Isotopes and Average Atomic Mass
advertisement

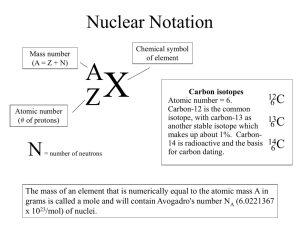
SCH 3U1 Isotopes and Average Atomic Mass Atomic mass units are a relative measure used to express the masses of atoms and molecules. All atomic masses relate to the carbon-12 atom Therefore 1u = 1/12 of the mass of a carbon atom Isotopic abundance: the relative amount in which each isotope is present in an element. The average atomic mass (mass number) of carbon on the periodic table is 12.01u, why? ___________________________________ There are three isotopes of carbon, they are carbon-12, carbon-13 and carbon-14 Each is present in differing amounts Carbon-12 makes up 98.9% Carbon-13 makes up 1.1% Carbon-14 makes up 1X10-10 % We calculate average atomic mass using weighted averages. Example 1: Lithium exists as lithium-7 and lithium-6 Lithium-7= 7.015u and accounts for 92.58% Lithium-6= 6.015u and accounts for 7.42% 7.015 u (0.9258) + 6.015 u (0.0742) = _____