Equations & Reactions
advertisement

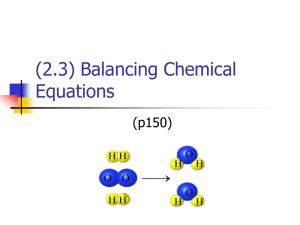
Equations & Reactions 8.1 Describing Chemical Reactions A. Chemical Changes and Reactions 1. New substances are produced. 2. Chemical reaction – chemical bonds between atoms or ions break, and new bonds form between atoms or ions. B. Evidence of a Chemical Reaction 1. color change 2. formation of a precipitate 3. temperature change 4. formation of a gas C. Mechanics of a Chemical Reaction 1. Starting Materials – reactants 2. Ending Materials - products 3. reactants → products Arrow = yields or produces 4. Many reactions occur to complete a set of valence electrons. 5. Symbols above the yield sign represent conditions necessary for a reaction to proceed. Ex) Δ = delta = heat elec = electrolysis 6. Some reactions occur spontaneously. 7. Symbols represent the state of the reactants and products. Liquid = l Gas = g Solid = s Crystal = cr Aqueous = aq (solids in water solution) DEMO Ex) 2Al(s) + 3CuCl2(aq) → 2AlCl3(aq) + 3Cu(s) silver blue gray red 8. Complete chemical equations include the subscript to indicate the physical state of each substance. 9. Diatomic molecules – certain elements exist in nature as diatomic molecules (X2) List them: N2 O2 F2 Cl2 Br2 I2 H2 Natural States of the Elements • Diatomic Molecules Nitrogen gas contains N2 molecules. Oxygen gas contains O2 molecules. Highlight your Periodic Table Natural States of the Diatomic Elements 8.2 Balancing Equations A. Equations in Chemistry 1. Chemical equation: an expression that uses symbols and formulas to describe a chemical reaction. 2. + means “reacts with.” 3. → means produces (called the yield sign.) B. Balancing Chemical Equations 1. Conservation of mass leads to balancing equations – the number of atoms of each element must be the same before & after the reaction. 2. The Law of Conservation of Mass also states that the total mass before and after the reaction must be the same. You cannot lose or gain mass. 3. Therefore the MASS OF THE PRODUCTS = MASS OF REACTANTS 4. Subscript – indicates number of atoms of an element present in a compound. 5. Coefficient – indicates the number of atoms or molecules involved in the reaction. 6. Steps to Balance Equations: A. Write equation with symbols. B. Count # of atoms on each side of the reaction. C. Balance atoms using coefficients. D. General Rule: Balance all elements first. Then, balance C, H, and O. E. NEVER CHANGE SUBSCRIPTS!!!! 3 H2 + N2 → 2 NH3 3 Na2SO4 + Ca3(PO4)2 → 2 CaSO4 + 2 Na3PO4 2 NaNO3 → 2 NaNO2 + O2 2 C8H18 + 25 O2 → 16 CO2 + 18 H2O 8.3 Classifying Chemical Reactions A. Synthesis Reactions (direct combination) 1. Two or more elements or compounds combine to form a more complex product. A + B → AB 2. Ex. Fe + S → FeS CaO + H2O → Ca(OH)2 sodium reacts with chlorine 2 Na + Cl2 → 2 NaCl Synthesis Reaction Sodium Metal plus Chlorine Gas Video 2 Na + Cl2 2 NaCl Synthesis Reaction B. Decomposition Reactions (analysis) 1. A single reactant breaks down into simpler compounds or elements. AB → A + B 2. The opposite of a synthesis reaction. 3. Carbonates (compounds ending in CO3) break down into metal oxides and carbon dioxide. 4. Ex. 2 HgO CaCO3 → → 2 Hg + O2 CaO + CO2 Decomposition Reaction CuCO3(s) CO2(g) + CuO(s) flame goes out copper (II) carbonate metal oxide What is one of the products? 2HgO → 2Hg + O2 mercury (II) oxide mercury (II) oxide C. Single Replacement Reactions 1. Atoms of an uncombined element replace atoms of another element in a compound. A + BX → AX + B 2. A more active element will replace a less active element. (See activity series.) 3CuCl2 + 2Al 2AlCl3 + 3Cu 3. An Activity Series is a way of ranking elements (usually metals) in order from greatest to least reactivity. It can be used to predict whether a reaction will occur or not. 2 Al + 3 CuSO4 Fe + MgCl2 PbSO4 + Au AgCl2 + Cu → 3 Cu + Al2(SO4)3 → No Reaction → No Reaction → CuCl2 + Ag D. Double-Replacement Reactions 1. Atoms or ions from two different compounds replace each other. AX + BY → AY + BX 2. These types of reactions will (A) form precipitates (↓) (B) form gases (↑) (C) are acid-base neutralizations Double Replacement Examples: A. Pb(NO3)2 + 2 KI → PbI2 ↓ + 2 KNO3 B. CaCO3 + 2 HCl → CaCl2 + H2CO3 C. NaOH + HCl → NaCl + HOH 3. In letter B above, carbonic acid, H2CO3, is unstable and will immediately decompose into carbon dioxide and water. CaCO3 + 2 HCl → CaCl2 + CO2↑ + H2O E. Combustion Reactions 1. One substance reacts with oxygen, O2 to produce oxide compounds. 2. Occurs during burning or oxidation (rusting.) 3. The reactions that only add oxygen are classified as synthesis reactions. Ex) S + O2 → SO2 Hydrogen Burning Video H2 + 2 O2 2 H2O Synthesis Reaction 4. Combustion reactions are exothermic, releasing a large amount of energy as light, heat, or sound. 5. A true Combustion reaction occurs when a hydrocarbon (compound containing H & C ) reacts to form carbon dioxide and water that are always the products. CxHx + O2 → CO2 + H2O 2 2O + 803 kJ 2 2 → __CO2 + __H 6. Ex. __CH4 + __O C6H12 O6 + 6 O2 → 6 CO2 + 6 H2O + heat Combustion Reaction Combustion Reaction 5 Types of Chemical Reactions Video