1 4000 2000 0 1800 1600 1400 1200 1000 800 600 -1 Wavenumber / cm Figure S1: Overlay of the SERS spectra of sterile yeastolate (5 g/L, BD-Difco-UF) and 10-5 M guanine solution. The guanine spectrum was extracted/reproduced from the paper by Giese and McNaughton.1 The overlay plot above shows that the SERS bands from guanine overlap very well with several of the yeastolate SERS bands confirming the suspicion that guanine is a major contributor to the overall SERS signal. 4 x 10 Raman Intensity / Arbitr. Units 2.5 a 2 1.5 1 0.5 0 1800 1600 1400 1200 1000 800 600 -1 Wavenumber / cm Figure S2: Temporal changes of SERS spectra of a 5 g/L yeastolate (BD-Difco-UF) solution when mixed with Ag colloid over time from 0 to 180 min. Page 2 of 15 S1.1: ROBPCA screening of same source yeast extract batches using normalized SERS data. SERS spectra collected from all 22 samples in triplicate were first subjected to sequential baseline correction, background removal, first derivative, and normalization pre-processing. Then, an ROBPCA analysis was undertaken on the fingerprint region (480~1870 cm-1) of the resulting data. A ROBPCA model was created with three principal components (PCs) which explained 96.02% variance in the mean-centered data. A 95% confidence level was applied to determine the threshold values of two critical parameters of score distance (SD) and orthogonal distance (OD) for each measurement. Using these thresholds (SD=3.06 and OD=0.33) we are able to identify the anomalous measurements (outliers). Raman Intensity / Arbitr. Units 1.0 a 0.5 0.0 -0.5 -1.0 1800 1600 1400 1200 1000 800 600 -1 Wavenumber / cm Figure S3: (a) Overlaid first-derivatized-normalized SERS spectra (480~1870 cm-1) of 66 measurements from 22 yeastolate solutions (5 g/L) in triplicate data collections, (b) ROBPCA outlier map diagnosing the outliers by defining two threshold values of score distance (SD) =3.06 and orthogonal distance (OD) = 0.33 (straight lines), (c) and (d) PC scores plots showing the significant sample discrimination. The first three robust PCs and a confidence level of 95% were employed for the ROBPCA analysis. Solid ellipse specifies a distribution of normal measurements at the 95% confidence level. Page 3 of 15 The samples #12, #13, #16, #17, #22, and #6, #7 each gave three outlying measurements, thus they have a significantly different composition to the other samples. Samples #12, #13, #16, #17, and #22 gave stronger SERS bands and could be modelled well by the three PCs (i.e., the cumulative explained variance was >98% for 3 PCs, Table S2.), with PC2 and PC3 explaining most of the spectral variances. Samples #6 and #7, are different, and have much higher residual information which could not be explained by these three PCs. In contrast to the un-normalized data, we also note that the 2006-vintage were not clearly discriminated from the 2007-vintage yeastolate samples. Comparison of the variance pattern and the difference spectra shows that there are three groups of outliers: Group 1: #13, #17, and #22; Group 2: #12 and #16; and Group 3: #6 and #7. The spectral comparison of these outlier samples with sample #22 in terms of difference spectra regarding the background subtracted data shows these common trends. For example it looks like #12 and #16 have lower adenine concentrations that the #22 sample. Loadings on PC1 (96.43%) 0.25 Loadings on PC2 (3.04%) 0.2 Loadings on PC3 (0.21%) 0.15 PC loadings 0.1 0.05 0 -0.05 -0.1 -0.15 -0.2 -0.25 1800 1600 1400 1200 1000 800 600 -1 Wavenumber / cm Figure S4: Loadings plots of the first three principal components in the ROBPCA model of the firstderivatized SERS spectra (not normalized) of 66 measurements from 22 yeastolate solutions. Table S2: Variance explained by the individual PCs with respect to the normalized SERS data of seven outlier yeastolate samples from run1. Variance explained (%) Sample #13 Sample #17 Sample #22 Sample #12 Sample #16 Sample #6 Sample #7 PC1 2.23 3.77 2.58 16.12 11.90 86.14 83.57 PC2 24.32 23.41 20.80 24.82 24.29 4.94 3.56 Page 4 of 15 PC3 73.12 72.34 74.66 58.22 63.41 1.68 3.59 Cumulative 99.67 99.52 98.05 99.16 99.60 92.76 90.71 1.0 a Raman Intensity / Arbitr. Units Raman Intensity / Arbitr. Units 1.0 0.5 0.0 -0.5 Sample #13 Sample #22 Diff spectrum (#13-#22) -1.0 b 0.5 0.0 -0.5 Sample #17 Sample #22 Diff spectrum (#17-#22) -1.0 1800 1600 1400 1200 1000 800 600 1800 1600 -1 1.0 c Raman Intensity / Arbitr. Units Raman Intensity / Arbitr. Units 1000 800 600 800 600 800 600 Wavenumber / cm 0.5 0.0 -0.5 Sample #12 Sample #22 Diff spectrum (#12-#22) -1.0 d 0.5 0.0 -0.5 Sample #16 Sample #22 Diff spectrum (#16-#22) -1.0 1800 1600 1400 1200 1000 800 600 1800 1600 -1 1.0 1400 1200 1000 -1 Wavenumber / cm Wavenumber / cm 1.0 e Raman Intensity / Arbitr. Units Raman Intensity / Arbitr. Units 1200 -1 Wavenumber / cm 1.0 1400 0.5 0.0 -0.5 Sample #6 Sample #22 Diff spectrum (#6-#22) -1.0 f 0.5 0.0 -0.5 Sample #7 Sample #22 Diff spectrum (#7-#22) -1.0 1800 1600 1400 1200 1000 800 600 -1 1800 1600 1400 1200 1000 -1 Wavenumber / cm Wavenumber / cm Figure S5: Comparison of the outlier samples #13/#17/#12/#16/#6/#7 with #22 (BD-Difco-UF yeastolate) in terms of difference spectra (normalized, background subtracted data). All of the data was collected from the run1 data collection. Page 5 of 15 Raman Intensity / Arbitr. Units Raman Intensity / Arbitr. Units a 30k Sample #13 Sample #22 Diff spectrum (#13-#22) 20k 10k 0 b 30k Sample #17 Sample #22 Diff spectrum (#17-#22) 20k 10k 0 1800 1600 1400 1200 1000 800 600 1800 1600 -1 1200 1000 800 600 800 600 Wavenumber / cm 30k 30k c Raman Intensity / Arbitr. Units Raman Intensity / Arbitr. Units 1400 -1 Wavenumber / cm 20k Sample #12 Sample #22 Diff spectrum (#12-#22) 10k 0 d 20k Sample #16 Sample #22 Diff spectrum (#16-#22) 10k 0 -10k -10k 1800 1600 1400 1200 1000 800 600 -1 1800 1600 1400 1200 1000 -1 Wavenumber / cm Wavenumber / cm Figure S6: Comparison of the outlier samples with #22 (BD-Difco-UF yeastolate) in terms of both the background subtracted SERS data (not normalized) and their difference spectra: (a) sample #13, (b) #17, (c) #12, and (d) #16 from the run1 data collection. Page 6 of 15 S1.2: Reproducibility of SERS spectra collected from sterile yeastolate solution samples stored at different temperatures for 30 days Figure S7 shows the SERS spectra collected from sterile solution samples stored at different temperatures for 30 days. Raman Intensity / Arbitr. Units 30k a 20k Day 30 Day 20 Day 15 10k Day 11 Day 8 Day 6 Day 1 0 1800 1600 1400 1200 1000 800 600 800 600 -1 Raman Intensity / Arbitr. Units Wavenumber / cm 30k b 20k Day 30 Day 20 Day 15 10k Day 11 Day 8 Day 6 Day 1 0 1800 1600 1400 1200 1000 -1 Wavenumber / cm Page 7 of 15 Raman Intensity / Arbitr. Units 30k c 20k Day 30 Day 20 Day 15 10k Day 11 Day 8 Day 6 Day 1 0 1800 1600 1400 1200 1000 800 600 -1 Wavenumber / cm Figure S7: SERS spectra of sterile yeastolate (5 g/L, BD-Difco-UF) solution recorded over 30 days stored at: (a) -70°C, (b) 2~4°C, and (c) room temperature. Spectra shown were just baseline-corrected. Page 8 of 15 Page 9 of 15 Figure S8: SERS spectra of sterile yeastolate (5 g/L, BD-Difco-UF) solution recorded over 30 days stored at: (a) -70°C, (b) 2~4°C, and (c) room temperature. Spectra have been baseline-corrected and background-subtracted. A comparison of Figures S7 and S8 shows the spectral differences induced by the background subtraction. Figure S9: Overlay plot of SERS spectra of sterile yeastolate (5 g/L, BD-Difco-UF) solution recorded over 30 days stored at: (a) -70°C, (b) 2~4°C, and (c) room temperature. Spectra are baseline-corrected, background-subtracted, and then normalized. Page 10 of 15 Table S3: Similarity coefficients between the SERS spectra (480~1870 cm-1 range) obtained from 5 g/L sterilized yeastolate (BD-Difco-UF) solution over a period of 30 days when stored at -70°C, 2~4°C, and room temperature. The similarity was calculated using the baseline-corrected and background-subtracted spectra after being subjected to maximal normalization. Storage -70°C 2~4°C room temp Day1 Day6 Day8 Day11 Day15 Day20 Day30 Day1 Day6 Day8 Day11 Day15 Day20 Day30 Day1 Day6 Day8 Day11 Day15 Day20 Day30 Day1 Day6 1 0.99 0.98 0.99 0.98 0.98 0.98 1 0.99 0.98 0.98 0.98 0.98 0.98 1 0.98 0.98 0.98 0.98 0.98 0.97 0.99 1 0.99 0.98 0.99 0.98 0.99 0.99 1 0.99 0.99 0.99 0.99 0.98 0.98 1 0.99 0.99 0.99 0.99 0.99 Day8 Day11 Day15 Day20 Day30 Similarity coefficients 0.98 0.99 0.98 0.98 0.98 0.99 0.98 0.99 0.98 0.99 1 0.98 0.98 0.98 0.99 0.98 1 0.97 0.97 0.98 0.98 0.97 1 0.99 0.99 0.98 0.97 0.99 1 0.99 0.99 0.98 0.99 0.99 1 0.98 0.98 0.98 0.98 0.98 0.99 0.99 0.99 0.99 0.98 1 0.99 0.99 0.99 0.98 0.99 1 0.99 0.99 0.98 0.99 0.99 1 0.99 0.99 0.99 0.99 0.99 1 0.98 0.98 0.98 0.99 0.98 1 0.98 0.98 0.98 0.98 0.97 0.99 0.99 0.99 0.99 0.99 1 0.99 0.99 0.99 0.99 0.99 1 0.99 0.99 0.99 0.99 0.99 1 1 0.99 0.99 0.99 1 1 0.99 0.99 0.99 0.99 0.99 1 Table S4: Within- and between-group variances obtained from SERS spectra of sterile yeastolate solution (BD-Difco-UF) stored at -70°C, 2~4°C, and room temperature for 30 days. The variance was calculated using the baseline-corrected spectra. Variance (×107) -70°C 2~4°C room temp RMSw -70°C *4.23 0.06 0.32 2.13% 2~4°C 0.06 *5.37 0.24 2.65% room temp 0.32 0.24 *4.78 2.13% * These values correspond to within-group variance. This table (Table S4) should be compared with Table 1 in the manuscript. Page 11 of 15 Table S5: Similarity coefficients between SERS spectra (480~1870 cm-1 range) obtained from unsterilized yeastolate solution (5 g/L, BD-Difco-UF) stored at 2~4°C over 21 days. Values were calculated from the baseline-corrected and background-subtracted SERS spectra Similarity Day 1 Day 5 Day 9 Day 21 Day 1 1 0.97 0.86 0.73 Day 5 0.97 1 0.80 0.69 Day 9 0.86 0.80 1 0.92 Page 12 of 15 Day 21 0.73 0.69 0.92 1 S1.3: Spectral differences between outliers and normal yeastolate EEM spectra. Figure S10 shows the difference spectra between the six outlier samples #13, #17, #22, #12, #16, #8 and the mean EEM spectrum (calculated by averaging the main group of normal measurements specified by the MROBPCA model). The triplicate EEM measurements were first averaged into one spectrum for each sample then the mean subtracted. It is evident that the major outlier samples #13/#17/#12/#16 are significantly more fluorescent than the main group of normal samples, whereas samples #22 and #8 are appreciably less fluorescent. Figure S10: Comparison of the outlier samples #13/#17/#22/#12/#16/#8 with the mean spectrum of the main group of normal measurements specified by the MROBPCA model in terms of difference spectra Page 13 of 15 (Rayleigh and Raman scattering removed data). The difference spectra were obtained by first averaging the triplicate EEM measurements of each of the individual samples #13/#17/#22/#12/#16/#8 into one spectrum, and then respectively subtracting the mean EEM spectrum of the main group of normal measurements. S1.4: Multiway ROBPCA of normalized fluorescence EEM data The EEM data obtained from triplicate measurements of 22 yeastolate solutions (5 g/L) was first treated by removal of both Rayleigh and Raman scattering using the interpolation method. The resulting data were then normalized using the Frobenius matrix norm. The MROBPCA model was generated using four PCs and a 95% confidence level was used to set SD = 3.34 and OD = 0.032. Both score and orthogonal distances were calculated for each measurement in the model. The model-generated outlier diagnostic map (Figure S11b) shows 9 outlier measurements, of which #16/#20 had 2 of 3 spectra outside the model limits. We would be confident that these two samples represent samples with a significant compositional difference to the main group samples. The other outliers (#22/#8/#12/#11/#18) are singular events where one EEM spectrum out of three is outside the model limits, and thus we suggest that these are due to measurement errors, although sample #22 is the BD Difco sample. In this normalized dataset, the 2006-vintage yeastolate samples were not clearly discriminated from the 2007-vintage yeastolate samples using the 4-PC MROBPCA model. This indicates that the overall fluorescence intensity is a factor that needs to be retained in the analytical methodology. This incurs a penalty in terms of method robustness, but can be addressed by appropriate control measurements. Page 14 of 15 Figure S11: (a) Representative EEM landscape plot (not normalized) of a 5 g/L yeastolate solution after Rayleigh and Raman scattering removal. The local maxima are marked by arrows and the excitation/emission values; (b) MROBPCA outlier diagnostic map (orthogonal distance versus score distance) for the triplicate measurement of 22 yeastolate solutions (5 g/L). The straight lines define two threshold values: SD = 3.34 and OD = 0.032 at a confidence level of 95% for the MROBPCA analysis with four PCs; (c) and (d) PC scores plots showing the significant sample discrimination (red markers indicate outliers). The solid ellipse signifies the 95% confidence level. References: [1] B. Giese, D. McNaughton, Phys. Chem. Chem. Phys. 2002, 4, 5171. Page 15 of 15