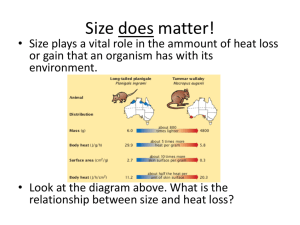
Physiology of Kidney Renin
advertisement

Physiol Rev 90: 607– 673, 2010; doi:10.1152/physrev.00011.2009. Physiology of Kidney Renin HAYO CASTROP, KLAUS HÖCHERL, ARMIN KURTZ, FRANK SCHWEDA, VLADIMIR TODOROV, AND CHARLOTTE WAGNER Institute of Physiology, University of Regensburg, Regensburg, Germany I. Introduction II. The Renin Gene A. Regulatory noncoding sequences of the renin gene B. Regulation of renin mRNA stability C. Control of renin gene expression at the cellular level D. Sites of renin expression E. Development of renal renin expression F. Recruitment of renin expression in the adult kidney III. The Juxtaglomerular Cell A. Processing and secretion of renin in juxtaglomerular cells B. Electrical properties of juxtaglomerular cells C. Intercellular contacts of juxtaglomerular cells D. Intracellular signals controlling renin release IV. Local Control of Renin Secretion A. Tubular control of renin secretion B. Vascular control of renin secretion C. Neural control of renin secretion V. Systemic Control of Renin Synthesis and Secretion A. Blood pressure B. Salt intake C. Hormones, autacoids, and other mediators V. Action of Renin A. AT1 and AT2 receptors B. Renin/pro-renin receptor C. The ANG-(1–7)-ACE2-Mas axis VII. Conclusion 607 608 608 611 611 613 613 615 615 615 617 618 620 623 623 628 630 630 630 633 633 643 643 645 646 649 Castrop H, Höcherl K, Kurtz A, Schweda F, Todorov V, Wagner C. Physiology of Kidney Renin. Physiol Rev 90: 607– 673, 2010; doi:10.1152/physrev.00011.2009.—The protease renin is the key enzyme of the renin-angiotensinaldosterone cascade, which is relevant under both physiological and pathophysiological settings. The kidney is the only organ capable of releasing enzymatically active renin. Although the characteristic juxtaglomerular position is the best known site of renin generation, renin-producing cells in the kidney can vary in number and localization. (Pro)renin gene transcription in these cells is controlled by a number of transcription factors, among which CREB is the best characterized. Pro-renin is stored in vesicles, activated to renin, and then released upon demand. The release of renin is under the control of the cAMP (stimulatory) and Ca2⫹ (inhibitory) signaling pathways. Meanwhile, a great number of intrarenally generated or systemically acting factors have been identified that control the renin secretion directly at the level of renin-producing cells, by activating either of the signaling pathways mentioned above. The broad spectrum of biological actions of (pro)renin is mediated by receptors for (pro)renin, angiotensin II and angiotensin-(1–7). I. INTRODUCTION Since its discovery 100 years ago, an impressive quantity of information about renin has been compiled. Notably, researchers have determined that the renin-driven www.prv.org renin-angiotensin-aldosterone system (RAAS) plays a major role not only in the homeostatic processes, such as blood pressure and fluid volume control, but also in the development and progression of fibrotic/hypertrophic diseases. We have learned to distinguish between the systemic RAAS, 0031-9333/10 $18.00 Copyright © 2010 the American Physiological Society 607 608 CASTROP ET AL. which is mainly controlled by the production and release of renin from the kidneys into the circulation, and the more locally acting renin-angiotensin systems that are present in a variety of organs. This review predominantly focuses on kidney renin, and it is thus a follow-up of the milestone review published in Physiological Reviews 30 years ago (181). Since then, an influx of exciting new information about the renin gene and its regulation, the renin-secreting cells in the kidney and their regulation, and the activity of the renin-angiotensin cascade has accumulated and will be considered in this review. Among these new discoveries is the identification of the local renin-angiotensin systems, which were recently extensively reviewed in this journal (674) and will therefore not be the focus of the present review. Our review concentrates on the organization and transcriptional control of the renin gene and on the nature of renin-producing cells in the kidney. It further considers the regulation of renin secretion on the cellular level and its control by local or systemic factors. Finally, we consider the different biological signaling pathways and their biological effects initiated by renin activity. Some inbred mouse strains are unique in having two renin genes. Mouse strains with a single renin gene possess Ren-1C, while mouse strains with two renin genes have Ren-1D and Ren-2 (3, 228). The Ren-1 and Ren-2 genes are closely related and probably originated from a gene duplication that occurred about three million years ago (197, 352). The functional significance of this gene duplication remains unknown. Ren-2 is strongly expressed in the submandibular gland (197, 229). The expression pattern of Ren-1, however, correlates best between the rat and human renin genes (214, 246). In addition, the product of Ren-2 lacks key glycosylation sites and is consequently sorted to the constitutive secretory pathway (163) (see sect. IIIA). Originally, it was believed that mice with two renin genes should exhibit higher plasma renin levels. However, in contrast to this hypothesis, mouse strains expressing Ren-2 have normal blood pressure (76, 578). A later systematic study demonstrated that plasma renin concentrations are actually not different between mouse strains with one or two renin genes (309). II. THE RENIN GENE A. Regulatory Noncoding Sequences of the Renin Gene The structure of the renin gene (Ren-1) has been determined for several different species, including sheep, rats, humans, dogs, and mice (13, 103, 245, 313, 341, 671). Sequence analysis has revealed high interspecies homology in the structural elements of the gene. More specifically, the mouse and rat renin genes possess nine exons and eight introns, while the human and sheep renin genes contain one additional exon (exon 5A) that encodes three additional amino acids. In both humans and mice, the renin gene was mapped to chromosome 1. The 5⬘-flanking noncoding region plays a central role in regulating the renin gene in all species studied. This region is therefore considered as a classical promoter, i.e., a 5⬘-cis-acting regulatory element (Fig. 1). Most of the information on the function of the renin promoter has been obtained from cell culture models. The isolation of the clonal renin-producing cell line As4.1 from the kidney of simian virus 40 (SV40)-T antigen transgenic mice has provided an important tool for studying the cell-specific regulation of renin transcription (802a). The isolation of FIG. 1. Structural organization of the renin promoter. Schematic map of the 5⬘-flanking regulatory elements of the renin gene and the binding of the transcription factors. Double-headed arrows bind the conserved regulatory regions in mouse (mRen) and human (hRen) renin genes. Physiol Rev • VOL 90 • APRIL 2010 • www.prv.org PHYSIOLOGY OF KIDNEY RENIN the native renin-expressing juxtaglomerular (JG) cells in primary cultures also represents a relevant model; however, it has two limitations. First, the expression of renin is turned off within several days in the culture, and second, JG cells usually represent only a minority of the isolated cells. These disadvantages of the primary JG cell cultures appear to have been overcome recently by a double-transgenic labeling of cells of renin lineage in vivo and their subsequent isolation and cultivation in vitro (681). However, this model is still awaiting a broader application to confirm its efficiency and reliability. The function of renin gene regulatory sequences in vivo has been intensively studied during the last few years. Recently, a number of transgenic mice with various deletions in the renin promoter have been generated. Surprisingly, some in vivo results have deviated from the models predicted on the basis of the existing cell culture data (269a, 857, 989, 990). Some of these discrepancies may reflect species-specific differences; however, it also appears possible that the in vitro findings may not be completely relevant for the in vivo situation. In any case, there is still a great number of cell culture data on the function of the renin promoter that needs to be validated at the level of the whole organism. Therefore, in the following sections, which concern the molecular and cellular mechanisms of renin gene expression (sect. II, A–C), we have focused on the results from cells with endogenous renin production, followed by discussion of the in vivo data if available. 1. The proximal renin promoter A 123-bp sequence from ⫺117 to ⫹6 in the mouse Ren-1C gene (all positions hereafter are relative to the transcription starting site) was originally defined as a “proximal renin promoter” (690). This sequence was found to be necessary for the maximal cell-specific expression of mouse and human renin genes (83, 690). The proximal promoter is highly homologous in human, mouse, and rat renin genes and contains a perfectly conserved TATA box. Further upstream, the homology is interrupted by an ⬃500-bp insertion that is only present in mouse renin genes (106). Currently, the term proximal renin promoter refers to the 5⬘-regulatory sequences located roughly downstream to position ⫺200, since a row of important cis-regulatory elements has subsequently been identified in this region. The general transcription factor binding site pattern of the proximal renin promoter suggests that this region is important for the basal activity of the renin gene, for the transcriptional regulation by cAMP and nuclear receptors and for the control of renin gene expression during ontogenesis. A) PROXIMAL PROMOTER CIS-ACTING ELEMENTS TARGETED BY CAMP. Renin is a cAMP-inducible gene. The presence of a Physiol Rev • VOL 609 functional cAMP response element (CRE) is a characteristic feature of the renin promoter in all species tested (83, 264, 442, 620, 663, 794, 975). A proximal CRE sequence is located at ⫺226 to ⫺219 in the human renin gene (975). However, only the 3⬘ half of this motif is evolutionarily conserved, which makes its role in the regulation of the renin gene uncertain. There is substantial evidence suggesting that several CRE-unrelated proximal promoter sequences are also involved in the cAMPdependent regulation of the renin gene. A Pit-1 transcription factor binding site at ⫺77 to ⫺67 in the human renin promoter is necessary for stimulation of renin transcription by cAMP (265, 833, 834). The homologous sequence in the mouse Ren-1C gene, which spans ⫺72 to ⫺50, was also shown to be important for transcriptional activity. This DNA motif is bound by the homeodomain proteins HOX and PBX (135, 665, 669). The HOX/PBX core binding sequence was recently found to be indispensable for the basal renin expression level in the kidney in vivo (269a, 857). Interestingly, the very same sequence is targeted by the retinoblastoma gene product (RB) to stimulate the Ren-1C promoter activity in human embryonic cells, which do not however express endogenous renin (852). Therefore, the role of RB in the cell-specific control of renin transcription remains to be further elucidated. The nuclear receptor LXR-␣ also mediates the cAMP effect via a motif called CNRE (cAMP and negative regulatory elements). The CNRE is located at position ⫺128 to ⫺115 in the human renin gene and at ⫺611 to ⫺599 in the mouse Ren-1D gene (356, 357, 850, 851). This sequence displays little homology to either the CRE or the classical DR4 LXR-responsive element (DR4/LXRE). However, the partial deletion of the proximal promoter LXR-␣ binding motif affected neither the basal expression nor the regulation of the renin gene in vivo, thus contradicting the physiological significance of the CNRE (see also sect. IIC1) (857). B) FURTHER CIS-ACTING ELEMENTS IN THE PROXIMAL RENIN PROMOTER. The human renin gene was recently identified as the first gene regulated by the transcription factor peroxisome proliferator-activated receptor-␥ (PPAR-␥), which occurs through a nontypical Pal3 (palindrome with a 3-bp spacer) binding site located at ⫺148 to ⫺134 (881). Although the renin Pal3 site resides in an evolutionarily conserved promoter region, we found that mouse and rat renin Pal3 elements are transcriptionally silent (882). The critical bases of the Pal3 motif that confer full inducibility by PPAR-␥ were mapped to the 5⬘-repeat and to the last base of the spacer (882). The human renin Pal3 sequence is unique in selectively binding PPAR-␥, which permits the maximal agonist-dependent stimulation of transcription, particularly at low cellular levels of PPAR-␥. Thus the human renin gene appears to be more sensitive to PPAR-␥ than its murine orthologs. 90 • APRIL 2010 • www.prv.org 610 CASTROP ET AL. The nuclear effector of the Notch signaling pathway (CBF1) bound to a sequence in the proximal promoter (⫺210 to ⫺191, ⫺679 to ⫺660, and ⫺185 to ⫺166 in human, mouse, and rat renin genes, respectively) was recently identified to be a partner of the HOX/PBX transcription factors (665, 669). Both the Notch system and the HOX proteins are important during embryonic development; more specifically, they are believed to participate in regulating the tissue-specific expression of the renin gene (494, 559, 665). In addition, the developmentally active Ets transcription factors have also been reported to interact with sequences within the proximal renin promoter (665). Another important regulatory sequence lies within the proximal promoter of the mouse Ren-1C gene, just upstream of the HOX/PBX-binding site (667). The deletion of this DNA-fragment (⫺197 to ⫺70) resulted in an almost complete loss of renin transcriptional activity. At least two transcription factor families, namely, NFI and Sp1/ Sp3, bind to this region of the proximal promoter (667). 2. Renin gene enhancers In addition to the proximal promoter region, the renin gene is controlled by two known 5⬘-flanking enhancer elements. An enhancer is typically defined as a regulatory DNA sequence that strongly induces transcription in an orientation and position-independent manner. A) RENAL ENHANCER. A 242-bp enhancer element was identified at ⫺2866 to ⫺2625 in the mouse Ren-1C gene. This sequence was shown to stimulate renin transcriptional activity up to 100-fold in the renin-producing cell line As4.1 (690). Since the As4.1 cells are of renal origin, the Ren-1C enhancer is called the “renal” or “kidney” enhancer (690, 802a). Emerging evidence suggests that the mouse renal enhancer directs the on/off switching rather than the transactivation rate of the renin gene in the single cell in vitro (601). Such a variegation model of renin expression control is feasible because the reninproducing cells are “recruited” during chronic stimulation in vivo (see sect. IIF). However, additional studies are necessary to confirm or reject this concept. The renal enhancer is also present in the human renin promoter, although at a more distal position (about ⫺12 kb) (966). The human and mouse kidney enhancers are highly homologous in their 202-bp distal portions, while the similarity in their 40-bp proximal fragments (m40) is only 45% (794). The heterology in the m40 sequence explains the lower transactivating capacity of the human enhancer compared with the mouse enhancer (379; see below). The role of the renal enhancer in the regulation of renin expression in vivo is still not completely understood. Studies of transgenic mice expressing human renin from an artificial chromosome have demonstrated that Physiol Rev • VOL the renal enhancer of the human renin gene is dispensable for the stimulation of renin gene expression by angiotensin converting enzyme (ACE) inhibition (989). In contrast, deletion of the renal enhancer of the endogenous renin gene in mice has revealed that it is necessary for the full activation of renin transcription by the combination of a low-salt diet and the ACE inhibitor (7, 562). On the other hand, both the human and mouse renal enhancers appear to be critical for the basal transcription of renin, both in vitro and in vivo (7, 989). B) CIS-ACTING ELEMENTS IN THE RENAL ENHANCER. The downstream enhancer portion of the renin gene contains a CRE and a more proximal E-box, which bind the CREB/CREM and USF1/USF2 transcription factors, respectively (442, 663). These binding sites are critical for the function of the renal enhancer, since a mutation of either one results in an almost complete loss of its activity in As4.1 cells (663). Moreover, CRE mediates the stimulatory effect of the cAMP/PKA cascade on renin expression. Thus cAMP signaling has at least two targets in the renin gene regulatory sequences, the nonconsensus CNRE site located in the proximal promoter (see above), and a canonical CRE located in the renal enhancer. It is still not understood whether the distinct cAMP-targeted cis-acting elements are redundant, thus underscoring the role of cAMP in the transcriptional control of the renin gene, or whether each has specific functions. Two directly repeated TGACCT sequences are located downstream and adjacent to the E-box (379, 796). This hexamer repeat represents the DNA binding site for the superfamily of nuclear receptors and is generally known as the hormone responsive element (HRE) (29). Consistently, the nuclear receptors retinoic acid receptor/ retinoid X receptor (RAR/RXR), PPAR-␥, and the orphan receptor Ear2 were found to bind to this enhancer element (521, 796, 882). It is important to note that the human renin HRE differs from the mouse sequence both in the spacer between the hexamer repeats and in the proximal hexamer repeat (794). While the deviations in the spacer are not critical for the transcriptional activity of the enhancer, the substitution from A to G in the proximal hexamer of the HRE is responsible for the lower transactivating capacity of the human kidney renin enhancer (379). In the mouse renin kidney enhancer, the downstream TGACCT motif is overlapped by an NF-Y transcription factor binding site (794, 795). It has been suggested that NF-Y prevents the binding of transcriptional regulators to the hexamer motif, thus inhibiting the enhancer’s activity (795). There are at least seven important transcription factor binding sites in the more distal enhancer fragment. One of these motifs binds the Wilms’ tumor suppressor, WT1, which inhibits renin transcription (826). The other six motifs are bound by the NFI and Sp1/Sp3 transcriptional regulators (664). Notably, the same transcription 90 • APRIL 2010 • www.prv.org PHYSIOLOGY OF KIDNEY RENIN factors bind to the proximal renin promoter (667). It remains to be clarified whether these multiple renin promoter binding sites are redundant or have different functions. The mutation of either the enhancer or the proximal NFI/Sp1/Sp3 binding sequences almost completely eliminates the renin promoter activity, thus indicating a crucial role for these transcription factors in the control of renin gene expression (664, 667). C) CHORIONIC ENHANCER. A second enhancer was identified in the human renin 5⬘-flanking region between ⫺5777 and ⫺5552 (263). This enhancer delivered an ⬃60-fold induction of the human renin promoter activity in chorionic cells and, therefore, is known as the “chorionic” enhancer (263). However, the chorionic enhancer is not evolutionarily conserved and was consequently found to be transcriptionally silent in transgenic mice (990). 3. Intron I regulatory sequences In addition to the promoter sequences, regulatory DNA elements have been identified in intron I of the human and rat renin genes (262, 919). Intron I has been shown to exert a silencing effect on the renin gene expression. Closer characterization of the rat intronic silencer sequence in vitro has led to the identification of two positive and five distinct negative cis-acting elements (918). B. Regulation of Renin mRNA Stability In addition to the tight transcriptional control described above, renin gene expression is also regulated at the posttranscriptional level (147, 499, 809). In general, the mechanisms that stabilize or degrade mRNAs are not completely understood. For renin mRNA, it is known that its stability is controlled by mRNA binding proteins, which interact with the 196-bp-long 3⬘-untranslated region (UTR) of renin mRNA. Multiple mRNA binding proteins have been shown to bind to the renin mRNA 3⬘-UTR (6, 811). HuR, CP1/hnRNP E1 [poly(C)-binding protein-1], nucleolin, dynamin, and YB-1 are thought to stabilize renin mRNA. The HADHB [hydroxyacyl-CoA dehydrogenase/3-ketoacyl-CoA thiolase/enoyl-CoA hydratase (trifunctional protein)--subunit] protein is believed to destabilize renin mRNA, while the effects of the MINThomologous protein and the hnRNP K protein on renin mRNA stability are unknown. Thus, in addition to the transcriptional mechanisms, posttranscriptional modifications of renin mRNA are also important for the control of renin gene expression. However, it is currently difficult to evaluate to what quantitative extent each of these two mechanisms contribute to the regulation of renin mRNA abundance. In fact, the single cell produces more transcripts than the accumulated RNA steady-state level, demonstrating the existence of constitutive RNA degradation Physiol Rev • VOL 611 mechanisms (reviewed recently in Ref. 358). Therefore, it has been proposed that changes in the mRNA stability provide a faster response of renin gene expression to regulatory signals (602). C. Control of Renin Gene Expression at the Cellular Level Altogether, our knowledge of the intracellular mechanisms that regulate renin transcription suggests that the cAMP/PKA/CREB and calcium/PKC cascades are employed by physiological cues, whereas STATs, NFB, and members of the nuclear receptor superfamily probably only become relevant in pathological situations. Similar to the renin gene regulatory sequences, most of the information on the cellular control of renin transcription comes from cell culture models. Consequently, not much is known about the relevance of the in vitro identified molecular mechanisms for the regulation of renin gene expression in the whole organism. Therefore, transgenic animals with selective and cell-specific gene inactivation are expected to deliver novel information on this issue. 1. cAMP and protein kinase A The second messenger cAMP is produced by adenylyl cyclases, which in turn are regulated by extracellular stimuli through G protein-coupled receptors. cAMP exerts its effects on transcription by activating protein kinase A (PKA) (497). cAMP binds to the two regulatory subunits of PKA to release two catalytic subunits from the inactive PKA tetramer complex. The free catalytic subunits (referred to as activated PKA) translocate to the nucleus and phosphorylate transcription factors of the cAMP response element binding protein/activating transcription factor (CREB/ATF) family. These transcription factors are constitutively bound to CRE in the regulatory sequences of cAMP-driven genes and are transactivated by the PKAinduced phosphorylation (596). cAMP signaling is believed to be the central stimulatory pathway in renin gene transcription (442, 663). cAMP mediates the stimulation of renin expression by catecholamines/sympathetic activation and prostaglandins (146, 401, 473). In addition, the cAMP/PKA cascade essentially determines the basal transcription rate of the renin gene (663). CREB is a prototypical representative of the CREB/ATF transcription factor family that mediates cAMP signaling to the renin gene (265, 442, 497, 663, 975). CREB was found to bind to the renin CRE sequences identified in the proximal promoter and in the kidney enhancer. CREM (cAMP response element modulator) and possibly ATF1, which are close relatives of CREB, also interact with the CRE enhancer (442, 663). Notably, all three of these proteins share the conserved target sequence for PKA. 90 • APRIL 2010 • www.prv.org 612 CASTROP ET AL. Studies in transgenic animals have unambiguously confirmed the central role of cAMP in the physiological control of basal renin gene expression. Chen et al. (146) generated a transgenic mouse line with a specific deletion of the Gs␣ protein in renin-producing cells (146). Gs␣ is an essential subunit of the stimulatory G protein that couples adenylyl cyclase with activating receptors such as the IP, EP4, and the -adrenergic receptors. These receptors, in turn, mediate the effects of prostacyclin, prostaglandin E2, and catecholamines on renin expression (241, 423). The local knockout of Gs␣ in renin-producing cells has been shown to sharply diminish renal renin mRNA levels and plasma renin concentrations (PRC) (146). Unfortunately, the G protein receptor agonist-induced stimulation of renin gene expression was not tested in the JG-specific Gs␣ knockout mice. Thus the role of cAMP signaling in the physiological regulation of renin transcription in vivo remains to be definitely confirmed. The binding of the nuclear factor LXR-␣ as a monomer to the CNRE proximal promoter motif was also reported to mediate the cAMP-dependent stimulation of renin transcription (356, 357, 850, 851). PKA has been suggested to phosphorylate LXR-␣ at two potential sites within its ligand binding domain (LBD) (426). Additionally, the COOH-terminal AF-2 transactivation domain in the LXR-␣ molecule was found to be necessary for responsiveness to cAMP (850). The renin gene in LXR-␣ ⫺/⫺ mice is insensitive to adrenergic stimulation (599); however, the CNRE sequence is dispensable for the regulation of renin expression in vivo (857). Therefore, the mechanisms of regulation of renin transcription by LXR-␣ need to be further investigated. cAMP also increases the stability of renin mRNA in cultured cells (see sect. IIB) (147, 499, 811). In support of this observation, the adenylyl cyclase activator forskolin increased binding of the stabilizing factors HuR and CP1 to the 3⬘-UTR of human renin mRNA (6). 2. Calcium and protein kinase C In contrast to the stimulatory function of cAMP, cytosolic calcium and/or protein kinase C (PKC) are involved in the suppression of renin expression (188, 606). This unusual role for calcium/PKC is termed the “calcium paradox” of renin production. Hormones such as angiotensin (ANG) II and endothelins inhibit renin gene expression and secretion by increasing the cytosolic calcium and/or activation of PKC (293, 485, 606, 727, 728). However, the mechanism by which calcium and/or PKC inhibits renin synthesis remains, to a large extent, unknown. Calcium was recently found to inhibit the transcription of the human renin gene by interacting with a calcium response element in the promoter region (243). ANG II targets the proximal 2.8 kb (606), while endothelin-1 targets the enhancer element of the mouse Ren-1C promoter Physiol Rev • VOL (737). In addition, like cAMP, calcium influences renin mRNA stability (see sect. IIB). The RNA-binding protein dynamin has been suggested to destabilize renin mRNA in response to increased intracellular calcium (443). 3. STAT and NFB transcription factors Inflammatory cytokines, such as interleukin-1 (IL1) and tumor necrosis factor-␣ (TNF-␣), were found to be potent inhibitors of renin production (52, 399, 668). Cytokines appear to be relevant as factors that regulate the renin expression during acute or chronic inflammation. The cardiovascular response to inflammation includes severe hypotension or septic shock (when hypotension is accompanied by organ failure), resulting from the dysregulation of vasoconstrictor and vasodilator systems, whereby the activity of the latter becomes dominant (92–94, 345). Since renin is the limiting factor for the generation of ANG II, which, in turn, is a potent systemic vasoconstrictor, it has been proposed that the cytokinemediated inhibition of renin expression is an important mechanism in the pathogenesis of hypotension/septic shock upon inflammation (52, 691). Accordingly, mice treated with bacterial lipopolysaccharide (LPS) in an acute model of sepsis express significantly less renin mRNA in the kidneys, compared with their controls (52). Neither cAMP nor the calcium signaling pathways are currently known to be involved in the intracellular mechanism of action of the cytokines on renin gene expression. At least two transcription factors have been reported to mediate the suppression of renin transcription by cytokines in cultured renin-producing cells. A) STATS. STAT (signal transducers and activators of transcription) transcription factors play key roles in interferon- and hematopoietic growth factor-dependent stimulation of transcription (179, 947). The inflammatory cytokine oncostatin M was first found to suppress renin transcription through STAT5 (52). More recently, the cytokines IL-1 and interleukin-6 were demonstrated to inhibit renin promoter activity via the extracellular signalregulated kinases (ERKs) and STAT3 (522, 668). B) NFB. We found that the transcription factor NFB is involved in the inhibition of renin gene expression through the cytokine TNF-␣ (880, 883, 884). NFB interacts with CRE in the renal enhancer without directly binding to the DNA. TNF-␣ employs at least two mechanisms to suppress renin gene expression. TNF-␣ inhibits the transactivating capacity of the NFB-p65 subunit and attenuates the binding of CREB to the CRE motif of the renal enhancer. The latter mode of trans-inhibition is particularly interesting because it could represent one of the underlying enhancer-dependent mechanisms for switching on/off the transcription, according to the variegation model of regulation of the renin gene. Moreover, a similar mechanism is utilized by the vitamin D3 90 • APRIL 2010 • www.prv.org PHYSIOLOGY OF KIDNEY RENIN receptor (see sect. IIC4) to suppress the renin transcription. 4. Nuclear receptors Vitamin A (retinoic acid) has been found to increase renin mRNA levels in vivo (796). The molecular basis for the effect of vitamin A on the renin gene expression is the presence of a HRE sequence that is generally targeted by the nuclear receptors in the renal renin enhancer (29). The retinoic acid receptor-␣ and the RXR-␣ were identified as members of the protein complex bound to the renin enhancer HRE, and treatment with retinoic acid stimulated renin promoter activity in an enhancer-dependent manner (796). RXR-␣ also binds to a proximal cisacting element in the human renin promoter (⫺148 to ⫺134), known as Pal3 (881). Although the role of this motif in the regulation of renin gene expression by vitamin A is still unknown, Pal3 was found to be targeted by another member of the nuclear receptor superfamily, PPAR-␥ (881). Interestingly, PPAR-␥ stimulates human renin transcription through the proximal promoter Pal3 sequence, while the human renin enhancer HRE seems to be dispensable for this effect (881). PPAR-␥ most likely targets murine renin genes through the HRE enhancer sequence. Together with their different protein-binding properties (881; see also sect. IIA, 1 and 2), the PPAR␥targeted sites of the renin gene (HRE and Pal3) are important examples for the single transcription factorbinding nonredundant cis-acting elements, which may mediate species-specific regulation. Therefore, we are currently generating several transgenic mouse strains to study the mechanisms of the PPAR␥-driven renin transcription in vivo. Unexpectedly, the vitamin D3 receptor (VDR) does not interact with the renin HRE enhancer site. Instead, the ligand-activated VDR represses the renin transcription by heterodimerizing with CREB and preventing it from binding to the renin enhancer CRE (980). Vitamin D3 is considered to be a (patho)physiologically relevant inhibitor of renin gene expression (516, 980). Accordingly, VDRdeficient mice have increased renin production, which has led to increased water intake, hypertension, and cardiac hypertrophy (516). D. Sites of Renin Expression It is generally accepted that the active renin found in the circulation of mammals almost exclusively originates from the kidneys. However, significant amounts of plasma (pro)renin are produced in the submandibular salivary gland of some mouse strains. As previously mentioned, these mouse strains possess two renin genes, of which Ren-2 is highly expressed in the salivary glands (3, 197, 229). This renin gene duplication is specific to the mouse, Physiol Rev • VOL 613 and its functional significance remains unknown. Notably, even in nephrectomized and sialectomized animals, substantial pro-renin levels can be measured (405), and the origin of the residual pro-renin remains unclear. In addition to systemic renin, there are a number of extrarenal tissues that express renin as a part of the local or tissue-specific RAASs. In these tissues, renin is expressed at a much lower level than in the kidney; its effects are therefore believed to be auto- and/or paracrine (293). Renin is also produced locally in organs that exhibit a blood-tissue barrier, such as the brain or the testes (190, 208, 214, 255, 670). In these organs, the local renin operates independently of the systemic RAAS. On the other hand, in organs such as the heart and the large arterial vessels, significant amounts of renin are taken up from the plasma to support the paracrine activity of the locally produced enzyme (627, 685). Within the kidney, renin is predominantly produced by the JG cells. Interestingly, renin is, to a lesser extent, also synthesized in the renal proximal tubule, connecting tubule and collecting duct, and it is believed to be involved in the local regulation of salt reabsorption and cellular proliferation by ANG II (448, 858, 860). In particular, the expression and regulation of (pro)renin in the connecting tubule and collecting duct have attracted attention over the last few years (729). Renin expression in this localization may be important under conditions of high ANG II states like diabetes (417, 688) and renovascular hypertension (702). Renin mRNA was detected in the organs of the gastrointestinal tract, the immune system, and the female reproductive system, as well as in the adrenal gland, adipose tissue, eye, and skin (214, 229, 246, 270, 383, 420, 433, 735, 803, 842, 929). The local effects of tissue renin are cell and tissue specific. These local RAASs were recently assessed in several excellent reviews (448, 512, 674). E. Development of Renal Renin Expression During nephrogenesis, renin expression changes in a uniform manner in mammals (115, 200, 204, 272, 273, 275, 457, 458, 584, 694, 803), as recently illustrated in a detailed three-dimensional view of renin expression during the development of mouse kidneys (756). Renin first appears in the undifferentiated metanephric blastema of embryonic kidneys (789) before vessel formation has begun, suggesting that renin cells originate within the metanephric kidney from renin progenitor cells rather than from an extrarenal source. Once vascularization of the kidney has begun (around embryonic day 14 in mice and rats), reninproducing cells are exclusively distributed along the walls of the arteriolar vessels (133, 584, 726). Renin in association with the vasculature is first present on day 15 of 90 • APRIL 2010 • www.prv.org 614 CASTROP ET AL. embryonic development, as it begins to be expressed in distinct cells scattered throughout the distal part of the evolving vascular tree. From this initial expression site, renin begins to expand bidirectionally into larger renal vessels, in which renin expression is not continuous and does not resolve into a clearly predictable expression pattern. As nephrogenesis proceeds, the arterial tree continues to mature, and the expression of renin shifts from larger vessels to newly developed afferent arterioles. Subsequently, the renin completely disappears from the larger vessels, becomes more and more restricted to the afferent arterioles, and is finally localized at the classic juxtaglomerular position. Recent data suggest that the acquisition and maintenance of the renin cell identity is mediated by cAMP and histone acetylation at the CRE of the renin gene (681). The embryonic cells expressing renin can also give rise to other cell types, and as a consequence, inhibition of the development of renin-producing cells leads to developmental defects of the whole kidney (682). Interestingly, cells that have switched off renin expression during kidney development can be reactivated to produce renin in adult life (788) (see sect. IIF). Among the afferent arterioles, renin expression is first observed in the afferent arterioles of the juxtamedullary zone of the kidney, and it later increasingly appears in the midcortical and finally in the cortical zones (Fig. 2). The functional relevance of this characteristic shift in renin expression during metanephrogenesis is not entirely clear. It has been speculated that renin-producing cells might guide the development of intrarenal blood vessels, since in rat kidneys the development of a new arterial branch is preceded by the appearance of renin expression at the point of branching (720). In contrast, presumptive bifurcations of the developing arteries in the mouse kidney are free of renin, while robust expression is detected at the mature branch points. The absence of renin in the branching arterial tips, as well as intense expression in the more established vessels, also suggests that renin expression is not a prerequisite for the formation of the vascular tree in the mouse kidney (756, 844). The cellular mechanisms responsible for the characteristic developmental changes in intrarenal renin expression are not well understood. One may assume that the spatiotemporal appearance of direct regulators of renin gene expression may coincide with the initiation or termination of renin expression during nephrogenesis. Some studies have shown that the distribution of intrarenal renin expression parallels the development of sympathetic innervation of the kidney (703), which reflects the centrifugal pattern of renin expression and renal vasculature. In addition, the expression of the transforming growth factor (TGF)- type II receptor shifts in a manner similar to that of renin during renal development (520), suggesting that TGF- might also be involved in triggering developmental renin expression. The evidence for a disPhysiol Rev • VOL FIG. 2. Renin during embryonic kidney development. Reconstruction of the ␣-smooth muscle actin immunoreactive structures (red) and of the renin immunoreactive areas (green) in the developing mouse kidney. The distribution of renin is shown in whole organ reconstructions (A–C) and in developing branches of arcuate arteries (D–F) on embryonic day 16 (A and C), postpartal day 1 (B and E), and in the adult kidney (C and F). The light green color indicates glomeruli. tinct role of cell-to-cell communication via gap junctions in the distribution of renin-producing cells has recently been obtained. Renin-producing cells at all developmental stages express the gap junction protein connexin40 (492). Mice lacking connexin40 display a normal pattern of renin expression in the larger vessels, but they are unable to maintain renin-producing cells in the afferent arterioles (493). Instead, renin-producing cells in the mature kidneys are located outside the vessel walls in the periglomerular interstitium. The proper regulation of developmental renin expression requires switching on and off the relevant transcription factors. It has been reported that the putative mRNA splicing protein Zis may be of relevance for the development of renin-expressing cells (419). However, its mode of action in renin-producing cells has not yet been elucidated in detail. The renin gene promoter contains 90 • APRIL 2010 • www.prv.org PHYSIOLOGY OF KIDNEY RENIN consensus binding motifs for many transcription factors (see sect. IIA), two of which might be considered favorite candidates. One is the Hox transcription factor, generally known to be relevant for development, while the second is the CRE factor, CREB (663). The deletion of both Hox binding motifs (665, 666, 669) and CRE sites (7, 146, 442, 666, 853) has been shown to strongly reduce the basal renin promoter activity and to blunt its stimulation in vitro. Preliminary evidence suggests that the interruption of the cAMP pathway in vivo can nearly prevent renin expression during kidney development (624). F. Recruitment of Renin Expression in the Adult Kidney usual, as calcium inhibits rather than facilitates renin secretion. A. Processing and Secretion of Renin in Juxtaglomerular Cells Renin shares the functions both of a hormone and of a protease. Therefore, on one hand, renin is intracellularly processed and glycosylated like a typical secretory protein. On the other hand, it is intracellularly processed and packed into vesicles like a typical lysosomal protein, which finally undergoes regulated secretion. 1. Renin secretory vesicles Plasma renin is produced and secreted by the renal JG cells (see sect. III). These epithelial-like cells are located in the wall of the afferent arteriole in close vicinity to the glomerulus (293). JG cells comprise a very small cell population in the kidney. Notably, the number of renin-producing cells in the afferent arteriole is variable, such that chronic stimulation of renin synthesis increases their number, and vice versa. These changes in the number of renin-producing cells are based on the unique ability of the smooth muscle(-like) cells of the afferent arteriole to acquire a secretory phenotype via a reversible metaplastic transformation. Thus, in states of increased renin production such as during impaired renal perfusion, salt depletion, or inhibition of ANG II formation, the larger portions of the afferent arteriole proximal to the JG region become renin-positive (114, 146, 788). The cellular mechanisms underlying the recruitment of renin-producing cells in the vessel walls of adult kidneys are not well understood. It was suggested that vascular smooth muscle cells may switch into renin producers, and vice versa, by a metaplastic transformation (114). An elegant study has recently provided evidence that not all smooth musclelike cells in the renal vasculature can undergo such a switch and that the cells that can do so are genetically programmed (788). III. THE JUXTAGLOMERULAR CELL Several aspects render the renin-producing JG cells somewhat mysterious. The first aspect is the yet unexplained special position of the cells in the media layer of afferent arterioles at the entrance into the glomerular capillary network. The second unusual feature is the cuboid, epithelial-like form, which coined the term juxtaglomerular epithelioid (JGE) cells. Third, the cells contain numerous, sometimes large, dense-core secretory vesicles, but they very rarely show signs of exocytotic events. Finally, the regulation of secretion appears rather unPhysiol Rev • VOL 615 Renin is initially synthesized as a pre-pro-renin protein. After cleavage of the presequence, the pro-renin is directed to the cis-Golgi cisternae. From there, pro-renin can be immediately secreted by the constitutive pathway or sorted to the dense-core secretory granules for regulated exocytosis (Fig. 3). The correct sorting of the prorenin to the regulated secretory pathway depends on the presence of a paired basic amino acid site in the pro-renin molecule, which serves as a protease processing site for granule-attached prohormone convertases (85). In addition, a decrease in the pH of the early renin granules may facilitate the aggregation of the granule core. Accordingly, the protogranules often contain paracrystalline cores (862). In mice with two renin genes (Ren-1D and Ren-2), the deletion of Ren-1D, but not Ren-2, is associated with a complete absence of the dense-core secretory granules in JG cells (163, 607), suggesting that the ability to form the dense-core secretory vesicles depends on the structural characteristics of the Ren-1 protein. These characteristics may be related to the glycosylation of the protein because renin originating from the Ren-1D gene has three N-linked glycosylation sites, while the Ren-2-derived renin has none. Renin glycosylation may be necessary not only for renin trafficking to the dense-core secretory vesicles (607), but also for the mannose-6-phosphate receptor-mediated uptake of renin or pro-renin in other tissues, such as the heart (685, 900). In addition to pro-renin, the protogranules contain proteases such as prohormone convertases (85, 95) and cathepsin B (579, 625), which can cleave off the 43-amino acid NH2-terminal prosegment. These proteases require an acidic pH for optimal activity. The autoactivation of the proteases from their own pro-forms also depends on a low pH of 4 – 6. The low pH of renin secretory vesicles allows them to be stained with fluorescent dyes like quinacrine (17, 122, 687). Quinacrine staining of renin-containing vesicles has been used for dynamic visualization of live cells to trace the exocytosis of renin-containing vesicles (687). 90 • APRIL 2010 • www.prv.org 616 CASTROP ET AL. FIG. 3. Subcellular structure of juxtaglomerular (JG) cells. Electron photomicrograph of a renin-producing JG cell in a mouse kidney. A: overview of the cell. B: high-power magnification of the renin storage organelles. RER, rough endoplasmic reticulum. Even in renal juxtaglomerular cells in situ, only 25% of synthesized renin is directed to the dense-core secretory granules, while 75% is constitutively secreted as prorenin (699). This source, together with the pro-renin originating from extrarenal sources, accounts for the observation that the proform accounts for 80 –90% of the total renin in human circulation (824). The interaction of circulating pro-renin with tissue renin receptors may lead to proteolytic or nonproteolytic activation, as well as local generation of angiotensin and the activation of second messenger pathways (see sect. VIB). The release of pro-renin via the constitutive pathway depends on the activity of the renin synthetic pathway, i.e., the level of gene activation and transcription efficiency in the individual cell, as well as the total number of renin-producing cells. Therefore, the acute stimulation of renin release leads to an increase in the exocytosis of mature renin secretory granules that contain active renin only, whereas the chronic stimulation of the renin system increases circulating levels of both pro-renin and renin (885). Although the rates of renal renin synthesis and secretion can vary significantly within and between individuals, the density of renin granules in secretory cells is surprisingly constant. For example, in rats under normal laboratory conditions, one renin granule is found, on average, in a cell volume of 5 m3 (715, 813). A very similar density was observed during chronic salt depletion when the overall renin synthesis and secretion are strongly stimulated. Instead of elevating the number of granules per cell, the total number of renin-producing cells increases during salt depletion. The gross morphology of renin secretory granules of newly recruited JG cells is similar to that of the granules in the control situation. The mouse and rat renin granules have average volumes of 0.63 (400) and 0.35 m3 (715), respectively. Therefore, the long-term regulation of renin synthesis and secretion appears to be mediated by altering the number of renin-producing cells, rather than by modifying the processes involved in the control of renin synthesis and secretion in the individual cell (see sect. IIG). 2. Mechanisms of renin secretion As in other cells, the early secretory granules in renin-producing cells can take up extracellular protein by endocytosis, including horseradish peroxidase, which is targeted to the early granules without transit through the Golgi apparatus (865). The ability to segregate renin into dense-core secretory granules for regulated exocytosis seems to be specific to native JG cells in situ. After isolation, JG cells rapidly lose the ability to direct renin into this pathway, and the various renin-producing cell lines (e.g., As4.1, Calu-6) do not store active renin in secretory granules, but instead secrete it constitutively. Physiol Rev • VOL A substantial amount of evidence indicates that the regulated release of renin proceeds via traditional exocytotic mechanisms. Thus the spontaneous release of renin at the cellular level is discontinuous in the renin content per discharge, which corresponds to the calculated renin content of one secretory granule (813). Furthermore, the acute stimulation of renin secretion in vivo, for example, by renal artery constriction, leads to a marked decrease in the number of renin storage granules (715), while the average size of the individual granules does not change. Elegant in vitro studies using two-photon microscopy 90 • APRIL 2010 • www.prv.org 617 PHYSIOLOGY OF KIDNEY RENIN have confirmed the rapid disappearance of renin storage granules upon the acute stimulation of secretion (687). Recent support for exocytosis as the pathway for renin release is derived from capacitance measurements of single renin-producing cells of the kidney. The average capacitance of a mouse JG cell is ⬃3.1 pF (235), yielding a calculated cell volume of ⬃500 fl (500 m3). Rat cells have a smaller capacitance of 2.6 pF, reflecting a somewhat smaller cell volume (235, 239). The other conditions known to stimulate renin secretion in vivo and in vitro (see below) lead to as much as a 15% increase in capacitance, reflecting the insertion of the vesicle membrane into the plasma membrane (235, 236, 239). It has been estimated from whole cell patch-clamp studies that the number of granules that are released within minutes after maximal stimulation constitutes ⬃20% of the total vesicle number. This might indicate the presence of a pool of immediately available renin storage granules, the existence of which has also been postulated from studies of isolated glomeruli (812) and isolated perfused kidneys (486). The activation of renin secretion is accompanied by the formation of deep plasma membrane invaginations. These invaginations likely result from the addition of membrane material, obtained from many secretory granules, to the cell surface and to already-fused secretory granules (compound exocytosis) (715, 734). These invaginations may explain why the discharge of the renin secretory vesicles does not necessarily require movement of the vesicles to the cell perimeter (687). The molecular events that drive and regulate the exocytosis of the renin storage vesicles remain unknown. A single finding in this context is that the renal reninproducing cells express the calcium-dependent activator protein for secretion (CAPS). This family of proteins is specifically involved in the secretory process of large dense-core vesicles but not small clear secretory vesicles (822). B. Electrical Properties of Juxtaglomerular Cells The electrophysiology of renin-producing JG cells has been investigated in some detail using electrodes or TABLE 1. patch-clamp techniques on single JG cells or JG cells in situ in isolated glomeruli with attached afferent arterioles (recently reviewed in Refs. 236, 238; see also Table 1). Vasoconstrictors, which are known to suppress renin release, depolarize JG cells in situ (96, 97). In addition, an increase in the extracellular K⫹ concentration or an inhibition of K⫹ channels not only depolarizes JG cells, but also suppresses renin release (158, 269, 480, 487). On the other hand, the membrane hyperpolarization induced by K⫹ channel activation or Cl⫺ channel inhibition is accompanied by a stimulated renin secretion (227, 239, 396, 403, 615). Furthermore, the pharmacological inhibition of the Na⫹-K⫹-2Cl⫺ cotransport activity using furosemide leads to the hyperpolarization of single JG cells while concomitantly stimulating renin exocytosis (128). Both effects of furosemide appeared to depend on the Na⫹-K⫹-2Cl⫺ cotransporter (NKCC1) isoform, since neither was observed in NKCC1-deficient mice (128). Taken together, there is good reason to assume that changes of the membrane potential of JG cells are associated with renin release, such that depolarization is accompanied by suppressed renin exocytosis, while hyperpolarization is accompanied by stimulated renin exocytosis. Despite the clear association between both parameters, it is not clear whether changes in the membrane potential directly and causally control renin release. The depolarization of JG cells in response to vasoconstrictors, such as ANG II, probably results from the inhibition of inwardly rectifying K⫹ channels (Kir) by a G protein-mediated process (483). Besides the Kir (235, 483, 507), there is electrophysiological evidence of at least two additional potassium (K⫹) conductances in JG cells. The first conductance is associated with a delayed outwardly rectifying K⫹ current (235, 239, 483) that has been identified as the BKCa zero splice variant; additionally, this conductance is activated by cAMP and results in hyperpolarization (239). The second conductance is associated with the KATP channels (732). In addition to potassium channels, there is good evidence for the presence of NKCC1 and Ca2⫹-activated chloride channels in the plasma membrane of JG cells (128, 418, 483). Ca2⫹-activated Cl⫺ channels might contribute to membrane depo- Expression and function of ion channels in JG cells Channel Inward rectifier K⫹ channels; Kir Ca2⫹-activated K⫹ channels; BKCa zero variant ATP-sensitive K⫹ channels; KATP Regulation in JG Cells Inhibition by angiotensin II Activation by cAMP Na⫹-K⫹-2Cl⫺ cotransporter1; NKCC1 Ca2⫹-activated Cl⫺ channels Voltage-dependent Ca2⫹ channels, L type Activation by Ca2⫹ Activation by depolarization Physiol Rev • VOL Function Reference Nos. Depolarization by angiotensin II Hyperpolarization Pharmacological activation: hyperpolarization, stimulation of renin secretion Pharmacological blockade: hyperpolarization, stimulation of renin secretion Depolarization, inhibition of renin secretion Calcium influx at positive membrane potentials 4, 92, 229, 468 4, 68, 229, 233 381, 706 90 • APRIL 2010 • www.prv.org 123 388, 468 233 618 CASTROP ET AL. larization in response to vasoconstrictors. Thus Ca2⫹ mobilization by vasoconstrictors could stimulate Ca2⫹activated Cl⫺ channels, Cl⫺ efflux, and membrane depolarization. JG cells are strongly electrically coupled to the neighboring cells of the afferent arteriole (96, 483, 732) (see sect. IIID). Interestingly, their resting membrane potential changes in situ from ⫺60 to ⫺80 mV in nonpressurized arterioles (96, 483, 732) to approximately ⫺40 mV in pressurized arterioles (539). Since the depolarization of JG cells is accompanied by the suppression of renin release, the depolarization in response to an increase in perfusion pressure might directly or indirectly contribute to the known pressure-dependent inhibition of renin secretion. Since Ca2⫹ inhibits renin secretion, it is clearly possible that the potential-operated Ca2⫹ channels mediate an influence of membrane potential on renin secretion. However, functional studies on the role of potential-operated Ca2⫹ channels have produced conflicting results (159, 487, 764; for a detailed discussion, see Ref. 779). A recent study by Friis et al. (239) might shed some light on this controversy; these authors provided evidence for the presence of L-type Ca2⫹ channel mRNA, protein, and functional activity in JG cells. However, because these channels are only activated at very positive membrane potentials (239), they are unlikely to be operational in JG cells under physiological conditions. These data might explain why several studies have failed to detect a direct functional role of the L-type Ca2⫹ channels in the regulation of renin release (779). Finally, there is good, albeit indirect, evidence in JG cells for the store-operated Ca2⫹ entry triggered by the filling state of the internal Ca2⫹ stores (283, 483, 973). The identity of the channels underlying this Ca2⫹ influx is currently unclear. It will be interesting to investigate the role of ORAI proteins (“calcium release-activated calcium modulator,” pore-forming subunit of a calcium release activated calcium channel), as well as TRPC channels, in the regulation of Ca2⫹ influx and renin release in JG cells, since these channels contribute to or mediate store-operated Ca2⫹ entry in other cell types (817). C. Intercellular Contacts of Juxtaglomerular Cells The different cell types that form the juxtaglomerular apparatus (JGA) are abundantly coupled via gap junctions (861, 864). The intense intercellular connection creates a functional syncytium within this multilayered complex and may contribute to the regulation of the preglomerular arteriolar tone, the glomerular filtration rate (GFR), and the activity of the renin system. Within the JGA, JG cells form a number of gap junctions among themselves (95), as well as with their neighboring endothelial, smooth Physiol Rev • VOL muscle, or extraglomerular mesangial cells (583, 859, 861, 864). Gap junctions are made up of connexin (Cx) proteins, which comprise a family of at least 20 protein isoforms (818). It was recently shown that JG cells predominantly express connexin 40 (Cx40) and, to a lesser extent, Cx37 and Cx43 (31, 296, 364, 983). The coexpression of Cx40 with Cx37 and Cx43 is specific to renin cells in the typical juxtaglomerular position, as renin-producing cells situated outside the classic juxtaglomerular position express only Cx40. Thus fetal renin-producing cells, which are still located in the wall of the larger renal arteries (756), and renin cells recruited upstream along the afferent arteriole by chronic challenge of the renin system showed only a Cx40-mediated coupling. For this reason, Cx40 may be considered as a fundamental feature of renin-producing cells (492). In addition to being found in renin-producing cells, Cx40 has been detected as one of the four main connexins (Cx37, Cx40, Cx43, Cx45) expressed in the wall of renal vessels (191, 290), where it is found almost exclusively in the endothelium. Our knowledge of Cx40 expression in renin-producing cells has been mainly obtained from morphological analyses, and the functional relevance has not yet been extensively studied. However, one could imagine that the Cx40-mediated junctions between JG cells and the adjacent endothelium or the extraglomerular mesangium might provide a suitable pathway for the propagation of blood-borne or macula densa-derived signals involved in the control of the renin system. Mice harboring a genetically disrupted Cx40 gene have been useful tools for investigating the functional importance of this connexin. Cx40 knockout mice are hypertensive (185) and show a high renin status (465, 922). An obvious explanation for the unusual combination of high renin levels with high blood pressure is that the transmission of inhibitory signals to JG cells does not work in the absence of Cx40. In fact, studies have shown that the classic negative-feedback mechanisms of renin secretion and renin gene expression exerted by ANG II or intrarenal blood pressure are disturbed in Cx40-deficient mice (465, 922); these data support the model of Cx40dependent transmission of inhibitory signals to JG cells. Experimental unilateral renal stenosis in Cx40 knockout mice did not lead to the increase in blood pressure or elevated plasma renin levels that would typically be observed in wild-type mice. Furthermore, the typical increase in renin mRNA in the kidney with impaired perfusion is absent in Cx40 knockout kidneys. However, whether the special importance of Cx40 in JG cells is related to the unique properties of this connexin remained uncertain and was subsequently addressed by Cx40 knock-in Cx45 (Cx40KI45) mice. In these mice, the coding region for Cx40 was replaced by Cx45 under the control of the native Cx40 promoter (12). Cx45 exhibits a lower 90 • APRIL 2010 • www.prv.org PHYSIOLOGY OF KIDNEY RENIN conductance than Cx40, and it is not normally expressed in JG cells (492, 780). Interestingly, Cx40KI45 mice showed a reestablishment of the interrupted physiological feedback control by ANG II and intrarenal pressure, and the mice were consequently protected against hypertension, which was observed in the general Cx40 knockout model (780). Renin-producing cells in the Cx40KI45 mice are, at first glance, associated with the juxtaglomerular regions of the afferent arterioles. A more detailed analysis, however, revealed that the arrangement of the renin-producing cells is not completely normal (490). Other previous studies in the Cx40KI45 mice described the unique functions of Cx40 in certain tissues (12, 956). The compatibility of Cx40 with Cx45 in JG cells, however, shows that at least the conduction of inhibitory signals involved in the control of renin secretion and renin mRNA expression does not rely on the specific properties of Cx40. Intriguingly, the regulation of the renin system by salt intake (see sect. VA) appears not to be fundamentally deteriorated in the absence of Cx40. In both wild-type mice and Cx40-deficient mice, the plasma renin concentrations and renin mRNA levels were inversely related to the salt load (465, 922). At the same time, catecholamines, the classic stimulators of the renin system, appear to function quite normally (922), suggesting that renin does not escape from physiological control in the absence of Cx40. The question remains as to whether other regulators of the renin system, such as prostanoids or nitric oxide (NO), function normally when the Cx40 gene is disrupted. Investigations into the involvement of cyclooxygenase (COX)-2-derived prostanoids have revealed a clear increase in the expression of COX-2 mRNA and protein levels in mice lacking Cx40 (464, 921), which underlines the parallel control mechanism of renin and COX-2 genes by the macula densa cells (127, 769). The treatment with COX-2 inhibitors markedly reduced renin mRNA levels in Cx40-deficient mice, similar to wild-type mice, and diminished the increase in the number of renin-producing cells (921). Similar results were obtained in experiments examining the involvement of the macula densa-derived NO in the regulation of renin expression in mice lacking Cx40. The kidneys of the Cx40 knockout mice demonstrate a large increase in the expression of the neuronal nitric oxide synthase isoform (nNOS) and show decreased renin levels after the pharmacological inhibition of NOS activity (464). These observations demonstrate that COX2-derived prostaglandins and NO act as tonic stimulators of the renin system in the kidney of both normal and Cx40 knockout mice. One could speculate that the strongly elevated levels of COX-2 and nNOS contribute to the excessive renin secretion and synthesis observed in the absence of Cx40. Physiol Rev • VOL 619 In addition to these functional consequences, the structure and positioning of renin-producing cells in the JG position is strikingly altered in Cx40 knockout mice (493). Reninproducing cells are typically located in the media layer of the afferent arteriole, close to the vascular pole of the glomerulus. Although renin-producing cells display a high degree of plasticity during renal development (756) and chronic stimulation of the renin system (see sect. II, E and F), they are normally integrated into the wall of the arterial vessels. Ectopic renin expression in cells outside the medial layer of arteries has been described as a rare event that occurs, if at all, in the extraglomerular mesangium (457). In kidneys lacking Cx40, renin-producing cells are absent from the wall of the afferent arterioles. Instead, they are found around the wall of the afferent arterioles, extending into the region of the extraglomerular mesangium and the periglomerular interstitium. These ectopic renin-producing cells do not show the typical epithelioid appearance, instead exhibiting a more irregular mesenchymal-like shape. Interestingly, the expression of renin in the developing kidney of Cx40 knockout mice is restricted to the walls of the larger arteries, and it does not appear in the interstitial space until the wave of renin expression shifting along the arterial tree during nephrogenesis finally reaches the glomerulus (493). One may, therefore, speculate that Cx40 is required for the correct “homing” of renin-producing cells in the JGA. It remains to be determined whether the Cx40-mediated contacts act as transmembrane channels that conduct unidentified signals relevant for the differentiation and proliferation of JG cells or whether they function as scaffolds that enable the correct assembly of other proteins relevant to the positioning of renin-producing cells in the classic location (216). Apart from Cx40, JG cells also express Cx37 and Cx43 (492). Cx37 expression is not coregulated with Cx40 when the renin system is stimulated (465), but it cannot be demonstrated in renin-producing cells in the absence of Cx40. In contrast to mice lacking Cx40, Cx37-deficient mice are normotensive (807), suggesting that Cx37 might be less relevant for JG cell function than Cx40. In fact, our preliminary information suggests that the renin system works quite normally in the Cx37-deficient mice (926). The role of Cx43 in the function of JG cells is at present less clear. Mice in which Cx43 is replaced by Cx32 (Cx43KI32 mice) displayed markedly lower renin levels than the wild-type controls (295). Notably, the reduction of renal perfusion pressure by unilateral renal artery stenosis, which normally leads to an increase in renin secretion and synthesis in the affected kidney and decreases renin content in the contralateral intact kidney, did not affect the renin expression in Cx43KI32 mice. This observation might indicate that, in addition to Cx40, Cx43 might also be critically involved in the control of the renin system. In summary, the intercellular signaling via con- 90 • APRIL 2010 • www.prv.org 620 CASTROP ET AL. nexins appears to be an important determinant for the control of the renin system and for normal localization of JG cells. D. Intracellular Signals Controlling Renin Release At the cellular level, the numerous systemic and local factors that regulate renin release merge into three intracellular second messenger systems: the cAMP, cGMP, and calcium (Ca2⫹) pathways (Fig. 4). 1. cAMP Undoubtedly, cAMP is the central intracellular stimulator of renin release. This conclusion is supported by several lines of indirect and direct evidence. For instance, the activation of -adrenoreceptors, which are known to enhance adenylyl cyclase activity, stimulates the renin secretion in a broad range of experimental in vitro models, including single JG cells as well as in vivo (54, 235, 423, 484, 903, 944). Moreover, the renin release in response to renal nerve stimulation is mediated by the -adrenoreceptors (349), and 1/2-adrenoreceptor knockout mice possess low plasma renin concentrations (PRC) (434). [Note that PRC is measured as the rate of ANG I generation in the presence of excess renin substrate. Plasma renin activity (PRA) is measured as the generation of ANG I without the addition of exogenous renin substrate.] In addition to catecholamines, other hormones, such as the prostaglandins E2 and I2 (prostacyclin) (402), dopamine (476), calcitonin gene-related peptide (482), pituitary adenylyl cyclase activating polypeptide (PACAP) (322), and adrenomedullin (397), stimulate the renin release from isolated JG cells and increase the intracellular cAMP concentrations. The stimulation of adenylyl cyclase activity in response to activation of G protein-coupled receptors is mediated via the stimulatory G protein Gs␣. In line with the stimulatory role of Gs␣ and cAMP for renin release, the PRC is markedly reduced in mice carrying a conditional deletion of Gs␣ in JG cells (146). Notably, PRC is not stimulated in the Gs␣ KO mice in response to catecholamines, inhibition of the macula densa mechanism, or a drop in blood pressure, underscoring the central role of the cAMP pathway in the regulation of renin release (146). Additionally, the direct activation of adenylyl cyclase activity by the diterpen forskolin strongly increases cAMP levels and renin release in isolated JG cells (283, 484), as well as in the renin-producing cell line As4.1 (283, 442). Finally, a direct link between cytosolic cAMP levels and renin release was demonstrated by the fact that mem- FIG. 4. Intracellular signaling pathways controlling the renin exocytosis. ANP, atrial natriuretic peptide; AC5/6, adenylate cyclases 5/6; cGK, protein kinase G; DAG, diacylglycerol; GC-A, guanylate cyclase A (particulate guanylate cyclase); GP, GTP-binding protein; IP3, insitol 1,4,5trisphosphate; NO, nitric oxide; PDE3a, cAMP-phosphodiesterase 3a; PKA, protein kinase A; PKC, protein kinase C; PLC, phospholipase C; sGC, soluble guanylate cyclase. Physiol Rev • VOL 90 • APRIL 2010 • www.prv.org PHYSIOLOGY OF KIDNEY RENIN brane-permeable cAMP analogs and direct intracellular applications of cAMP via a patch pipette rapidly stimulate renin secretion (235). Intracellular cAMP levels are determined not only by cAMP formation, but also by cAMP hydrolysis to 5⬘-AMP through the activity of cAMP phosphodiesterases (PDEs). The inhibition of cAMP degradation, either by nonselective inhibition of PDE activity using xanthine derivatives, such as 3-isobutyl-1-methylxanthine (IBMX) or theophylline, or by selective blockers of the isoforms PDE-1, PDE-3, and PDE-4, stimulates renin release (154, 156, 157, 479). Moreover, strong expression of PDE-3 and PDE-4 was confirmed in single JG cells and in the JG cell line As4.1 (237, 442). PDE-3 activity is regulated by cGMP, with an increase in the cytosolic cGMP inhibiting PDE-3 activity, which eventually results in elevated cAMP levels (57). Via this pathway, cGMP can indirectly stimulate renin secretion, which is of major physiological importance for the influence of NO on renin release (see below). In conclusion, numerous studies using different approaches have clearly indicated that cAMP is the central stimulator of renin release. However, little is known about the mechanisms through which cAMP triggers the exocytosis of renin storage vesicles. While it has been shown that the stimulation of renin release by cAMP involves a PKA-dependent step (237, 477), the phosphorylation targets of PKA in JG cells are currently mostly unknown. It has recently been reported that JG cells express functional cAMP-sensitive BKCa channels and that the activation of these channels by cAMP results in hyperpolarization of the cells (239). As discussed earlier, the hyperpolarization of JG cells appears to favor exocytosis, suggesting that the activation of BKCa channels by cAMP might lead to enhanced renin exocytosis. However, the pharmacological inhibition of the BKCa channels does not attenuate the stimulation of renin release in response to cAMP (239). Therefore, a future challenge will be to identify the downstream signaling events that mediate the stimulation of renin exocytosis by the cAMP/PKA pathway. 2. Calcium While cAMP is the main stimulator of renin release, the free cytosolic Ca2⫹ concentration is considered as the primary inhibitor. Notably, an increase in the intracellular Ca2⫹ concentration initiates and supports exocytosis in all other secretory cells, excluding parathyroid gland cells (165). Accordingly, the unusual effect of Ca2⫹ in the reninsecreting JG cells has been termed as the “calcium paradox” of renin release. Early indirect evidence for an inhibitory influence of calcium on renin secretion was obtained from the observation that classical vasoconstrictors suppress renin rePhysiol Rev • VOL 621 lease (293, 779). Thus vasoconstrictors such as angiotensin II, endothelin, vasopressin, and norepinephrine not only increase the Ca2⫹ influx or intracellular Ca2⫹ levels in JG cells (283, 369, 484, 485), but they also inhibit the renin release in a Ca2⫹-dependent fashion (587, 843). Other measures that result in an elevation of the cytosolic Ca2⫹ concentration also suppress renin release. For instance, thapsigargin, which blocks the sarcoendoplasmic Ca2⫹-ATPases and induces the store-operated Ca2⫹ influx, elevates the intracellular Ca2⫹ concentration in JG cells and suppresses renin release from isolated perfused kidneys and cultured JG cells (283, 781, 973). Conversely, the chelation of intracellular Ca2⫹ using 1,2bis(2-aminophenoxy)ethane-N,N,N⬘,N⬘-tetraacetic acid (BAPTA) stimulates renin release from isolated juxtaglomerular cells (587, 654, 655). Extracellular and intracellular calcium concentrations are positively correlated in JG cells (483). Therefore, it was correctly predicted that lowering the extracellular Ca2⫹ concentration would stimulate renin secretion in several in vitro models (293, 488, 863) and that the infusion of Ca2⫹ would suppress renin secretion from the nonfiltering kidneys of dogs in vivo (940). In addition to the parallel changes in the extra- and intracellular Ca2⫹ concentrations, the Ca2⫹-sensing receptor, which also controls the release of the parathyroid hormone in a Ca2⫹-dependent fashion, may participate in the regulation of renin release by the extracellular Ca2⫹ concentration (653). Similar to the stimulation of renin release by cAMP, the downstream signaling pathways that mediate the suppression of renin exocytosis in response to an elevation in the cytosolic Ca2⫹ concentration are still under investigation. There is evidence to suggest that the suppression of renin release by Ca2⫹ involves the following: 1) Ca2⫹/ calmodulin-dependent processes, such as the activation of myosin light chain kinase; 2) the modulation of ion channel activities in the plasma membrane or in the membrane of the renin-containing granule; or 3) the activation of PKC. Since these studies have been the subject of previous reviews (293, 473, 779), we focus on a further downstream target of Ca2⫹, namely, the Ca2⫹-inhibited adenylyl cyclases AC5 and AC6 (283, 655). Both AC5 and AC6 are inhibited by physiological increases in the intracellular calcium concentrations and are therefore well positioned to link the inhibitory calcium pathway to the stimulatory cAMP pathway in an inverse manner in JG cells. In fact, the expression of AC5 and AC6 mRNA has been found in JG cells, while protein expression in these cells has so far been demonstrated only for AC5 (283, 654, 935). Moreover, an inverse relationship between the cytosolic Ca2⫹ and cAMP levels has been found in primary cultures of JG cells. While the increase in intracellular Ca2⫹ that occurred in response to endothelin-1, ANG II, or thapsigar- 90 • APRIL 2010 • www.prv.org 622 CASTROP ET AL. gin was paralleled by a reduction of the intracellular cAMP levels and renin release (283), the intracellular Ca2⫹ chelator BAPTA-AM had the opposite effect (654, 655). Since the inverse relationship between intracellular Ca2⫹ and renin release was prevented by clamping the intracellular cAMP levels or by the inhibition of adenylyl cyclase activity, these data suggest that the modulation of cAMP by Ca2⫹ is an important determinant of the inverse relation between calcium and renin release (283, 655). The functional role of AC inhibition by Ca2⫹ has been further corroborated in gene knockdown studies (283) and in pharmacological studies using the adenylyl cyclase inhibitor NKY-80, which shows preferential binding to AC5 compared with the non-calcium-inhibited isoforms AC2 or AC3 (648, 654). Although the inhibition of cAMP formation by Ca2⫹ provides a possible explanation for the calcium paradox, future work should attempt to verify the functional roles of AC5 and AC6 in more complex models and in vivo, since the regulation of adenylyl cyclase activity does not appear to completely explain the calcium paradox at the whole organ level (283). Moreover, further studies are needed to integrate other factors known to contribute to the Ca2⫹-dependent inhibition of renin release, such as the activation of the myosin light-chain kinase or the modulation of channel activity, into this model of Ca2⫹inhibited adenylyl cyclases mediation of the calcium paradox. 3. cGMP In contrast to cAMP and Ca2⫹, which have been unequivocally shown to either stimulate or suppress renin release, the effects of cGMP are more complex, given that it can both stimulate and inhibit renin secretion. On one hand, the application of membrane-permeable cGMP analogs at high concentrations suppresses renin release in a variety of models, including dispersed JG cells (276, 332, 475, 477, 633, 771). On the other hand, unmodified cGMP stimulates renin exocytosis upon direct injection into single JG cells (237). These contradictory effects can be best explained by the different affinities of each type of cGMP for intracellular target molecules. While cAMP-degrading phosphodiesterases such as PDE-3 are inhibited more efficiently by cGMP than by the stable cGMP analogs, the latter show a much higher affinity for cGMP-activated kinases (cGKs) (108). Therefore, these data indicate that cGMP can either stimulate renin release via the inhibition of PDEs or suppress renin secretion via cGK-dependent processes. In fact, the cGMP-inhibited PDE-3 plays a mediator role in the stimulation of renin release by NO. Although the data are not unequivocal, NO has been shown to stimulate renin release in a variety of in vitro models, as well as in vivo (489). This stimulatory effect of NO is Physiol Rev • VOL mimicked by the pharmacological inhibition of PDE-3, a PDE isoform that hydrolyzes cAMP (156, 157, 237, 479). Moreover, NO donors increase both intracellular cAMP concentration and renin secretion in primary cultures of JG cells, similar to the effects of PDE-3 inhibitors (477). These data suggest the existence of a cascade of signaling events connecting the cGMP and cAMP pathways in JG cells, where NO stimulates the formation of cGMP, which in turn suppresses PDE-3 activity and cAMP hydrolysis. Since cAMP is the central stimulator of renin release, the resulting intracellular accumulation of cAMP eventually enhances renin exocytosis. In fact, this interplay between the cGMP and cAMP pathways has been confirmed in vivo (59) in isolated perfused kidneys (479) and in patch-clamp experiments using single JG cells, in which cGMP stimulated renin exocytosis in a PDE3-, cAMP-, and PKA-dependent manner (237). In addition to PDE-3, JG cells express cGK. cGKI is found in the cytosol, while cGKII is associated with renin granules (253, 254). Stable cGMP analogs suppress renin release in isolated perfused kidneys and in microdissected glomeruli with attached afferent arterioles (254, 927). As mentioned above, the stable cGMP analogs show a high affinity for protein kinase G. Accordingly, the suppression of renin release by these compounds is completely prevented by the pharmacological inhibition of the cGKs (254). Moreover, cGMP analogs inhibit renin secretion in the primary cultures of JG cells isolated from wild-type or cGKI-deficient kidneys, but not from cGKIIdeficient kidneys (254, 927). Therefore, it can be concluded that cGMP can inhibit renin release via the activation of cGKII. The downstream targets of cGKII that eventually interfere with the exocytosis of renin storage granules are presently unknown. The dual effects of cGMP on renin release raise the question of which factors determine whether endogenous cGMP stimulates or inhibits renin secretion. It is possible that the effects of cGMP depend on its intracellular concentration. Since low concentrations of cGMP preferentially inhibit PDE-3, the stimulation of renin release would occur. In contrast, high concentrations of cGMP can also activate cGKII. Since cGKII activation can, in principle, attenuate the stimulation of renin release in response to cAMP (489), a high concentration of cGMP would suppress renin release. Moreover, it appears likely that the stimulatory and inhibitory cGMP pathways are spatially segregated from each other. This hypothesis is consistent with the intracellular localization of the cGMP-generating guanylate cyclases and cGMP target enzymes. Soluble guanylate cyclase (sGC) is a cytosolic enzyme like PDE-3; as a result, the cGMP derived from sGC may predominantly stimulate renin secretion via the cAMP pathway. Since NO activates sGC, this pathway could provide an explanation for the stimulation of renin release by NO. In contrast to sGC, the membrane-bound particulate guany- 90 • APRIL 2010 • www.prv.org PHYSIOLOGY OF KIDNEY RENIN 623 late cyclase (pGC) is found in close proximity to the vesicles associated with cGKII. Since cGMP derived from pGC would therefore inhibit renin secretion, this pathway could explain the inhibition of renin release in response to atrial natriuretic peptide (ANP) (see sect. VC4). Such a spatial segregation of cGMP signals induced by ANP and NO occurs in both HEK cells and vascular smooth muscle cells (623, 695), although no data supporting this hypothesis in JG cells are presently available. IV. LOCAL CONTROL OF RENIN SECRETION As will be outlined in section V, renin secretion is modulated by multiple systemic factors. In addition, the presence of renin-generating granular cells at the distal portion of the afferent arteriole enables the adjacent or nearby cells to exert a local control over the renin secretion. These local control mechanisms of renin secretion comprise tubular, vascular, and nervous components (Fig. 5). In addition to unambiguously locally generated factors, there are hormones such as ANP and ANG II that may be of local and/or systemic origin; those factors will be covered in section V. A. Tubular Control of Renin Secretion Renin secretion from the granular cells of the afferent arteriole is locally influenced by the tubular system of the corresponding nephron. The anatomical basis of this regulatory pathway for renin secretion resides in the close linkage of the tubular and vascular system within the confines of the JGA. Specifically, the thick ascending limb of Henle’ loop (TAL) emerging from the renal medulla comes into close contact with its parent glomerulus. This anatomical link between the tubular system and the vasculature of the afferent and efferent arterioles enables the specialized tubular cells of the cortical thick ascending limb of Henle’s loop (cTAL) and the macula densa (MD) cells located adjacent to the extraglomerular mesangium to influence renin secretion. In this local control system, changes in the tubular NaCl concentration are translated into inverse changes in renin secretion. Early experimental evidence for a tubular control mechanism of renin secretion has been reviewed elsewhere (293), but the most striking and direct evidence was provided by the following in vitro experiment. Rabbit JGAs were isolated, and TALs were perfused with artificial tubular fluid containing various concentrations of NaCl. Simultaneously, the entire JGA was superfused, and renin release was determined by microassay. In this experimental setup, a decrease in the tubular NaCl concentration resulted in “a prompt stimulation of the renin release rate” (815). This fundamental finding sparked interest in the signaling pathways that link the tubular NaCl concentration and Physiol Rev • VOL FIG. 5. Local control of the renin secretion. The renin-generating granular cells are located at the distal portion of the afferent arteriole. The cells adjacent to the granular cells or in their close vicinity exert a local control on the renin secretion. These local control mechanisms of renin secretion comprise vascular, nervous, and tubular components. The stimulatory factors like nitric oxide (NO; formed by endothelial NO synthase) and prostacyclin/prostaglandin E2 are formed in the endothelium adjacent to the granular cells. They are counteracted by the inhibitory factors like endothelins. The overall effect of the sympathetic input derived from local nerve endings and circulating catecholamines is stimulatory. The prostanoids (stimulatory) and ATP (inhibitory) are generated by the tubular macula densa cells under the control of the NKCC2-dependent salt transport activity. The macula densa salt transport in this system is translated into an increased formation and release of prostanoids and ATP in response to the low and high tubular salt concentration, respectively. In addition, the tubular macula densa cells constitute a rich source of NO (formed by neuronal NO synthase), which provides a background stimulus for the renin secretion. renin secretion. The initial step of the MD-dependent control of renin secretion is the detection of the NaCl concentration in the tubular lumen by the MD cells. The transport activity of the Na⫹-K⫹-2Cl⫺ cotransporter NKCC2 (BSC1) in the apical membrane of these cells is thought to be the primary mechanism by which the MD cells detect the tubular NaCl concentration (64, 65, 500, 538, 640, 758, 958). In addition, the NaCl transport activity mediated by NHE2 might contribute to the MD salt sensing (306). The MD cells subsequently transmit information about the tubular NaCl concentration to the granular cells of the afferent arteriole to influence renin secretion. Three likely candidate molecules have been proposed to serve as mediators of the MD-dependent control of renin secretion: prostanoids, NO, and adenosine/ATP. These molecules meet the key criteria to serve as signal mediators between tubular epithelial cells and JG cells of the afferent arteriole: 1) MD cells have the capacity to gener- 90 • APRIL 2010 • www.prv.org 624 CASTROP ET AL. ate each molecule, 2) their formation depends on the tubular salt concentration, and 3) there is solid experimental evidence to suggest that they directly influence renin secretion. 1. Prostanoids Prostanoids are generated from arachidonic acid in a two-step reaction. The first step, the formation of prostaglandin H2 (PGH2), is catalyzed by two isoforms of cyclooxygenases, the constitutively expressed COX-1 and the inducible COX-2 (171, 660). PGH2 is subsequently modified by the various prostanoid synthases to produce specific prostanoids. Of those, prostaglandin E2 (PGE2) and prostacyclin (PGI2) were shown to directly stimulate renin secretion in isolated JG cells (241, 401). A) ACTIVATION OF EP2, EP4, AND IP RECEPTORS STIMULATES RENIN SECRETION. In vitro investigations and studies in mice with a specific deletion of single prostanoid receptors revealed that the stimulation of renin secretion by PGE2 and PGI2 are mediated via EP2, EP4, and IP receptors (241, 244, 778). EP2, EP4, and IP prostanoid receptors are all coupled to Gs-mediated increases in intracellular cAMP, which act as the main stimulus of renin secretion (see sect. IIC1). B) PROSTANOID GENERATION IN THE TAL. Regarding the concept of local tubular control of the renin system, Harris et al. (315) first described the constitutive expression of COX-2 in MD cells of the rat. This finding suggested that COX-2-derived prostanoids might be involved in the MD control of renin secretion. An in-depth analysis of COX-2 expression in the cortical TAL by immunohistochemistry revealed that COX-2 expression in rats and rabbits is not restricted to the MD cells but is also found in the adjacent tubular cells of the TAL (151, 558, 866, 916). COX-2 expression in the TAL of primates and humans appears to be low compared with rodents, at least under normal conditions (429, 452, 621, 867). It should be noted in this context, however, that the typical diet in the Western hemisphere is rich in NaCl and that, as outlined later, COX-2 expression is suppressed by a high oral salt intake, which may thus impair the detection of COX-2 in human renal cortices by immunohistochemistry. In addition to COX-2, the MD cells also express the microsomal isoform of PGE2 synthases (867), rendering MD cells capable of generating PGE2 (113, 250, 867, 917). The expression of PGE2 synthases in MD cells has been detected in both humans and rodents (113, 867). Functional evidence for the generation of PGE2 by MD cells was obtained using biosensor cells in isolated JGA preparations. In this setup, HEK293 cells transfected with the PGE2 receptor EP1 were positioned close to MD cells, and a release of PGE2 was detected via an increase in intracellular calcium of the biosensor cell; furthermore, this effect was specific for MD cells (689). Physiol Rev • VOL C) COX-2 EXPRESSION AND ACTIVITY IN THE TAL ARE REGULATED. The constitutive generation of prostanoids by MD cells would allow for a tonic influence on renin secretion by JG cells. However, the expression of COX-2 in the MD cells and the acute formation of prostanoids are regulated by physiological stimuli, making it feasible that the MD-derived prostanoids could be involved in the signal transmission between the epithelial MD and vascular JG cells. Most strikingly, the regulation of COX-2 is usually paralleled by corresponding changes in the activity of the renin system. Thus COX-2 mRNA expression in the MD is upregulated upon chronic administration of loop diuretics (131, 414, 415). Similarly, Bartter patients (these patients suffer from loss-of-function mutations of NKCC2, or other crucial proteins for TAL NaCl reabsorption, such as ClCNKB, ROMK, or Barttin) show markedly increased COX-2 expression in the cTAL, indicating a relationship between the NKCC2-dependent salt transport and COX-2 expression (453). COX-2 expression was also found to be increased under conditions of reduced oral salt intake or diminished renal perfusion pressure, both of which might result in a reduced distal TAL salt concentration (315, 318, 398, 955, 972). In addition, the enhanced expression of COX-2 observed in conditions of both reduced oral salt intake and compromised TAL salt transport activity (loop diuretics, Bartter’s patients) was paralleled by an increase in the expression of the microsomal PGE2 synthase, the downstream enzyme of PGE2 synthesis (113, 454). Whereas changes in prostanoid production due to the altered expression of COX-2 and accessory downstream enzymes are likely to influence renin secretion over longer time frames, an acute reduction of salt concentration in MD cells was shown to result in rapid changes in the prostanoid formation, presumably related to changes in COX-2 and PGE2 synthase activity. In this context, in vitro experiments using the aforementioned biosensor approach indicated a release of PGE2 across the basolateral membrane of the MD cells in response to a decreased tubular salt concentration or in response to the presence of loop diuretics (689). Such acute changes in the prostanoid formation require the presence of sufficient amounts of substrate, i.e., arachidonic acid. Whether these changes in phospholipase A2 activity are causally involved in the modulation of the tubular prostanoid formation awaits experimental evaluation. D) COX-2-DERIVED PROSTANOIDS AND RENIN SECRETION. Both in vitro and in vivo studies were performed to assess the functional relevance of prostanoid release from the MD cells in the control of renin secretion. The in vitro experiments using isolated TAL/JGA preparations indicated a crucial role for MD prostanoid formation in the stimulation of renin secretion in response to a reduction in the salt concentration in the tubular perfusion fluid; that is, the nonselective blockade of COX activity blunted the increase in renin secretion following a reduction of the 90 • APRIL 2010 • www.prv.org PHYSIOLOGY OF KIDNEY RENIN tubular salt concentration (277). Similar results were obtained with the COX-2 inhibitor NS-398, whereas COX-1 inhibition had no effect (890). All attempts to assess the in vivo role of locally generated prostanoids in the regulation of renin secretion have been hampered by the lack of experimental tools to specifically or locally modulate the prostanoid formation within the confines of the JGA without inducing systemic effects. For example, the blockade of COX-2 activity during the inhibition of NKCC2 transport activity by the administration of loop diuretics markedly reduced the typical stimulation of renin secretion (414, 768). However, the reduced renin secretory response under loop diuretic treatment was accompanied by a considerably moderated diuretic effect of the loop diuretic, thus permitting no clear conclusion as to whether the blunted stimulation of renin secretion was directly or indirectly related to the COX inhibition. Similar results were found in humans with genetic inactivation of NKCC2: in type 1 Bartter’s patients, plasma renin activity and abnormal natriuresis/diuresis were both ameliorated by the specific inhibition of COX-2 with rofecoxib (723). In healthy volunteers, COX-2 inhibition by rofecoxib was shown to block the increase in PRA in response to a salt-restricted diet (414). Initial animal experiments in rats and mice have suggested that the stimulation of renin secretion in response to various experimental maneuvers, including renal artery stenosis, ACE inhibition, AT1 receptor antagonism, and a salt-restricted diet, all depend on the intact COX-2-dependent prostanoid formation (311, 312, 934). In these early studies, experimental COX-2 inhibitors like NS398 and SC58236 were used. Follow-up studies using the Food and Drug Administration-approved COX-2 inhibitors rofecoxib or celecoxib were not able to fully confirm the initial results of reduced stimulation of renin secretion after oral salt restriction and after angiotensin antagonism (343, 346, 414). The somewhat contradictory results of the studies that sought to assess the in vivo role of prostanoids in the MD control of renin secretion might be attributed to a problem that appears to be inherent to all attempts to investigate the tubular control of renin secretion. More specifically, to date, we are not aware of any experimental protocol to specifically manipulate (which means reduce in the case of the stimulatory signaling pathway) the salt concentration at the MD segment of the TAL without causing systemic effects that, in turn, also affect the renin secretion. The availability of COX-2-deficient mice provided a fresh impetus for the investigation of the tubular control of renin secretion. COX-2-deficient mice show markedly reduced baseline renin expression and secretion (152, 435, 969). In addition, the stimulation of renin secretion due to reduced oral salt intake, ACE inhibition, angiotensin AT1 receptor antagonism, and reduced renal perfusion pressure was reduced in COX-2 ⫺/⫺ mice compared with controls (152, 435, 969). Thus it was tempting to suggest Physiol Rev • VOL 625 that at least the MD-dependent component of the regulation of renin secretion under these conditions is highly dependent on the COX-2-dependent formation of prostanoids. However, the interpretation of the results was hampered by the fact that the COX-2 ⫺/⫺ mice suffer from retardation of renal development and considerable deterioration of renal function during adulthood (199, 600); additionally, the latter was shown to be highly dependent on the genetic background of the COX-2-deficient strain (971). Therefore, the regulation of renin secretion in COX-2 knockout mice was recently revisited in a study using COX-2-deficient mice of various genetic backgrounds (435). The baseline plasma renin concentration was reduced to some degree in all COX-2 ⫺/⫺ strains compared with the corresponding wild-type mice. Similarly, the renin secretory response to acute challenges like the administration of furosemide, ACE inhibitor, AT1 receptor antagonist, or isoproterenol was unanimously compromised in the COX-2 ⫺/⫺ mice compared with controls. However, when the baseline PRC of the COX-2deficient mice was chronically augmented by pretreatment with a combination of an ACE inhibitor and a low-salt diet, the acute stimulation of renin secretion in response to furosemide was partially reestablished, suggesting that the COX2-derived prostanoids are responsible in vivo for determining the size of the releasable pool of renin, which eventually defines the amount of renin that can be secreted during acute stimuli (435). Similar results were obtained in rats using the COX-2 inhibitor SC58236 (569), again indicating that COX-2-derived prostanoids act as general enhancers of the renin system, rather than as mediators of the regulatory input. How can these results be reconciled with the credible in vitro data unequivocally showing that intact COX-2 activity is an indispensable prerequisite for the modulation of renin secretion in response to acute changes in the tubular salt concentration? Although this question cannot be answered comprehensively at the moment, accumulating evidence indicates that the role of the tubular control of renin secretion during chronic variations of the tubular salt concentration (e.g., chronic changes in oral salt intake) is limited, or it can be compensated for by other presumably systemic control mechanisms, which are absent in the situation of the in vitro JGA preparation; conversely, the MD seems to play a crucial role in the acute (inhibitory) control of renin secretion in vivo (435, 651, 652). We will further address this issue when we discuss the role of adenosine in the tubular control of renin secretion. In summary, prostanoids like PGE2 and PGI2 are formed by tubular MD cells, and their generation is dependent on tubular salt concentration. PGE2 and PGI2 are potent stimulators of renin secretion and determine the operating point of the renin system in vivo. Their relevance as stimulatory mediators of a communication path- 90 • APRIL 2010 • www.prv.org 626 CASTROP ET AL. way between the TAL and JG cells in vitro (i.e., in the absence of other regulatory mechanisms) is well established, although their significance as regulatory factors of renin secretion in vivo is less clear. 2. NO NO activates both inhibitory and stimulatory pathways of renin secretion in the granular JG cells, as outlined in detail in section III. However, with all of the relevant data taken together, it now appears well established that the overall effect of NO on renin secretion, at least as far as the in vivo situation is concerned, is stimulatory (407, 683, 765, 770). A) NOS IN THE VICINITY OF RENIN-GENERATING CELLS. The formation of NO from L-arginine is catalyzed by three different NOS isoforms (936, 937). At least two of the three known NOS isoforms are expressed in close vicinity to the granular cells of the afferent arteriole. Endothelial NOS (eNOS) is localized to the endothelial cells of the afferent arteriole, and nNOS is found in the MD of the TAL (608, 952). Commensurate with a possible role of NO as the mediator of MD-dependent regulatory input on renin secretion, the expression of nNOS in the MD was shown to be regulated in parallel with the expression of renin under various conditions, including the variation of oral salt intake, administration of loop diuretics, and renal hypoperfusion (84, 126, 611, 772, 808, 886). B) MACULA DENSA NO FORMATION AND RENIN SECRETION. A role for NO as a mediator of the MD-dependent stimulation of renin secretion was first suggested in a study that utilized isolated perfused JGA preparations (326). The increase in renin secretion observed in response to a low-salt concentration in the artificial perfusate of the TAL was markedly attenuated in the presence of the nonspecific NOS inhibitor NG-nitro- L-arginine (326). For in vivo studies with the specific nNOS inhibitor 7-nitroindazole (7-NI), the results were less clear. More specifically, some studies reported that 7-NI was capable of eliminating or at least reducing the effect of a low-salt diet on the renin system (60, 854), whereas another study found no influence of 7-NI on the renal renin content given a salt-restricted diet (312). Several studies have assessed the effect of NOS inhibition on the stimulation of renin secretion caused by loop diuretics, which were used as a measure to address the MD-dependent control of renin secretion (325). In an isolated renal vessel preparation, NOS inhibition substantially reduced the increase in renin release after pretreatment with furosemide (142). Similar results were obtained in vivo, where renin secretion was reduced in response to loop diuretics under concomitant NOS inhibition (61, 722, 768). The interpretation of the results derived from studies with a pharmacological inhibition of NOS is complicated because it is unclear to what extent the agents specifically inhibit each Physiol Rev • VOL of the three NOS isoforms. In particular, the specificity of the widely used agent 7-NI for nNOS over eNOS and inducible NOS (iNOS) is based on its preferential in vivo uptake by neuronal cells compared with endothelial cells, rendering its specificity for nNOS in cells other than neurons questionable (597). To circumvent these issues and to assess whether MD-derived NO is a functional stimulatory mediator of renin release in response to changes in the tubular salt content, the effect of a loop diuretic on renin secretion was investigated in nNOS- and eNOSdeficient mice (130). The loss of either nNOS or eNOS activity had little impact on the magnitude of renin secretion in response to furosemide application. However, after the nonspecific inhibition of all NOS isoforms by NGnitro-L-arginine methyl ester (L-NAME), the furosemideinduced renin secretion was substantially reduced in wild-type, nNOS ⫺/⫺, and eNOS ⫺/⫺ mice. These data suggest that NO acts as an enhancer of renin secretion irrespective of its source, and therefore, NO is not required to mediate the MD control of renin secretion. Similarly, in the isolated perfused kidney, the effect of bumetanide on renin secretion was blunted by NOS inhibition. However, clamping the NO concentrations in the isolated perfused kidney by exogenous NO administration with the concomitant inhibition of all NOS isoforms fully restored the renin secretory responsiveness to bumetanide, suggesting again that the mere presence of NO is sufficient and that a fluctuation in NO concentrations is not required for the activation of the MD control of renin secretion (130). In summary, NO is generated in the vicinity of the JG granular cells by nNOS and eNOS and serves as a stimulatory, permissive factor in renin secretion. On the basis of the present data, it seems unlikely that NO derived from MD cells functions as a mediator of the tubular control of renin secretion. 3. Adenosine/ATP Adenosine meets the key requirements to serve as a mediator of the local tubular control of the renin system. First, there is solid evidence that adenosine is generated in the JGA. Second, the formation of adenosine in the JGA depends on the tubular salt concentration in the MD segment of the TAL. Third, adenosine exerts a direct effect on renin secretion from JG granular cells of the afferent arteriole. A) TUBULAR NACL CONCENTRATION AND ADENOSINE FORMATION IN THE JGA. The function of adenosine as a paracrine factor in the JGA has been the subject of intense research over the past several years. The focus, however, has been on the role of adenosine in the MD control of preglomerular resistance (the so-called tubuloglomerular feedback mechanism; Ref. 874) rather than its function in the local tubular control of renin secretion. Nevertheless, progress 90 • APRIL 2010 • www.prv.org PHYSIOLOGY OF KIDNEY RENIN made towards the elucidation of the signaling pathways that connect the tubular MD cells with the vascular smooth muscle cells of the afferent arteriole also expanded our knowledge of the local tubular control of renin secretion. The data indicate that the following chain of events is initiated by an increase in the tubular salt concentration at the macula densa. First, MD cells detect increases in the tubular NaCl concentration via enhanced NKCC2-dependent NaCl reabsorption, the MD cells subsequently release ATP via their basolateral membrane, and the ATP is successively dephosphorylated in the extracellular space to eventually form adenosine, which in turn causes afferent arteriolar vasoconstriction and a concomitant drop in the GFR of the respective nephron (66, 90, 125, 455, 650, 832). A1 adenosine receptors (A1AR) are expressed in vascular smooth muscle cells of the afferent arteriole, with particularly high expression levels in the most distal portion of the vessel (390), and they mediate the vasoconstriction through the Gi-dependent activation of phospholipase C (308). Since the granular JG cells also express A1AR (11), it is conceivable that adenosine may also influence or suppress renin secretion in response to increases in the tubular NaCl concentration. In fact, the dose-dependent inhibition of renin secretion by adenosine was demonstrated for isolated JG cells and renal cortical slices (160, 474). The A1AR antagonists such as 8-cyclopentyl-1,3 dipropylxanthine (DPCPX) in turn result in a stimulation of renin secretion, suggesting that the renin system is tonically suppressed by endogenous adenosine formation (388, 469, 470). The assumptions that renin secretion is controlled by an endogenous “adenosine-brake” (388) and that adenosine in this context is derived from tubular sources, i.e., tubular ATP, are further supported by the observation that renin secretion in the isolated JGA is lower in the presence of the tubular MD segment than in its absence. This difference in the renin release rate was eliminated by the nonspecific adenosine receptor antagonist theophylline (381). Similarly, the PRA was increased in humans after the application of the A1AR antagonist FK-453, again suggesting a tonic inhibition of renin release by adenosine (43). The involvement of adenosine in the mediation of the MD-dependent suppression of renin secretion in response to high tubular NaCl was first suggested by in vitro experiments using the isolated TAL/JGA preparation. In this experimental setup, as mentioned above, an increase in the salt concentration of the tubular perfusion fluid resulted in a reduction of the renin release rate (815). The application of DPCPX to the bath markedly reduced the magnitude of this decrease in renin secretion, indicating that the activation of A1AR by adenosine constitutes a crucial step in the signaling cascade between tubular cells and the effector cells of the afferent arteriole (943). The in vivo significance of adenosine in the tubular control of renin secretion was assessed in studies using Physiol Rev • VOL 627 A1AR-deficient mice (90, 832). The effects of acute changes in the tubular salt concentration were investigated in these mice by acute salt loading to raise the NaCl concentration at the MD or by the application of loop diuretics to mimic a low tubular salt concentration. The acute salt loading was achieved by the intravenous application of isotonic saline, an experimental maneuver that has been shown to result in increased distal tubular salt concentration and a concomitant suppression of renin secretion (440, 537). In A1AR ⫺/⫺ mice, the acute suppression of renin secretion provoked by acute salt loading was completely absent, whereas the PRC was reduced in wild-type controls by ⬃40% (436). The converse of salt loading, a blockade of the MD salt transport by the use of furosemide, resulted in a comparable stimulation of renin secretion in both wild-type and A1AR-deficient mice, suggesting that the adenosine-dependent signaling is responsible for the inhibitory arm of the tubular control of the renin system (436). In contrast to acute changes in the tubular salt, the chronic modulation of oral salt intake does not appear to be dependent on intact adenosine signaling. Thus the stimulation and suppression of the renin system under dietary salt-deprived conditions were fully preserved in the A1AR ⫺/⫺ mice (784). The assumption that this adenosine-dependent suppression of renin secretion derives from the tubular control of the renin system is further supported by the observation that the acute suppression of renin secretion due to salt loading is also absent in NKCC2A-deficient mice, a strain that lacks the isoform of NKCC2 responsible for MD salt-sensing in the high concentration range (651, 652). However, both these and the A1AR-deficient mice display a reduction in the PRC after chronic high salt intake, similar to that observed in wild-type controls. Thus A1AR- and NKCC2A-deficient mice maintain an inverse regulation of PRC after a high-salt diet, but they have lost the ability to suppress PRC after acute salt loading (652). Since NKCC2 expression is restricted to the TAL (211, 640, 970), these data again suggest that the negative tubular control of renin secretion is more relevant for acute changes in tubular NaCl than for chronic changes and that a lack of tubular control can be compensated for by other factors when salt intake is chronically increased. B) ADENOSINE AND PRESSURE-DEPENDENT REGULATION OF RENIN SECRETION. With respect to the pressure-dependent control of renin secretion, the contributions of local tubular, vascular, and systemic factors remain to be determined (see also sect. IVB). The experimental evidence suggests a crucial role for the adenosine-dependent signaling in the tubular domain. Thus the acute and chronic phenylephrine infusion in vivo resulted in a suppression of PRC in wild-type mice, but not in A1AR-deficient mice, despite similar increases in the arterial pressure (783). Similarly, in the isolated perfused kidney, the A1AR deficiency and 90 • APRIL 2010 • www.prv.org 628 CASTROP ET AL. inhibition of A1AR by DPCPX eliminated the suppression of renin secretion in response to a stepwise increase in the renal perfusion pressure (783). Conversely, renin secretion was enhanced by a decrease in the perfusion pressure, irrespective of whether functional A1AR was present or not (783). The results of this in vitro study resemble an in vivo experiment in which the vasodilator agent hydralazine was used to reduce the arterial pressure. Again, PRC was enhanced to a similar extent after hydralazine administration in A1AR ⫺/⫺ mice and wildtype controls (436). These data suggest that intact adenosine signaling is critical to the pressure-dependent inhibitory arm of renin secretion, whereas other factors (rather than decreased adenosine formation) appear to be responsible for the stimulatory arm, which is activated by low arterial pressure. B. Vascular Control of Renin Secretion JG granular cells in the afferent arteriole are surrounded and influenced by neighboring vascular smooth muscle cells and endothelial cells (see also sect. IIIC). Multiple local factors derived from the vasculature may influence renin expression and secretion, as summarized in detail elsewhere (293). We focus on four major local factors that appear to be primarily involved in the communication among vascular smooth muscle cells, endothelial cells, and JG cells: NO, adenosine/ATP, endothelins, and prostanoids. In this section, we also assess the data that relate to the regulation of renin secretion by the local “baroreceptor,” although it is still difficult to define whether and to what degree the local baroreceptor is related to vascular or tubular events or events that occur within JG cells. 1. NO Due to the expression of eNOS, the endothelium is a rich source of NO in most vascular beds, including the kidney. Specifically, the expression of eNOS in the afferent arteriole has been shown for a number of mammalian species (40, 41, 896). A coculture of JG cells with endothelial cells revealed both inhibitory and stimulatory signaling derived from the endothelial cells, with the stimulatory component related to endothelium-dependent NO formation (462, 481, 773). In contrast, the NOS inhibition in the absence of endothelial cells had no effect on the renin secretion from JG cells, presumably due to the lack of NOS expression in JG cells (773). The relevance of endothelium-derived NO for the release of renin was further supported by studies in the isolated perfused kidney. The addition of acetylcholine, a known stimulator of endothelial NO release (248), to the perfusion fluid increased the renin release rate, which was blunted by a concomitant NOS inhibition (610, 765). Furthermore, Physiol Rev • VOL eNOS was shown to have a considerable tonic stimulatory influence on renin transcription in vivo without being involved in the regulation of renin mRNA expression under conditions such as a high-salt or low-salt diet in combination with the ACE inhibitor ramipril (923). Finally, as mentioned above, in vivo data from eNOS- and nNOS-deficient mice suggest that the adequate NO generation in the vicinity of JG cells, irrespective of whether the source is endothelial (eNOS) or tubular (nNOS), constitutes a permissive factor that allows other regulatory mechanisms of PRC to operate at full magnitude (130). 2. Adenosine/ATP The function of adenosine in the local control of renin secretion was touched upon in connection with the tubular mechanisms that account for the adenosine-dependent suppression of renin secretion. The formation of adenosine in the confines of the JGA, however, is not limited to the ATP release by MD cells and the subsequent extracellular dephosphorylation resulting in the formation of adenosine (66, 455). ATP is also known to be released by endothelial cells and vascular smooth muscle cells, e.g., in response to increased shear stress (80, 582, 678, 786, 938). In fact, the renal extracellular ATP concentration as determined by microdialysis has been shown to be directly related to the renal perfusion pressure, which was most likely related to both tubular and vascular ATP release (631, 632). Of note, some regulatory capacity of renin secretion by the renal perfusion pressure was preserved in hydronephrotic kidneys, i.e., in the absence of functional tubular-JG signaling (766). Thus the vasculature-dependent ATP release plays a key role in mediating the renal autoregulation in response to increased perfusion pressure (375, 376, 554). In addition, JG cells were shown to release ATP in response to mechanical stretching (973); in this context, ATP could subsequently either influence JG cells in an autocrine manner, or ATP might be subject to dephosphorylation in the extracellular space. In terms of an increased renal perfusion pressure, the overall effect of ATP released by the endothelial, smooth muscle, or JG cells is supposedly the suppression of renin secretion (973). Since ATP, however, has been shown to stimulate renin secretion from renal cortical slices, presumably via enhancing endothelial NO generation (162), it is likely that the bulk of the ATP present in vivo is locally hydrolyzed to form adenosine, which then acts on A1AR (160, 474), or that the ATP acts directly on the P2 purinoreceptors present on JG cells (973). In summary, the vascular release of ATP and subsequent formation of adenosine in the extracellular space appears to contribute to the local adenosine formation, which results in a suppression of renin secretion mediated by the activation of A1AR. This may be of particular 90 • APRIL 2010 • www.prv.org PHYSIOLOGY OF KIDNEY RENIN relevance during situations of increased renal perfusion pressure. 3. Adenosine/ATP and renal baroreceptor The term renal baroreceptor describes the inverse relationship between the intrarenal (perfusion) pressure and renin secretion. Although it is still difficult to pinpoint the exact nature of the renal baroreceptor, it appears likely that the adenosine/ATP from vascular and tubular sources is crucially involved in the suppression of renin secretion during increased intrarenal pressure, as mentioned above. In addition, mechanical stretching results in the release of ATP from JG cells (973). This release of ATP from JG cells may provide a tubule-independent link between renal perfusion pressure and renin secretion and may participate in the still elusive local “baroreceptor.” Multiple studies describe a stretch or transmural pressure dependency of JG function. Thus in isolated JG cells, the mechanical stretch reduces both the basal and prestimulated renin secretion in parallel with reduced renin transcription (117). In addition, the processing of pro-renin into active renin is attenuated by transmural pressure on JG cells (339). Similar results were obtained for the reninexpressing cell line As4.1 after mechanical elongation (736). In this study, the activity of a renin promoter reporter construct was diminished upon mechanical stretching. The mechanical stretching of the As4.1 cells also induced a rise in the intracellular calcium, the wellestablished intracellular inhibitor of renin formation (see sect. IIID) (738). Contrary to this report, however, the mechanical stretch of mouse JG cells in situ in isolated afferent arterioles did not affect the JG cell calcium levels (761). In summary, the investigation into the mechanisms underlying the local renal baroreceptor and the pressuredependent control of the renin system requires further experimental efforts. It appears most likely that the baroreceptor is composed of several factors, including tubular, vascular, and JG cell-related components. 4. Endothelins Multiple studies have suggested that endothelins serve as negative regulators of renin expression and secretion. A primary culture of JG cells revealed that endothelin-1, -2, and -3 all inhibit the increase in renin secretion that occurs after the stimulation of the cAMP pathway, whereas they have little influence on the baseline renin secretion. The in vitro effect of endothelins on the cAMP-stimulated renin secretion was not modulated by the ETA receptor antagonist BQ123. Conversely, the ETB agonists like IRL-1620 and sarafotoxin S6b had similar effects on the renin secretion as those elicited by endothelins, suggesting that the endothelins influence renin secretion via activation of the ETB receptors (4, 728). Physiol Rev • VOL 629 Similar results were obtained with regard to renin gene expression (727). For the isolated perfused kidney, a slight increase in the renin release rate was observed at a low endothelin concentration (10 pM), whereas higher concentrations consistently suppressed renin secretion; once again, this effect was primarily mediated by ETB receptors (763). It is conceivable that the activation of the ETB receptors exerts a dual effect on renin secretion, with a stimulatory component related to the enhanced endothelial NO formation (218, 337) and a direct inhibitory component that depends on the ETB receptors present on JG cells. The correlation between endothelins and the renin system in vivo was assessed during chronic hypoxia in rats (782). The ET receptor antagonist LU135252 administered during chronic hypoxia resulted in a marked increase in renin gene expression, suggesting a negative effect of endothelins on the renin system and basically confirming the in vitro data (782). At present, our knowledge of the functional impact and relevance of the endothelin system on the regulation of renin expression and secretion is limited. Endothelins apparently act as endothelial factors that tonically counteract the stimulatory effects of other endothelium-derived factors, most notably NO. Since the endothelial generation of endothelin-1, in particular, was shown to be related to vascular shear stress (733), it appears reasonable to suggest an involvement of the endothelin system in the pressure-dependent regulation of renin secretion, a possibility that awaits further experimental evaluation. 5. Prostanoids The role of prostanoids in the control of renin expression and secretion was discussed in detail in the context of tubular prostanoid formation. However, in addition to the tubules, the renal vasculature also appears to be a significant source of prostanoids, particularly prostacyclin. In humans, the substantial expression of COX-2 was observed in the afferent arteriole in close vicinity to the granular JG cells, which exceeded the expression of COX-2 in the TAL (867). In rodents, conversely, no COX-2 but some COX-1 was found in the renal vasculature (113). The vascular expression of COX-2 in humans was enhanced in patients with renal artery stenosis, and therefore, vascular COX-2 activity may contribute to the increased renin generation during renal hypoperfusion. Similarly, in mice, the formation of prostacyclin and the activation of the IP receptors appear to be crucial for the activation of renin secretion during renal hypoperfusion. Accordingly, the stimulation of the renin system during renal artery stenosis was markedly reduced in IP receptordeficient mice (244). In addition, stimulation of the renin system after the application of loop diuretics may be at least partly related to the enhanced vascular prostacyclin formation (518). Conversely, the attenuation of renin secretion in 90 • APRIL 2010 • www.prv.org 630 CASTROP ET AL. response to loop diuretics by inhibitors of cyclooxygenases appears to be partially mediated by the reduced vascular prostacylin synthesis (344, 414, 415, 827). In conclusion, prostanoids derived from vascular sources in the vicinity of JG cells (or from the systemic vasculature, as outlined in section V) constitute potent stimulators of renin secretion and expression. Their contribution to overall prostanoid formation may be considerably species dependent. Changes in the formation of prostanoids, e.g., in response to decreased renal perfusion pressure, are not restricted to changes in the tubular prostanoid formation, but they are often paralleled by regulated vascular prostanoid formation. C. Neural Control of Renin Secretion The presence of 1-adrenergic receptors in the JG granular cells has been established by multiple receptor binding studies and by in situ hybridization. The results of these investigations are summarized in detail elsewhere (293). More recently, the expression of 1-receptors on JG cells was shown by immunohistochemistry (81). The activation of 1-receptors by catecholamines results in an augmented formation of cAMP, the central intracellular stimulus of renin secretion (see sect. III). Thus the pharmacological inhibition of 1-receptors results in reduced renin secretion (130, 423). Similarly, renal denervation to excise sympathetic input was shown to lower renin expression (271, 354), but it should be noted that renal denervation also removes the input of the sympathetic system mediated by ␣-receptors, including hemodynamic and tubular effects (335). Most notably, double-knockout mice deficient in 1- and 2-receptors have substantially reduced basal PRC, ⬃85% lower than that observed in wild-type controls (434). Thus the sympathetic input from local nerve endings or from circulating catecholamines may represent the most powerful background stimulus for renin secretion. In view of the significance of the sympathetic nerve tone for the maintenance of basal renin secretion, it appears reasonable to also suggest an involvement of sympathetic input in the regulation of renin secretion triggered by changes in oral salt intake. It should be mentioned in this context that the renal sympathetic nerve activity is higher in rats on a low-salt diet than in those on a high-salt diet (118). However, most studies suggest that modulation of the activity of the renin system is not crucially dependent on intact -adrenergic input. That is, most reports indicate that neither renal denervation nor pharmacological -blockade considerably influence the stimulation of the renin system by a salt-deficient diet or its suppression by a high-salt diet (271, 353, 354). In some studies in humans, dogs, and rats, however, the -receptor antagonist propranolol was shown to reduce the magnitude of the renin system stimPhysiol Rev • VOL ulation during a salt-deprived diet (423, 879). These somewhat contradictory results might be attributed to differences in the species or in the specific experimental setups used. However, for most in vivo studies utilizing -antagonists, it has remained unclear to what extent -inhibition was achieved. Those issues were circumvented by the use of 1/2 double-knockout mice (434). As mentioned above, basal renin expression and PRC in 1/2 ⫺/⫺ mice were both substantially reduced compared with wild-type controls. In 1/2 ⫺/⫺ mice, the changes in renin secretion that occurred in response to both acute and chronic stimuli, such as oral salt intake, loop diuretics, ACE blockers, and AT receptor antagonists, were maintained; however, the magnitudes of these changes in the PRC were markedly diminished (434). In summary, activation of JG cell 1 receptors is required to sustain basal renin expression and secretion. Furthermore, the 1 receptors are necessary to drive the renin synthesis needed to maintain a releasable renin pool, which provides the basis for the full-magnitude secretory responses to external stimuli. V. SYSTEMIC CONTROL OF RENIN SYNTHESIS AND SECRETION The RAAS is critically involved in the regulation of salt and volume homeostasis and in the control of blood pressure. These variables in turn influence renin synthesis and renin release via negative-feedback loops. In addition, renin synthesis and release are also influenced by sympathetic nerves, tubular mechanisms, renal autacoids, and hormones. We discuss, in this section, the newest advances relevant to the regulation of renin release and synthesis. A. Blood Pressure The regulation of blood pressure involves a variety of organ systems including the central nervous system (CNS), cardiovascular system, kidney, and adrenal glands. These systems modulate cardiac output, fluid volume, and peripheral vascular resistance as the major determinants of blood pressure. The renin-angiotensin system is a key regulator of blood pressure and salt and water homeostasis (946). The inhibitors of ACE or ANG II receptor antagonists are potent blood pressure-lowering drugs that may also attenuate cardiovascular and renal injuries (945, 954). Due to changes in the formation of the vasoconstrictor ANG II, alterations in renin release can also cause changes in the arterial blood pressure, such that an increased rate of renin release increases blood pressure and vice versa. Accordingly, renin inhibitors have been shown to lower blood pressure to similar levels as ANG II-AT1 receptor blockers or ACE inhibitors (544). Furthermore, mice with genetic deletions of angiotensinogen, renin, ACE, and 90 • APRIL 2010 • www.prv.org PHYSIOLOGY OF KIDNEY RENIN AT1a receptors (in contrast to humans and all other mammalian species, rodents posses two AT1-receptor isoforms: AT1a and AT1b) are hypotensive (220, 380, 432, 466, 967). Virtually all tissues presumed to have a major role in blood pressure control, including the heart, the vasculature, the adrenal glands, the nervous system, and the kidney, express AT1 receptors (164). Via activation of the AT1 receptors, ANG II triggers multiple effects, such as vasoconstriction, stimulation of aldosterone release, central vasopressor responses, and increased renal sodium retention (8, 30, 182, 230, 877), which may potentially lead to an increase in the systemic blood pressure. It has been assumed that the body has several important systems for controlling blood pressure, which react within seconds (baroreceptors, chemoreceptors, and CNS ischemic response), minutes (the RAAS, stress relaxation, capillary fluid shift, and aldosterone release), and finally hours or days (renal volume control) (291). Nevertheless, it has been suggested that the kidney is the dominant mechanism for the long-term regulation of blood pressure; as such, an elevation in the renal perfusion pressure due to an increased arterial blood pressure enhances sodium and water excretion, leading to a decrease in body fluid volume and vice versa (“pressure natriuresis”) (291). Moreover, renal transplantation studies further support the central role of the kidney in regulating blood pressure (725). The RAAS modulates renal function such that the activation of the RAAS causes a shift of the pressurenatriuresis curve to the right and the inhibition of the RAAS causes a shift to the left. This was demonstrated, for example, by showing that the infusion of ANG II into the renal artery reduced the sodium reabsorption and increased blood pressure without impairing peripheral resistance (300, 302). A critical role for renal AT1a receptors derives from the AT1a receptor-deficient mice, which develop a salt-sensitive hypertension (646). Recently, renal cross-transplantation studies with AT1a receptor-deficient mice and wild-type mice further demonstrated the importance of renal AT1a receptors for blood pressure control. The specific absence of renal AT1a receptors (“renal knockout”) lowered blood pressure from ⬃118 mmHg in wild-type mice to 99 mmHg in renal knockouts, suggesting that the kidney is an important determinant of blood pressure control (173). However, there is also evidence of a role for the RAS in the control of blood pressure in nonrenal tissues. In this context, it has been found, for example, that the injection of ANG II into the brain increases blood pressure, likely via activation of the AT1a receptors (15, 182), and that the systemic infusion of ANG II induces vasoconstriction via the activation of vascular AT1a receptors. To address the role of extrarenal tissues in the control of blood pressure, Crowley et al. (173) also investigated a “systemic AT1a-receptor knockout” with intact renal AT1a receptors; they found that blood pressure decreased to the same extent as for renal Physiol Rev • VOL 631 knockouts. Because blood pressure was further reduced to ⬃86 mmHg in animals that completely lacked the AT1a receptors, these findings suggest that both renal and extrarenal AT1a receptors contribute to blood pressure control (173). Subsequently, the authors showed that ANG II-induced hypertension depends on the presence of intact renal AT1a (172). As part of a negative-feedback loop, blood pressure in turn affects the synthesis and release of renin from the JG cells of the kidney: an increase in the arterial blood pressure inhibits, whereas a decrease stimulates, the synthesis and secretion of renin (925). This pressure-dependent mechanism, which controls renin synthesis and release, was denoted the “long negative-feedback loop” (293). Increases in the systemic blood pressure can inhibit renin release via intrarenal as well as extrarenal mechanisms. Both mechanisms have been suggested to be involved in the pressure-dependent regulation of the renin system. With regard to intrarenal mechanisms, high blood pressure 1) induces pressure-dependent natriuresis, which increases the NaCl load at the macula densa, and 2) increases the renal perfusion pressure and therefore influences the renal baroreceptor mechanism. Since the effects of blood pressure on renin secretion also occur in the isolated kidney (765), it seems likely that the renal baroreceptor mechanism could be involved in the pressure-dependent regulation of renin release. Although the precise identity and localization of the renal baroreceptor are still unclear, this sensing “receptor” may be located within the afferent arteriole. Much effort has been spent in attempting to discriminate between the pressure-dependent effects and the MD mechanism. However, the baroreceptor response was shown to be independent of the tubular (MD-mediated) effects (293), although the MD may still participate in the pressure-dependent effects (766, 767). In addition, it has been shown that the renal perfusion pressure, not the renal perfusion flow, is important for renin secretion (617). As outlined before, several renal autacoids have been shown to modulate the pressure-dependent regulation of the renin system, with roles for NO and prostaglandins in response to a drop in the renal perfusion pressure and for adenosine in response to an increased arterial blood pressure (see sect. IVB for details). The renal cross-transplantation studies with AT1a receptor-deficient mice may also provide further insight into the regulation of renin by the renal baroreceptor mechanism. The renal AT1a-receptor knockouts had normal renin mRNA levels, despite the absence of AT1a receptors at the JG cells and a significantly lower blood pressure, two factors that have been suggested to increase renin synthesis and renin release. However, renin strongly increased in the complete knockouts, which was paralleled by a further decrease in blood pressure (173). Therefore, one may assume that the renal baroreceptor mechanism may be primarily responsible for the 90 • APRIL 2010 • www.prv.org 632 CASTROP ET AL. strong rise in renin mRNA in the total AT1a-receptor knockouts. Because blood pressure reductions of ⬃20 mmHg did not increase renin mRNA, these data further suggest that a threshold of blood pressure lowering must be achieved to stimulate the renin system via the renal baroreceptor mechanism, as was anticipated in earlier studies (439). An important extrarenal mechanism involved in the control of renin secretion is the renal sympathetic nerve activity (RSNA). The autonomic nervous system, via the renal sympathetic nerves, adjusts kidney function dynamically in response to changes in the sensory information arising from the cardiovascular system, the soma, viscera, and the higher cortical centers. The renal sympathetic nerves innervate the vascular and tubular components, thereby regulating the renal hemodynamics, salt and water reabsorption, and renin secretion (868). The kidney has dense sympathetic innervations of vascular smooth muscle cells of the afferent and efferent arterioles, including the JG cells, as well as of most of the tubular segments in the cortex, including the proximal tubule, the loop of Henle, and the distal tubule (46, 194). Increases in the RSNA increase renin secretion, decrease urinary sodium excretion, and decrease the renal blood flow and GFR (194, 511, 557). With the use of graded frequency renal sympathetic nerve stimulation, it was found that the frequency response curve for renin secretion was to the left of that for decreases in sodium excretion, which, in turn, was to the left of that for decreases in renal blood flow. Therefore, it was assumed that an increase in renin secretion could be achieved before antinatriuresis and renal vasoconstriction occurs (194, 406). However, a resulting increase in ANG II due to the increased release of renin by the low levels of renal nerve activation could itself affect urinary excretion. In a recent study, it was shown that a low-level stimulation of renal nerves resulted in an ANG II-dependent increase in blood pressure and a decrease in the renal blood flow, urine flow rate, and sodium excretion, which was attenuated by ACE inhibition. Therefore, these data suggest that the changes in urinary excretion in response to activation of the renal nerves are mediated directly by the renal nerves and indirectly by an increase in ANG II, which may be due to an increase in renin secretion (502). Afferent inputs to the CNS from the somatosensory receptors, cardiovascular baroreceptors, visceral receptors, renorenal reflexes, and higher cortical centers modulate the RSNA. Cardiovascular baroreceptors have been suggested to be of major importance for afferent inputs. The deactivation of high pressure receptors of the carotid sinus and the aortic arch led to an increase in the RSNA in response to a fall in blood pressure (119), and the low pressure receptors of the cardiopulmonary area inhibit the RSNA in response to an increase in volume within the vascular system (196). It is well known that stimulation of Physiol Rev • VOL the left atrial receptors leads to a reflex reduction in the RSNA and the inhibition of renin release (298), and a fall in the systemic blood pressure unloads the carotid baroreceptors and leads to the stimulation of renin release (195, 868). However, the increased renin secretion in response to hypotensive hypovolemia does not seem to depend on the cardiac or arterial baroreceptors (704, 872, 873, 932). The arterial baroreceptors have long been considered to be only crucial for the short-term control of blood pressure (169, 869). However, in a new model of chronic baroreceptor unloading, it has been found that blood pressure, heart rate, and plasma renin activity increase in response to baroreceptor unloading, suggesting that arterial baroreceptors are also involved in the long-term control of blood pressure (870, 871). Additional supporting evidence derives from the studies of Lohmeier et al. (527), who investigated the responses to five days of ANG II infusion in dogs using a split-bladder preparation combined with the denervation of one kidney. During ANG II infusion, the sodium excretion from the innervated kidney increased compared with the denervated kidney, indicating a chronic decrease in the RSNA in response to ANG II. This effect seemed to be mediated by the baroreflexes. In a subsequent study, they found an increase in sodium excretion in the innervated kidney in response to chronic ANG II infusion (528). Similarly, it has been found that the decrease in RSNA in response to chronic infusion of ANG II in rabbits is baroreceptor mediated (48, 49). Thus these data further support the role of arterial baroreceptors in the long-term regulation of blood pressure. Moreover, these results suggest that the baroreflexes may actually inhibit the renal sympathetic nerve during ANG II-induced hypertension, and in the absence of these reflexes, ANG II has sustained renal sympathoexcitatory effects. Accordingly, it has been found that the chronic activation of the carotid baroreflex reduces blood pressure, heart rate, and plasma norepinehrine levels. However, the plasma renin activity was unchanged, despite a strong fall in blood pressure, suggesting the presence of an inhibitory influence, likely baroreflex-mediated renal sympathoinhibition, on the renin release during sustained activation of the carotid baroreflex (526). This assumption is supported by acute observations in chronically instrumented dogs (686). Surprisingly, bilateral renal denervation had no impact on the blood pressure response and on renin secretion following prolonged baroreflex activation (524). Therefore, the presence of renal nerves does not seem to be an obligate requirement for long-term reductions in the arterial pressure and renin release during prolonged activation of the baroreflex. Furthermore, the renal nerve activity, as well as arterial baroreflex control, can be modulated by ANG II within the CNS (193, 338, 566, 741). In addition, several other sensory receptors, including nociceptors, metabo-, mechano-, osmo-, chemo-, and thermoreceptors, can in- 90 • APRIL 2010 • www.prv.org PHYSIOLOGY OF KIDNEY RENIN fluence the level of sympathetic nerve activity and, consequently, the renin release and synthesis (195, 461). In summary, blood pressure is an important regulator of renin release and synthesis. The blood pressure-dependent renin release is influenced by the activity of the renal nerves, by baroreceptor reflex loops and renal perfusion pressure, which in turn control the renin release via the intrarenal baroreceptor mechanism. B. Salt Intake Oral salt intake is a well-known important regulator of RAAS activity, such that low salt intake increases and high salt intake decreases renin synthesis and secretion (925). Recently, it was found that sodium intake determines the activity of the renin system in a log-linear fashion (441). The salt-dependent changes in the RAAS activity have been suggested to be mediated by 1) systemic humoral factors, 2) intrarenal factors, 3) renal nerves, or 4) the intrarenal MD mechanism. At present, however, it is still unclear which factor(s) or mechanism(s) mediates the observed salt-induced changes. The role of local factors, including the intrarenal MD mechanism, locally acting mediators, and renal nerves, in the salt-induced changes in the renin system was discussed above in detail. As outlined there, the MD-dependent (tubular) control of renin secretion in response to changes in the luminal NaCl concentration seems to be more relevant for acute than for chronic changes in the tubular NaCl. Furthermore, locally generated mediators like the COX-2-derived prostanoids and NO act as modulators of the RAAS activity rather than as major mediators of the long-term salt-dependent control of the renin system (see sect. IVA). Several studies have indicated a suppression of the sympathetic nervous system with high salt intake (25, 196, 525). Therefore, the inhibition of the RAS in response to high salt intake may be due to a reduction of the RSNA. More recently, however, it has been found that a high-salt diet decreased the PRA independent of RSNA in rabbits (570, 571). Therefore, the renal nerve activity seems to serve as a stimulator of the renin system but not as a mediator of the control of the renin system by salt (see sect. IVC). Arguments against an intrarenal mechanism, especially in long-term control of the renin system by salt intake, derive from the following: 1) findings in nonfiltrating kidneys, in which the regulation of the renin system by salt intake is unaltered, although the MD mechanism is obviously not intact (50, 777, 986), and 2) results from mice with a genetic deletion of NKCC2A, where the longterm regulation of renin secretion by salt intake was not altered, although the suppression of renin secretion in response to acute salt loading was absent (652). Since this dissociation between acute and chronic regulation of the Physiol Rev • VOL 633 activity of the renin system in response to salt intake is also seen in A1 adenosine receptor-deficient mice (91, 436, 784), one may conclude that intrarenal factors, especially the MD mechanism, are not essential for the chronic control of the renin system by salt intake. Instead, extrarenal-derived factors are likely to be critically important. Changes in salt intake may lead to changes in systemic blood pressure in some individuals, such that high salt intake can increase and a low salt intake can decrease blood pressure (637). Because changes in blood pressure also lead to changes in the activity of the RAAS, it might therefore be plausible that the salt-induced changes in renin expression and secretion are due to changes in systemic blood pressure. However, there is evidence of blood pressure-independent natriuresis and regulation of the renin system (21–24, 353, 746, 747). It has been found that acute salt loading, either by isotonic or hypertonic solutions, was followed by natriuresis without changes in blood pressure, and in one study, this effect even occurred together with a fall in blood pressure (22). These findings suggest that the response to sodium load may take place in the absence of increases in blood pressure, and therefore, the pressure-natriuresis concept may be inadequate (75). In addition, it was shown that the natriuresis in response to sodium load, which increased blood pressure, was inhibited by the administration of ANG II, suggesting that a decrease in the RAS is a prerequisite for the natriuretic response to sodium load (21, 74). Subsequent elegant studies in humans and laboratory animals further suggest that the suppression of renin release in response to an acute modest sodium load is not only independent of changes in blood pressure, but also independent of cardiac output, GFR, ANP, 1-adrenergic receptors, plasma sodium levels, osmolality, renal nerves, and nNOS-derived NO (73, 456, 593, 717). Therefore, the question arises which mechanism(s) mediates natriuresis and suppression of renin in response to an acute modest sodium load. It seems likely that the MD mechanism is a major determinant of the renin release in response to an acute modest sodium load. Apart from the MD mechanism, a currently unknown mediator may be involved in the salt-induced changes of the renin system. Taken together, sodium balance is an important regulatory mechanism for the activity of the renin system. It is currently unclear which mechanism or mediator is principally responsible for the salt-dependent regulation of renin synthesis and release, especially during chronic alterations in salt intake. The existence of such a humoral factor remains elusive. C. Hormones, Autacoids, and Other Mediators A large variety of biological agents are known to influence renin synthesis and/or renin release. Many of these are 90 • APRIL 2010 • www.prv.org 634 CASTROP ET AL. of major relevance, and an established role in renin synthesis and/or secretion has been demonstrated; conversely, for others, there is only evidence on the cellular or isolated organ level or when given exogenously. These biological agents include classical hormones, autacoids, vitamins, cytokines, or the metabolite succinate (see Table 2). The role of cytokines and vitamins is described in section II, and the TABLE 2. role of rather locally acting mediators, such as adenosine, norepinephrine, NO, endothelins, and prostanoids, has been described in section IV. In addition, the review by Hackenthal et al. (293) provides a detailed overview about the influence of angiotensin, antidiuretic hormone (vasopressin), prostaglandins and leukotrienes, dopamine, ANP, kallikrein and kinins, vasoactive intestinal polypeptide, histamine, platelet- Signal molecules Substance Cellular Effect 12-Hydroxyeicosatetraenoic acid Not determined Acetylcholine Not determined Adenosine Inhibition Adrenomedullin Aldosterone Angiotensin II Atrial natriuretic peptide Bradykinin, kinins Calcitonin gene-related peptide Catecholamines (1-adrenoreceptor) Catecholamines (␣-adrenoreceptors) Dopamine Endothelins Estrogens Stimulation Stimulation Inhibition Inhibition Stimulation Stimulation Stimulation Inhibition Stimulation Inhibition No effect Glucocorticoids Growth hormone Stimulation No effect Histamine Insulin-like growth factor I Stimulation Not determined Interleukin-1 Interleukin-6 Leptin Inhibition Inhibition Not determined Neuropeptide Y Not determined Nitric oxide Stimulation Oncostatin M Oxytocin Inhibition Not determined Pituitary adenylate cyclaseactivating polypeptide Platelet activating factor Progesterone Stimulation Prostaglandins (PGE2, PGI2) Stimulation Substance P Succinate Inhibition Not determined Testosterone No effect Thyroid hormone Tumor necrosis factor-␣ Vasoactive intestinal polypeptide Stimulation Inhibition Not determined Vasopressin Vitamin A Vitamin D Inhibition Stimulation Inhibition Inhibition Not determined Physiological Function Pharmacological tool; inhibition using renal cortical slices Pharmacological tool; no effect using renal cortical slices Mediator, modulator Pharmacological tool Pharmacological tool Mediator, modulator Pharmacological tool Pharmacological tool Pharmacological tool Mediator, modulator Pharmacological tool Modulator Pharmacological tool Pharmacological tool; stimulation in animal models Pharmacological tool Pharmacological tool; stimulation in humans and rats Pharmacological tool Pharmacological tool; stimulation in renal cortical slices Pharmacological tool Pharmacological tool Pharmacological tool; no effect after intra-arterial or intravenous infusion Pharmacological tool; no effect using renal cortical slices; inhibition in animal models Modulator Pharmacological tool Pharmacological tool; stimulation in animal models Modulator Pharmacological tool Pharmacological tool; stimulation in man Modulator Pharmacological tool Modulator; stimulation in isolated superfused glomeruli Pharmacological tool; stimulation in animal models Pharmacological tool Pharmacological tool Pharmacological tool; stimulation in isolated superfused glomeruli Modulator Pharmacological tool Modulator Physiol Rev • VOL 90 • APRIL 2010 • www.prv.org Reference Nos. 27 184 43, 90, 382, 436, 470, 474, 783, 784, 814, 832, 943 141, 247, 397, 496 444 99, 606, 647, 696, 775, 802, 904 102, 213, 331, 475, 639, 696, 743, 797, 913 62, 63, 696, 839-841 482 9, 235, 299, 354, 355, 434, 598 484, 567, 603, 816, 905 350, 373, 476, 535, 586, 963, 985 4, 609, 714, 727, 728, 737, 763, 782 89, 444, 550, 551 444 175, 347, 413, 588, 589, 959 260, 696, 709, 785 408, 563, 564 399, 522, 691 668 793, 914 10, 38, 77, 168, 221, 292, 798 60, 61, 130, 142, 326, 407, 477, 479, 481, 489, 683, 722, 765, 768, 770, 773, 923 52 360, 361, 530, 810 322 692 641, 642 240, 241, 244, 401, 435, 569, 635, 778, 901, 934 696 53, 324, 887, 908 45, 149, 217, 256, 444, 719 144, 145, 207, 320, 367, 445-447, 560 880, 884 109, 198, 697 28, 99, 278, 334, 485, 556, 759, 776, 902 796 516, 980 635 PHYSIOLOGY OF KIDNEY RENIN activating factor, acetylcholine, calcitonin gene-related peptide, and endothelin in the release and synthesis of renin. If new findings have since been reported, the role of these mediators has been updated within this section. Otherwise, we refer readers to the review by Hackenthal et al. (293). For an overview of the putative receptors on JG cells, please see Table 3. It should be noted that Table 3 contains findings reported from cell cultures (As4.1 cells, isolated JG cells, and human transfected JG cells) and from histological approaches, which do not always definitively allow the conclusion that a receptor is colocalized with the native renin-producing JG cells. 1. -Adrenergic pathway It is well known that the sympathetic nervous system participates in the release of renin via the -adrenergic pathway (293), as outlined in detail in section IVC. JG cells contain 1-adrenergic receptors (81, 161). The systemic application of the -adrenoreceptor agonist isoproterenol by chronic infusion into rats, both time and dose dependently, stimulates the synthesis and secretion of renin (355, 598, 802). In addition, isoproterenol infusion increased renin mRNA expression and PRA in rats during normal and high salt intake, but not during low salt intake (355). Notably, COX-2-derived prostanoids do not seem to be involved in the isoproterenolinduced stimulation of the renin system (435, 569). TABLE 3. In agreement with the stimulatory effects of the -adrenoreceptor agonist isoproterenol on basal renin secretion, chronic -adrenoreceptor antagonism has been found to decrease basal renin synthesis and secretion (354, 987). However, -adrenoreceptor antagonism did not influence the increase in renin synthesis and secretion due to low salt intake (987), ANG II type 1 receptor blockade (924), or the combination of low salt intake with ACE inhibition (342), but it did attenuate the stimulation of the renin system in response to chronic stress (9), acute angiotensin AT1 receptor blockade (299, 662), and hypotensive hemorrhage (354). Additionally, oxytocin-induced renin release seems to be mediated via activation of the -adrenergic system (360), whereas thyroid hormone stimulates the renin system independent of the sympathetic nervous system (447). In summary, the sympathetic nervous system provides one of the most powerful background stimuli for renin synthesis and secretion. Its relevance as a regulatory factor of the renin system appears to be limited. 2. ANG II Circulating ANG II functions in a direct negativefeedback loop (“short negative-feedback loop”) to inhibit renin synthesis and secretion (293). Numerous in vivo studies have shown that inhibiting the effects of ANG II Expression of receptors on juxtaglomerular cells Mediator Adenosine Aldosterone Angiotensin II ANP Bradykinin Calcium Catecholamines CGRP, ADM Dopamine Endothelin EGF Glucocorticoid Histamine Neuropeptide Y Neutrophins Oxysterols Oxytocin PACAP PGE2 Prostacyclin TGF- Thyroid hormone Vasopressin Receptor Species Level of Evidence A1 MR AT1 AT2 NPR1 B2 CaR 1 CALCRL D1, D5 D3, D4 ETA ETB EGF-R GR H2 Y1 trkB LxR␣ OT PAC1 EP2, EP4 IP TGF- II TR V1a Rat, rabbit Mouse Human, rat, mouse, monkey Rat, monkey, swine Rat Human Mouse, rat Human, rat, dog Human Rat Rat Mouse, rat Mouse, rat Rat Mouse Human Human Mouse Mouse, human Human Mouse Rat, mouse Rat, mouse Rat Rat Rabbit, mouse, rat, human mRNA, functional (941, 943) mRNA, functional (444) mRNA, protein (258, 267, 316, 365, 437, 677, 718, 893, 991, 992) mRNA, protein (42, 267, 365, 731) Protein, functional (475, 622) mRNA, functional (696, 820) mRNA, protein, functional (553, 653) mRNA, protein, functional (81, 161, 327, 513, 831, 935) Protein (297) mRNA, protein, functional (18, 19, 476, 636, 963) mRNA, functional (745) Protein, functional (737, 948) Protein, functional (219, 463, 727, 948) Protein (888) Functional (444) Functional (696) mRNA (949) Protein (257) mRNA, protein, functional (599, 851) Protein (35) Functional (322) mRNA, functional (241) Functional (241, 401) Protein (520) Protein (545) mRNA, protein (35, 36, 672) CGRP, calcitonin gene-related peptide; ADM, adrenomedullin; ANP, atrial natriuretic peptide; EGF, epidermal growth factor; PACAP, pituitary adenylate cyclase-activating polypeptide; PGE2, prostaglandin E2; TGF-, transforming growth factor-. Nomenclature is in accordance with Alexander et al. (14). Reference numbers are given in parentheses. Physiol Rev • VOL 90 • APRIL 2010 • www.prv.org 636 CASTROP ET AL. with ANG II-AT1 receptor antagonists or reducing the level of ANG II with ACE inhibitors causes a strong increase in renin synthesis and release (127, 346). Likewise, the exogenous infusion of ANG II decreases renin synthesis and renin release (775), suggesting that ANG II may exert direct negative effects on this system. Further support derives from mice lacking AT1a receptors, which have an increased number of renin-positive JG cells (647). Since changes in the systemic ANG II concentrations, as well as alterations in the AT1 receptor density, modulate the systemic blood pressure, an indirect effect of ANG II on renin synthesis and secretion via the systemic blood pressure cannot be excluded. However, it has also been found that nonpressor doses of ANG II inhibit renin secretion, suggesting a blood pressure-independent inhibitory effect of ANG II on renin release (181, 293). Further support for a pressure-independent effect of ANG II derives from the observation that ANG II inhibits renin secretion from isolated perfused kidneys and from kidney slices (616, 904). Such an inhibitory effect of ANG II on renin is in accordance with the existence of AT1 receptors on the JG cells (Table 3). ANG II has also been found to decrease the COX-2 expression in MD cells via AT1 receptors at lower concentrations than needed for systemic hemodynamic actions (984). Thus an inhibitory effect of ANG II on renin synthesis and renin release via a suppression of COX-2-derived prostanoids from MD cells may also be conceivable. Nevertheless, since JG cells are equipped with ANG II AT1 receptors and because ANG II has been shown to decrease renin synthesis and release in As4.1 cells (105, 606), a direct negative effect of ANG II on renin release and renin synthesis can be postulated. In addition, it has been demonstrated that renal nerves are not essential for the ANG II negative-feedback loop (924), and this feedback loop is also relevant in various conditions of stimulated or reduced renin synthesis and secretion (802). However, a recent study in chimeric mice carrying a regional null mutation of the AT1a receptor questions the direct effect of ANG II on the JG cells. In this report, the JGA of the AT1a receptor-deficient mice were enlarged with intense expression of renin. In the chimeric mice, the changes in the JGA were proportional to the degree of chimerism, but the degree of JGA hypertrophy/hyperplasia and the expression of renin were unaltered in AT1a-expressing and AT1a-deficient JG cells (568). More recently, renal cross-transplantation has been combined with gene knockout technology. The AT1a receptor-deficient mice had increased renin mRNA and a lower blood pressure than wild-type controls. Mice that expressed the AT1a receptor only in the kidney or only in extrarenal tissues had similar blood pressures; these were lower than that of wild-type controls, but they were higher than that of the complete AT1a-deficient mice. Renin mRNA levels in renal or extrarenal knockouts were similar to wild-type controls but were significantly lower Physiol Rev • VOL than in complete AT1a-deficient mice (173). These data therefore suggest that the renal AT1a receptors are key determinants of blood pressure, but they may not be of major importance for the regulation of renin expression. Thus these two recent studies suggest that the regulation of renin synthesis and release by ANG II is indirectly mediated, likely via changes in systemic blood pressure. In addition to the effect of circulating ANG II on the renal renin synthesis and secretion, ANG II in the brain may also have an inhibitory effect on the renin release. The central administration of ANG II decreases renin secretion, and the central administration of losartan increases renin secretion (387, 573, 942, 968). This effect on renal renin secretion is likely due to a reduction in the renal sympathetic nerve activity (574). Moreover, it should be kept in mind that ANG II facilitates the release of norepinephrine from the renal sympathetic nerve terminals (193). In summary, ANG II inhibits renin release and synthesis through a direct short negative-feedback loop and also via indirect mechanisms. 3. ANP The 28-amino acid peptide ANP is mainly released by the cardiac myocytes of the atrium in response to atrial stretch. The cardiovascular and renal effects of ANP (hypotension, natriuresis, and diuresis) counteract the effects of ANG II (837). ANP can modulate the renin release through direct and indirect mechanisms. The activation of the natriuretic peptide receptor-1 (NPR1) can directly inhibit the renin release by increasing the cGMP formation. Although it is generally believed that the tubular action of ANP is predominantly exerted in the more distal segments of the nephron, ANP may also indirectly reduce the renin release via a possible effect on the proximal tubular salt reabsorption (314), which may lead to an increased NaCl load at the MD. In contrast, ANP can indirectly stimulate renin release by lowering the blood pressure. Indeed, somewhat conflicting data have been reported regarding the role of ANP in renin release, although most of these studies, including in vitro studies using isolated JG cells, suggest that ANP is an inhibitor of renin release (293). More recent studies found that the incubation of rat renal cortical slices with or intravenous infusion of ANP into humans did not affect the renin release (377), whereas the intrarenal and intravenous infusion of ANP in dogs stimulated and inhibited renin release, respectively (213). ANP-deficient mice have lower renal renin mRNA levels but increased plasma renin concentrations and are hypertensive (576, 638). In addition, the genetic deletion of the NPR1 receptor leads to hypertension, which is paralleled by a reduction in the plasma renin concentration and in renal renin expression (797). The increase in systemic blood pressure observed 90 • APRIL 2010 • www.prv.org PHYSIOLOGY OF KIDNEY RENIN in the ANP- and NPR1-knockout mice may indirectly mediate the inhibition of the renal renin gene expression. Taken together, an inhibitory effect of ANP on the renin secretion at the JG cell level has been demonstrated (411, 475). The inhibition of renin release by ANP may be modulated by the cardiovascular and renal effects of ANP in vivo. 4. Vasopressin Arginine vasopressin (AVP), also known as antidiuretic hormone (ADH), influences a wide variety of physiological functions; more specifically, the induction of antidiuresis and vasoconstriction are considered the most important effects of AVP. AVP acts on three G proteincoupled receptors, termed the vasopressin V1a, V1b, and V2 receptors (44, 891). In the kidney, the V1a receptors, which mediate the vasoconstrictor effect of AVP, are localized in the renal vasculature, the JG apparatus, the MD cells, the connecting tubule, and the cortical collecting duct (28, 35, 672). The V2 receptor expression, which mediates the antidiuretic effect of AVP, has been found in the TAL, the MD cells, the distal convoluted tubule, the connecting tubule, and the collecting duct (614, 672). The renal expression of the V1b receptor has also been reported, but the precise localization and function of this receptor are unclear (531, 595). AVP also modulates renin release, and the inhibitory effect of AVP on renin release was favored by older investigations (293). Among other findings, AVP has been shown to decrease renin release from primary cultures of renin-producing JG cells (485). Because vasopressin V1a but not V2 receptor expression has been described for the afferent arteriole (35, 614, 672), one may assume that this inhibitory effect could be mediated via vasopressin V1a receptors (776). In line with this assumption, it has been found that the preferential V1a agonist terlipressin decreased the plasma renin concentration in patients with hepatorenal syndrome (294, 897). However, terlipressin also improved the renal function and blood pressure (605, 897). Contrary to these findings, it was recently reported that PRA and renin mRNA were lower in the vasopressin V1a receptor-deficient mice compared with wild types (28). The decrease in the renin system in these mice was paralleled by a decreased expression of nNOS and COX-2 in MD cells and alterations in systemic and renal hemodynamics, such as lower blood volume, hypotension, decreased renal blood flow, and GFR (28, 459). Since the expression of vasopressin V1a receptors has been found in MD cells (28), AVP may also influence renin release via a MD-dependent mechanism. AVP may also modulate the renin system via the vasopressin V2 receptors. It has been found that the infusion of the vasopressin V2 receptor agonist desmopressin (dDAVP) decreases blood pressure and increases the PRA in humans (47, 72). Subsequently, Physiol Rev • VOL 637 it was reported that the stimulatory effect on renin release was inhibited by the COX inhibitor indomethacin, independent of the blood pressure-lowering effect of dDAVP (319). However, the infusion of dDAVP into humans and animals also increases the renal blood flow and GFR, probably due to a vasodilatation of the afferent arteriole (86, 231, 619, 701, 849). A stimulatory effect of dDAVP on renin release has been reported for the isolated perfused rat kidney and from rabbit renal cortical slices (319, 762), and chronic infusion of dDAVP has been shown to increase renin mRNA levels in Brattleboro rats, which lack AVP (20). Although these data support a stimulatory role for the vasopressin V2 receptor in renin release, it has recently been found that renin mRNA is also increased in vasopressin V2 receptor-deficient mice (759), which exhibit a clear renal phenotype (981). Furthermore, the vasopressin V2 receptor antagonist tolvaptan did not change the plasma renin activity in rats and humans (800, 801, 911). Due to its complex cardiovascular and renal effects in vivo, the role of vasopressin and the respective receptors in the control of renin release is still controversial and needs further evaluation. One could assume that AVP inhibits renin release under some conditions via the activation of the vasopressin V1a receptors, but it may also increase renin secretion via the activation of V2 receptors. 5. Oxytocin In addition to its well-known effects on uterine contraction and milk ejection, oxytocin has been shown to influence renal function, such that oxytocin increases the GFR and natriuresis in rats (167). Oxytocin activates a single G protein-coupled receptor, termed the OT receptor (268). Because the expression of OT receptors has been found in MD cells (35, 658, 828), a role for oxytocin in renin release was presumed. It has been reported that oxytocin increases the plasma renin concentration in rats and that this increase may be independent of the oxytocin-induced natriuresis (810). Subsequently, it was found that oxytocin-induced renin secretion was inhibited by -adrenergic receptor antagonism (360), but not by renal denervation (530). Therefore, circulatory catecholamines may be involved in renin release in response to oxytocin. Because oxytocin is released in response to hypotension and hypovolemia, it has been speculated that oxytocin could stimulate renin release under these conditions. The OT receptor antagonist atosiban attenuates the rise in PRA and heart rate in response to hydralazine or diazoxide, without affecting the blood pressure-lowering effect of these two vasodilatators (361). In contrast to these findings, the intravenous infusion of oxytocin decreased urine flow and reduced sodium excretion and PRA in humans (716). The opposite 90 • APRIL 2010 • www.prv.org 638 CASTROP ET AL. finding in humans may be due to a different expression of the renal OT receptors (35). In summary, oxytocin stimulates renin secretion in laboratory animals via a -adrenergic-dependent mechanism. The stimulatory effect may be species dependent, probably due to a different expression of renal OT receptors. 6. Aldosterone The stimulation of renin release increases the plasma levels of ANG II, which subsequently stimulates the secretion of aldosterone from the adrenal glands (821). The stimulation of angiotensin II and aldosterone formation increases the sodium reabsorption and consequently increases the body sodium content, which, in turn, inhibits the renin gene expression (293, 488, 821). Recently, it was shown that JG and As4.1 cells express mineralocorticoid receptors; additionally, aldosterone increased renin mRNA levels in primary cultures of mouse JG cells prestimulated with isoproterenol, but it had no effect on the exocytosis of stored renin (444). Conversely, mineralocorticoid receptor- and aldosterone synthase-deficient mice have a strong increase in plasma renin concentration, probably due to a sodium deficit caused by renal sodium wasting (71, 555). These data, therefore, suggest that aldosterone exerts a direct positive effect on renin gene expression at the cellular level, probably by stabilizing renin mRNA. However, the in vivo relevance of this finding remains unclear, and therefore, further investigation is necessary. Moreover, the in vivo effect of aldosterone may be indirectly due to changes in the body sodium content, extracellular volume, or blood pressure. 7. Glucocorticoids It is well known that the administration of glucocorticoids increases the GFR via a vasodilatation of afferent and efferent arterioles and increases the renal blood flow and blood pressure (56, 82, 183, 950). Several findings suggest that glucocorticoids also regulate renin synthesis and renin release. In sheep, a birth peak of cortisol seems to be responsible for the parallel activation of renin (231a). Furthermore, glucocorticoids have been found to directly activate renin synthesis in JG cells (444). Glucocorticoids act on glucocorticoid receptors (GR), which have been mainly localized in the glomeruli, proximal tubule, and TAL segments, including the MD cells (549, 879a). In addition, cortisol can bind and activate the mineralocorticoid receptor (MR). However, the 11-hydroxysteroid dehydrogenase type 2 enzyme (11-HSD2) is mostly coexpressed with the MR and inactivates cortisol to cortisone, which is not able to bind to the MR. Since afferent arterioles, isolated JG cells, and As4.1 cells express 11-HSD2, a direct mineralocorticoid effect of the Physiol Rev • VOL glucocorticoids on renin release and synthesis may be unlikely (444, 895a). In addition, the glucocorticoids may indirectly induce renin release via a possible effect on the proximal tubular salt reabsorption, which may lead to a decreased NaCl load at the MD (634). On the other hand, it has been found that cortisol can reduce the plasma renin activity in adult ewes (425). The treatment of rats with dexamethasone did not alter the PRA, although it did strongly increase the blood pressure (838). Furthermore, COX-2 expression in the TAL and in the MD cells is suppressed by endogenous glucocorticoids (549, 915, 984). Taken together, a direct stimulatory effect of glucocorticoids on renin synthesis has been shown in vitro. The stimulatory effect of glucocorticoids on renin release and synthesis may be modulated by an increase in blood pressure and by the suppression of the MD COX-2-derived prostanoids in vivo. 8. Thyroid hormone Several in vivo and in vitro studies have suggested that the thyroid hormone influences renin synthesis and secretion. Hyperthyroidism in response to L-thyroxine or triiodothyronine (T3) treatment increased, while hypothyroidism due to thyroidectomy or methimazole treatment reduced, the PRA and renin mRNA in rats and fetal sheep (144, 145, 207, 446, 560). Moreover, the stimulation of renin synthesis and renin secretion by thyroid hormone seems to be independent of sympathetic nerve activity, since the chemical sympathetic denervation with 6-hydroxydopamine did not affect the stimulatory effect of the thyroid hormone on renin synthesis and renin secretion (447). In vitro, thyroid hormone enhanced renin secretion from rat kidney slices and from primary cultures of JG cells (320, 367). In addition, renin mRNA was found to be increased in primary cultures of JG cells by T3 and in rats in response to L-thyroxine (367, 446). These observations suggest a possible direct effect of thyroid hormone on the activity of the renin system. Subsequently, it was found that thyroid hormone induces the promoter activity of the human renin gene through thyroid hormone response element-dependent mechanisms in Calu-6 cells (445). 9. Sex steroids: testosterone, estrogen, and progesterone Various studies have indicated that blood pressure is higher in men than in women and that blood pressure increases after menopause in women (107, 430, 565, 823). In addition, the increase in blood pressure starts with puberty, suggesting a role of the sex steroids (39, 317). Sex-dependent differences in blood pressure are also known for several animal models (289, 351, 431, 719). Male spontaneously hypertensive rats (SHRs), for example, have higher blood pressure than females, and orchi- 90 • APRIL 2010 • www.prv.org PHYSIOLOGY OF KIDNEY RENIN dectomy, but not ovariectomy, attenuated the increase in systolic blood pressure (148, 217). Since the renin-angiotensin system is a major system for the long-term control of blood pressure and salt and water homeostasis, genderdependent differences in the synthesis and release of renin from the kidney may be involved in the sex-dependent blood pressure regulation. It has been found that the PRA is higher in men than in women and that it increases in postmenopausal women (395, 774). The chronic treatment of gonadectomized SHRs with testosterone increases blood pressure and PRA in male and female SHRs, and PRA decreases with castration in male rats (149, 217). Furthermore, the administration of testosterone increases the PRA and blood pressure in normotensive rats (719), and application of the testosterone receptor antagonists cyproterone acetate or flutamide attenuated the development of hypertension in male stroke-prone SHRs and reduced the renin synthesis and secretion in female TGR(mREN2)27 rats and mice (45, 256). Thus it has been suggested that androgens may have a stimulatory effect on renin secretion. Androgens, such as testosterone and dihydrotestosterone (DHT), act on androgen receptors, which are expressed in mammalian kidneys (739). Although the precise localization of these receptors is not entirely clear, it seems likely that the androgen receptors are mainly expressed in proximal tubular cells where testosterone enhances the salt and water reabsorption, likely via an increased expression of the proximal tubular transporter NHE3 (705, 739). There is no evidence for the expression of testosterone receptors in JG cells or MD cells. In line with this, testosterone did not alter the levels of renin mRNA in the As4.1 cell line (444). Otherwise, no effect, or a decrease in renal renin mRNA expression, has also been reported for mice (132, 700, 928). As a result, testosterone may indirectly influence the renin system, likely via an increased absorption of salt and water at the proximal tubule, which may lead to a decreased NaCl load at the MD. This effect may be modulated by an increased extracellular volume and increased blood pressure in vivo. In contrast to androgens, estrogens tend to have antihypertensive effects, mainly via the control of local blood flow and peripheral resistance due to alterations in the release of the endothelial mediators (359, 551). Interactions between estrogens and renin have also been reported. Several studies support a stimulatory effect of estrogen on renin release. Increases in endogenous estrogen levels are associated with increased PRA. In healthy women, for example, the PRA increases during the luteal phase of the menstrual cycle, when plasma estrogen levels are highest (137, 153, 787). Furthermore, estrogen administration in animals increased the PRA, which was paralleled by a decrease in blood pressure (550, 551), and estrogen replacement increased the PRA in cynomolgus monkeys (89). On the other hand, it has also been found Physiol Rev • VOL 639 that, after menopause, blood pressure and PRA increase in women, and the renin levels in women with estrogen replacement therapy were lower than in those without estrogen therapy (178, 774). In animals, estrogen administration reduced the PRA and blood pressure in ovariectomized mRen(2) Lewis rats (138). The mechanism for a possible modulatory effect of estrogen on the renin secretion is not understood. Moreover, the information on the renal distribution of the estrogen receptor is rather limited (739), and no expression has been reported for the MD cells (698, 829). Whether the JG cells contain estrogen receptors has yet to be determined. Since estradiol did not alter the renin mRNA in the As4.1 cell line (444), it seems likely that indirect, rather than direct, mechanisms may be involved in the modulation of renin secretion by estrogens. In this regard, estrogen has been reported, for example, 1) to increase the renal oxytocin receptor gene expression (659), 2) to attenuate the response of ANG II on the AT1 receptors (34, 252, 628, 804), 3) to increase the renal NO synthesis (55), 4) to modulate the bradykinin B2-receptor gene expression and function (547), and 5) to inhibit the sympathetic nervous system (187, 234, 523). In addition, during low estrogen levels, the effect of androgens on renin release may be more apparent (719). Taken together, the influence of estrogen on the renal release of renin remains controversial mainly due to the complex influences on the cardiovascular functions in vivo. Progesterone acts on progesterone receptors, but the precise renal localization of these receptors is not clear (739). With regard to renin synthesis and renin release, it has been reported that the administration of progesterone increases PRA in humans (642). Furthermore, the increase in PRA during the menstrual cycle was attenuated by a synthetic progesterone (641). In addition to an action on the progesterone receptors, progesterone can also act as a mineralocorticoid receptor antagonist (98). Thus the natriuretic and blood pressure-lowering actions of progesterone have mainly been related to this antagonistic effect (136, 642, 707). Therefore, progesterone may also attenuate the action of aldosterone on renin synthesis and renin release. In addition, progesterone can be metabolized to testosterone and dihydrotestosterone in the kidney and may therefore have androgenic effects, probably also on renin release (706). 10. Adipokines: leptin and adiponectin Obesity is a major risk factor for the development of hypertension (232, 301). The activation of the sympathetic nervous system and of the RAAS, as well as the endothelial and renal dysfunctions, has been implicated in the association between obesity and hypertension (710). In the past few years, it has become apparent that adipose tissue is not only a reservoir for energy, but it is also an endocrine organ, which secretes a variety of bioactive 90 • APRIL 2010 • www.prv.org 640 CASTROP ET AL. peptides, known as adipokines (427). Among these, leptin and adiponectin in particular have been suggested to be involved in the development of obesity-induced hypertension and, therefore, also for the activation of the RAAS in obesity. Leptin, the product of the obesity gene, is a circulating peptide that is primarily synthesized and secreted by the adipocytes. Originally, it was described as an adipostat that controls energy balance (532, 585). Later on, it was observed that leptin also influences the cardiovascular and renal functions and that the increased levels of leptin in obesity may contribute to obesity-induced hypertension. It has been found, for example, that the infusion of leptin activates sympathetic nerve activity and increases the mean arterial blood pressure, heart rate, urine volume, and sodium excretion in rats (323, 389, 793, 914). Reports of a positive correlation between leptin and PRA in obesity and essential hypertension in humans suggest a potential role for leptin in renin release (5, 836, 894), but up to now, no direct effect of leptin on the release of renin from the kidney has been reported. In contrast, the carotid artery or intravenous infusion of leptin did not alter PRA in rats (793, 914). However, leptin may indirectly induce renin release via a central activation of the renal sympathetic nervous system (120, 323, 561, 711, 712, 910). In accordance with this, it has been reported that renal denervation inhibits the rise in PRA, at least in response to a high-fat diet in dogs (421). Adipocytes also secrete adiponectin, a peptide that has been described to enhance insulin sensitivity and prevent arteriosclerosis (644, 964). The plasma levels of adiponectin have been found to be lower during obesity and in hypertensive men, and they are negatively correlated with blood pressure (33, 385, 509). Adiponectin has been found to ameliorate obesity-related hypertension in mice (643) and to decrease blood pressure and renal sympathetic nerve activity in rats (856). Furthermore, the inhibition of the RAAS increases and infusion of ANG II decreases plasma adiponectin levels (249, 509). It is unknown whether there is a direct effect of adiponectin on renal renin release. However, since adiponectin decreases the renal sympathetic nerve activity, it may indirectly influence renal renin release. 11. Growth hormone and insulin-like growth factor I The administration of growth hormone (GH) into healthy men increases the PRA without changes in blood pressure or decreases in the sodium and water excretion (340, 590). In line with these findings, the inhibition of the RAAS prevented the GH-induced fluid retention in humans (592). Furthermore, GH was found to increase the PRA in GH-deficient humans and rats (175, 347, 588, 589, 959). Consistent with a stimulatory effect of GH on renin release, it has been found that a growth hormone receptor Physiol Rev • VOL deficiency results in reduced plasma renin concentration despite a decrease in blood pressure (212). The stimulatory effect of GH on renin release may be due to an increased formation of COX-derived prostanoids (310). It has been suggested that several effects of GH are at least partly mediated by insulin-like growth factor I (IGF-I) (591). Indeed, it has been found that IGF-I increases PRA in rats and fetal sheep and stimulates the secretion of renin from renal cortical slices (392, 408, 563, 564), but the precise role of IGF-I for GH-induced renin release is unknown. On the other hand, no effect of GH on the PRA and renin mRNA or on the secretion of renin from primary cultures of JG cells have been reported (51, 333, 413, 468). In summary, it can be concluded that GH stimulates renin synthesis. Since GH did not induce renin release from the JG cells, the effect of GH on renin secretion may be rather indirect and may depend on the formation of COX-derived prostanoids. 12. Prostanoids PGI2 and PGE2 are direct stimulators of renin synthesis and secretion that most likely function via enhanced cAMP formation (293). Due to their short half-life time, it is still unclear whether extrarenal-derived PGI2 and PGE2 could serve as stimulators of renin secretion and synthesis. Therefore, PGI2 and PGE2 have been suggested to function as local, rather than systemic, regulators of these processes (see sect. IV, A1 and B4). However, since PGI2 is not completely metabolized when it passes through the pulmonary system and since the half-life of PGI2 is between 30 s and several minutes (541, 594, 906), it may be theoretically possible that systemic-derived PGI2 is of relevance for renal renin release under certain circumstances, such as furosemide treatment or sepsis (93, 345, 518). 13. Dopamine The neurotransmitter dopamine modulates several physiological functions within the kidney, including renin release and sodium excretion. Dopamine can directly stimulate renin release via the activation of the D1-like receptors upon the dopaminergic innervation of the vascular pole of the glomerulus. Increases in the systemic dopamine concentration can also indirectly affect renin release by lowering blood pressure. In addition, dopamine can negatively influence renin release and synthesis via its effect on the proximal tubular sodium reabsorption. This effect is important because the main source of dopamine within the kidney is the proximal tubule itself. Here, the glomerular filtered dopamine precursor L-DOPA is reabsorbed and then converted into dopamine by the action of aromatic L-amino acid decarboxylase, which is strongly expressed in proximal tubular cells. Therefore, dopamine 90 • APRIL 2010 • www.prv.org PHYSIOLOGY OF KIDNEY RENIN signaling leads to an increase in the tubular sodium chloride concentration at the MD (293). Most investigators have observed a stimulatory effect of dopamine on the renin secretion at the level of kidney slices, isolated perfused kidneys, and in vivo (293). Two recent studies further support a stimulatory role for dopamine in renin synthesis and secretion. The authors reported that dopamine induces renin release from rat JG cells (963) and isolated glomeruli via DA1 receptors (307). The effect of dopamine on renin release was also recently investigated in mice carrying a genetic deletion of catechol-O-methyltransferase (COMT), the main enzyme in the renal metabolization of dopamine. These data suggest that dopamine inhibits renin release and synthesis indirectly via increased NaCl load at the MD during normovolemia or hypovolemia; moreover, the data suggest that dopamine directly stimulates renin release in situations of volume expansion (985). In summary, dopamine can directly stimulate renin release and synthesis, most likely via DA1 receptors at the JG cell level. However, the direct stimulatory effect of dopamine on renin release and synthesis is largely compensated for by the indirect inhibitory cardiovascular and renal effects of dopamine in vivo. 14. Succinate/GPR91 The orphan G protein-coupled receptor GPR91 has recently been shown to function as a receptor for succinic acid. GPR91 mRNA was found to be expressed predominantly in the kidney and, at lower levels, in the liver and spleen. The in situ hybridization revealed GPR91 expression in the proximal tubules, distal tubules, and the JGA of the mouse kidney. The intravenous injection of succinate increased PRA in Sprague-Dawley rats (324), confirming a report that examined the rate of renin release from viable JG cells during the superfusion of isolated rat glomeruli (53). Moreover, blood pressure also increased in response to succinate in a dose-dependent manner. The increase in blood pressure was eliminated in the bilateral nephrectomized animals, attenuated in the animals pretreated with the ACE inhibitor captopril, and absent in the GPR91 knockout mice. Because these authors found that GPR91 is a Gq- and Gi-coupled receptor, it seems unlikely that GPR91 directly influences renin secretion (324). Recently, it was demonstrated that MD cells express the GPR91 protein in the apical membrane and that the treatment of MD cells with succinate activates the mitogenactived protein kinase (MAPK) pathway, leading to an increase in the COX-2 derived PGE2. In line with these in vitro findings, succinate-induced renin release from microperfused JGA was eliminated by the inhibition of MAPK and COX-2 (908). Increased plasma succinate levels were found in several animal models of hypertension, including SHR, and Physiol Rev • VOL 641 diabetes, but they were not found in human hypertensive or diabetic patients (740). Animal studies suggest that the GPR91 receptor may be of relevance for the stimulation of renin release in response to high glucose levels (887), in experimental diabetes (908), and likely in response to low sodium intake (908). In summary, it can be concluded that the succinate/ GPR91 system enhances renin synthesis by a direct effect of succinate on GPR91 expressed in the JGA/glomerulus. 15. PACAP PACAP is involved in the regulation of the cardiovascular and central nervous system (909). PACAP was recently shown to dose-dependently stimulate renin secretion from isolated perfused kidneys of rats and from primary cultures of renin-producing JG cells. The PACAPinduced renin release from isolated kidneys was attenuated in pituitary adenylate cyclase 1 (PAC1) receptordeficient mice, which also exhibited lower basal PRC and lower blood pressure. Moreover, the PRC was lower in PAC1 receptor knockout mice fed a low- or high-salt diet, compared with the wild-type mice, and in response to treatment with the ACE inhibitor ramipril, although the principal regulation was not attenuated. Therefore, it may be inferred that PACAP is a tonic enhancer of the renin system in vivo via the activation of adenylyl cyclases (322). 16. Bradykinin The renal and cardiovascular activities of bradykinin, the enzymatic product of the kallikrein-kinin system, are primarily mediated via the activation of the bradykinin B2 receptor, which is constitutively expressed in the vascular endothelium and mediates the vasodilator effects of kinins (274, 721). Although some studies found a stimulatory effect of kinins on renin release, other investigators have observed no effect (293). Accordingly, studies using the B2 receptor-deficient mice have also produced conflicting results. The B2 receptor knockout mice on a 129SvxC57Bl/6J background were hypertensive compared with the wild-type mice on a 129Sv/J background. However, the PRA and renin expression did not differ between the strains (548). The B2 receptor-deficient mice that were backcrossed onto a C57BL/6 background were normotensive, had a lower heart rate, exhibited decreased renal blood flow, and possessed higher renal renin mRNA expression compared with wild-type controls (889). Mice lacking tissue kallikrein, which is the main kinin-forming enzyme in vivo, exhibited normal blood pressure and normal renal blood flow, but they displayed decreased renal renin mRNA expression (889). Another study examining the B2 receptor-deficient mice on a C57BL/6 background reported lower renal renin protein levels and decreased renin mRNA abundance. 90 • APRIL 2010 • www.prv.org 642 CASTROP ET AL. Renal tissue levels of angiotensin I were found to be diminished in these B2 receptor knockout mice, while the ANG II levels were reportedly unchanged. In addition, these authors observed decreased renal COX-2 gene expression in the B2 receptor knockout mice (374). They concluded that the B2 receptor-dependent regulation of COX-2 participates in the basal control of renin gene expression. With regard to the lower renin mRNA expression, a similar finding was reported for the newborn and adult B2 receptor-deficient mice (976). In addition, kidney renin mRNA levels did not differ between the B2 receptordeficient mice and wild-type controls during normal and chronic high salt intake (134). The role of the bradykinin B2 receptor was further investigated in mice overexpressing the human version of the protein. These hypotensive mice exhibited enhanced renal function with increased urinary excretion of nitrite/nitrate cGMP, cAMP, and kinin. However, the renal renin mRNA abundance was not altered in these mice (933). The treatment of rats with HOE-140 (Icatibant), a B2 receptor antagonist, or the kallikrein inhibitor aprotinin did not alter basal renal renin mRNA abundance (215, 977). The intravenous infusion of HOE-140 decreased the basal PRA in rabbits independent of any changes in blood pressure, but it did not attenuate the furosemide-stimulated elevation in the PRA (155). HOE-140 did not alter the basal PRA or the furosemideor valsartan-induced PRA changes in humans (504, 612). However, HOE-140 inhibited the increase in PRA and the decline in blood pressure observed in response to captopril in humans (251). In contrast, HOE-140 did not alter the basal or enalapril-induced PRA and PRC changes in rats with passive Heymann nephritis, although HOE-140 attenuated the decreased blood pressure effect of enalapril (363). Moreover, the DOCAsuppressed PRA was not altered by HOE-140 in rats (546). In summary, the precise role of bradykinin in renin synthesis and secretion is still controversial, probably because of the complex nature of the cardiovascular and renal effects of bradykinin in vivo. 17. Adrenomedullin Adrenomedullin (ADM) is a peptide with renal and cardiovascular activities, and the immunoreactivity of the proadrenomedullin NH2-terminal 20-amino acid peptide has been localized in the JG cells (536). The plasma levels of ADM have been shown to significantly correlate with the PRA in patients with heart failure (422) and cirrhosis (450), suggesting a potential role for ADM in the secretion of renin. Accordingly, the intravenous administration of ADM in rabbits and sheep caused a decrease in the mean arterial blood pressure, which was paralleled by increases in the heart rate and Physiol Rev • VOL PRA (141, 247, 673). In addition, ADM infusion into healthy volunteers or patients with heart failure, essential hypertension, or chronic renal impairment increased the PRA (495, 496, 572, 892). This effect was not observed in patients with pulmonary hypertension (618). Subsequent studies demonstrated that the renin release from isolated perfused rat kidneys is dosedependently increased by ADM (397) and that ADM stimulates renin release and renin mRNA synthesis in mouse JG cells, suggesting a direct effect in renin synthesis (397). On the other hand, it has been reported that the intrarenal infusion of ADM in dogs increased the renal blood flow, urine flow, and urinary excretion of sodium and potassium without alterations in blood pressure, heart rate, or PRA (209, 409). Similarly, ADM infusion attenuated the rise in PRA and blood pressure due to left renal artery clipping in rats (428). In addition, a negative correlation was found between the plasma ADM concentrations and PRA in patients with chronic glomerulonephritis (438). Recently, it was reported that the infusion of ADM-2, a new member of the calcitonin gene-related peptide superfamily, increased the PRA in normal conscious sheep (140). Taken together, a direct stimulatory effect of ADM on renin secretion at the JG cell level has been shown, although this effect may be modulated by the cardiovascular and renal effects of ADM under certain in vivo circumstances. The possible stimulatory effect of ADM-2 warrants further investigation. 18. Neuropeptide Y The vasoactive peptide neuropeptide Y (NPY) is coreleased with norepinephrine from sympathetic nerve terminals. In addition to the expression of the NPY receptor subtype Y1 in the collecting ducts and the loop of Henle, the Y1 receptor expression has been described for the JGA (949). Therefore, a possible role for NPY in the release of renin has been suggested. It has been shown that the infusion of NPY inhibited renin secretion in isolated perfused rat kidney and in animal models, which was paralleled by renal vasoconstriction (77, 168, 292). Furthermore, NPY was found to decrease the PRA in adrenalectomized rats (693), in postmyocardial infarction rats (982), in response to isoproterenol or epinephrine (10, 38, 221), and in two-kidney one-clip renal hypertensive rats (920). On the other hand, no changes in PRA in response to nonpressor doses of NPY have also been reported (37, 221, 684, 920, 982). In addition, no effect of NPY was observed on renin release from renal cortical slices (798). Furthermore, no renal phenotype has been reported in mouse models deficient in NPY or in Y1 receptors (953), although the administration of NPY has been found to decrease the renal blood flow and to induce diuresis and natriuresis (78). 90 • APRIL 2010 • www.prv.org PHYSIOLOGY OF KIDNEY RENIN 643 Taken together, NPY may inhibit renin release under some experimental conditions or with pharmacological doses, but it does not seem to be involved in the physiological control of renin secretion. V. ACTION OF RENIN Most of the major known effects of the reninangiotensin system (RAS) are related to the activity of angiotensin II (ANG II). Consequently, the end point of the renin-angiotensin cascade is generally assumed to be the generation of ANG II and related peptides. In this section, we briefly overview the data relevant to the signaling and function of the various angiotensin receptors, as reviewed in more detail elsewhere (575). In addition, we discuss data showing how angiotensin receptors are subject to regulatory processes, including changes in the angiotensin receptor expression, receptor desensitization/internalization, and dynamic interaction with angiotensin receptor-binding proteins. Furthermore, there is growing evidence that alternate peptides like ANG-(1–7) and their respective receptors broaden the functional range of the RAS. Finally, the action of renin is not restricted to its proteolytic activity and subsequent angiotensin generation; there is accumulating evidence that renin and/or pro-renin exert direct effects upon binding to renin/pro-renin receptors (Fig. 6). A. AT1 and AT2 Receptors Two receptor subtypes for ANG II have been characterized, AT1 and AT2. In rodents, AT1 exists as two highly homologous subtypes, AT1a and AT1b. While AT1a and AT1b have similar functions under most conditions, AT2mediated signaling differs considerably from AT1-dependent signaling, as it partly antagonizes the effects of ANG II on AT1 receptors. 1. AT1, AT1a, and AT1b receptor function and signaling AT1 receptors mediate most of the known effects of ANG II. The AT1 receptor, a 40-kDa protein consisting of 359 amino acids, is a member of the seventransmembrane domain G protein-coupled receptor family. The AT1 receptors typically couple to Gq complexes, resulting in the activation of downstream intracellular signaling pathways that lead to the activation of phospholipase C, phospholipase A2, and phospholipase D (898). The activation of phospholipase C triggers the enhanced formation of IP3 and diacylglycerol, promoting the subsequent release of calcium from intracellular stores and the activation of PKC, respecPhysiol Rev • VOL FIG. 6. Multiple effector systems of the renin-angiotensin system. ANG 0, angiotensinogen; ANG I, angiotensin I; ANG II, angiotensin II; ACE, angiotensin I converting enzyme; ACE-2, angiotensin converting enzyme type 2; ANG 1–9, angiotensin nonapeptide; ANG 1–7, angiotensin heptapeptide; Mas-R, Mas receptor. tively. For a comprehensive review of the manifold intracellular signaling processes triggered upon the binding of ANG II to AT1 receptors, see Reference 575. In most mammalian species, there is only one distinct type of AT1 receptor. However, in rats and mice, two isoforms of AT1 exist, AT1a and AT1b. They share high amino acid homology (⬎95%), and so far, no pharmacological tools have been available to specifically, or even preferentially, block one of the isoforms (280). ANG II exerts myriad functions via AT1, including but not limited to vasoconstriction, increased renal tubular salt reabsorption, stimulation of aldosterone release, and central effects like elicitation of thirst and vasopressin secretion. Under pathological conditions, ANG II was shown to promote vascular remodeling, cardiac hypertrophic remodeling, and extracellular matrix deposition (206). 90 • APRIL 2010 • www.prv.org 644 CASTROP ET AL. In view of these multiple functions and considering the broad expression pattern of AT1 receptors in almost all organs, it is not surprising that mechanisms have evolved to regulate AT1 receptor function, both spatially and temporally. These mechanisms include 1) regulation of AT1 receptor transcription, 2) regulation of AT1 receptor surface expression via internalization and membrane recycling, and 3) modulation of AT1 receptor activity by accessory proteins. 2. Regulation of AT1 receptor transcription The chronic elevation of ANG II levels in vitro has been reported to activate a feedback loop that results in the downregulation of the AT1 receptor expression (279, 284, 501). Unfortunately, the situation in vivo is less clear. During a low-salt diet, and by inference elevated ANG II levels, the downregulation of the AT1 receptor expression in the rat liver has been reported (150, 760). The regulation of AT1 expression in the kidney under a low-salt diet is spatially heterogeneous. AT1 expression was also found to be upregulated in the proximal tubule but downregulated in glomeruli (150). In the adrenal gland, AT1 expression was augmented during a low-salt diet (384, 506, 760). These diverse results clearly suggest that multiple factors affect AT1 receptor expression in response to a salt-restricted diet. In fact, AT1 expression was shown to be regulated by numerous factors other than ANG II. These include low-density lipoprotein (629), insulin (845), and progesterone (630); meanwhile, ANG II (284), NO (371), and estrogens (630) suppress AT1 expression. Thus modulation of AT1 expression may constitute a measure to locally adapt the sensitivity of target cells and organs when they are exposed to changes in systemic ANG II levels. For example, a salt-restricted diet, accompanied by enhanced systemic ANG II concentrations, stimulates various salt-conserving mechanisms, like aldosterone release and renal sodium reabsorption, but it has little, if any, effect on the vascular resistance. It remains to be determined to what extent the modulations of AT1 receptor expression are relevant for this apparent local fine-tuning of ANG II sensitivity. 3. Regulation of AT1 receptor surface expression by internalization and membrane recycling AT1 receptors are subject to internalization and desensitization. The continuous cycle of receptor internalization and cell surface retrafficking was shown to be shifted towards internalization upon binding of ANG II to the AT1 receptor. As a consequence, the number of available receptors in the cell membrane was reduced, resulting in cell desensitization for ANG II (205, 279, 284). In addition, AT1 receptors located in the cell membrane are also subject to direct desensitization. Various members of the G protein-coupled receptor Physiol Rev • VOL kinase family (GRK; Refs. 505, 529), including GRK2, GRK3, and GRK5, were found to be capable of phosphorylating serine and threonine residues located in the cytoplasmic domain of AT1 receptors. The phosphorylation of AT1 resulted in inactivation of the cell membrane-bound AT1 and promoted receptor internalization (412, 649). In summary, the AT1 receptor internalization and surface trafficking permit regulation of the AT1 receptor surface expression and, consequently, influence the magnitude of local ANG II effects. 4. Modulation of AT1 receptor activity by accessory proteins The set of molecular mechanisms that modulates AT1 receptor signaling also includes the interaction of AT1 with AT1-associated proteins. During the last few years, four different proteins that interact with the COOH-terminal, intracellular tail of the AT1 protein have been identified. These AT1-associated proteins include negative and positive regulators of AT1 signaling. The AT1-associated protein Atrap (angiotensin receptor-associated protein) was identified as a negative regulator of AT1 (180), whereas ARAP1 (angiotensin receptor-associated protein 1 ⫽ angiopoietin-related protein 2; Ref. 286) and GLP (GEF-like protein) exert positive regulatory functions. ARAP1 overexpression in the proximal tubule of mice results in hypertension and kidney hypertrophy, suggesting that ARAP1 may function as a positive regulator of the AT1 receptors (285). The function of the fourth known AT1-associated protein, EP24.15 (metalloendopeptidase 24.15), remains unknown (799). 5. Atrap: a negative regulator of AT1 receptors? The investigation of the function of AT1 receptorassociated proteins has only recently begun, with Atrap probably being the best characterized AT1 receptor accessory protein. Atrap is an 18-kDa protein with three putative transmembrane domains (534). It is expressed in various organs, with the highest expression levels observed in the kidney, heart, and testes; on the other hand, lower expression levels were found in the lung, liver, spleen, and brain (180). In the kidney, Atrap was detected by immunohistochemistry primarily in the tubular system, where it partially colocalized with the AT1 receptors. Little Atrap expression was detected in the renal vasculature, despite the marked expression of AT1 receptors, implying a specific function of Atrap in modulating the tubular effects of ANG II (893). The functional impact of the Atrap-AT1 interaction has been assessed in several studies. In vascular smooth muscle cells, the overexpression of Atrap resulted in enhanced internalization of the AT1 receptor upon exposure to ANG II (174). Increased AT1 receptor internalization was accompanied by marked 90 • APRIL 2010 • www.prv.org PHYSIOLOGY OF KIDNEY RENIN inhibition of the angiotensin-induced proliferation of vascular smooth muscle cells (174). Similar results were obtained for cardiomyocytes, in which the prohypertrophic effect of ANG II was reduced during the coexpression of Atrap and paralleled by reduced surface expression of AT1; once again, this indicates that Atrap promotes the internalization of AT1 receptors (855). It should be noted that the mutations in the COOH-terminal end of the AT1 receptor, the putative binding site of Atrap, result in a reduced internalization frequency of the AT1 receptor (166, 329). Thus far, it remains unclear whether the negative effect of Atrap on the AT1 signaling is merely due to a reduction of the AT1 surface localization or whether Atrap also interferes with the AT1-dependent intracellular signaling (517). A first attempt to address the in vivo function of Atrap was recently made via the generation of a transgenic mouse line that overexpresses Atrap under the control of the CMV promoter (Atrap-Tg) (657). Atrap mRNA expression in the heart, aorta, and femoral artery was increased three- to fourfold compared with the wildtype controls (657). The arterial blood pressure and heart rate were indistinguishable between Atrap-Tg and wildtype mice. However, the effect of cuff placement around the femoral artery as an experimental approach to assess vascular inflammation and remodeling was considerably ameliorated in Atrap-Tg mice. Similarly, the progression of heart hypertrophy after aortic banding was attenuated in Atrap-Tg mice compared with wild-type mice, indicating that the overall effect of Atrap is attenuation of the AT1-mediated signaling (657). 6. AT2 receptor function and signaling The AT2 receptor is a seven-transmembrane domain protein consisting of 363 amino acids with a molecular mass of 41 kDa (604). AT2 and AT1 share 34% homology (604). AT2 receptors are highly expressed in various tissues of the developing embryo, but expression declines after birth (791, 792). The range of the AT2-dependent functions is still not completely understood. Similarly, the AT2-linked intracellular signaling events are still a matter of intense research and appear to be far more diverse than those of the AT1 receptor (for a comprehensive review, see Ref. 825). However, there is growing evidence that AT2-dependent signaling may antagonize the effects of ANG II on AT1 receptors under various conditions. This AT1-antagonizing effect of the AT2 receptor might be related, in part, to a direct interaction between the AT2 and AT1 receptors. The AT2 receptors were shown to form heterodimers with AT1 receptors, resulting in an attenuation of the AT1-mediated effects of ANG II (2). The AT2-mediated effects of ANG II on vascular resistance may serve as an example of how AT2 receptor activation functionally counteracts the AT1 receptor. Thus, in contrast to AT1 receptor-deficient mice, the AT2 knockout Physiol Rev • VOL 645 mice were shown to exhibit increased arterial blood pressure (328, 370). Commensurate with the vasodilator effect of AT2 activation, the infusion of ANG II under concomitant AT1 receptor inhibition resulted in a decrease in arterial blood pressure in rats, which was dependent on the intact NOS activity (116). Several other studies confirmed these findings and provided evidence that the ANG II binding to AT2 results in enhanced formation of vasodilator agents like NO, prostanoids, and bradykinin, as reviewed in detail elsewhere (951). B. Renin/Pro-renin Receptor Traditionally, the function of renin was thought to be restricted to its enzymatic activity, i.e., cleavage of the renin-substrate angiotensinogen. This assumption was first challenged by the finding that human plasma contains considerable amounts of the enzymatically inactive prorenin (542). This phenomenon raised the question as to whether pro-renin in the circulation is somehow activated or transformed into active renin and/or whether pro-renin has a function that is entirely independent of the formation of enzymatically active renin, a function that might be related to the binding of pro-renin (and/or renin) to receptors. The first evidence for the existence of a renin/prorenin receptor was obtained by the finding that cultured human mesangial cells are able to bind renin, which subsequently induced the synthesis and release of the plasminogen activator inhibitor-1 (626). In a similar study, renin was shown to increase the expression of TGF-1 and extracellular matrix proteins upon binding to mesangial cells, an effect that was independent of the renin activity (362). A few years after the discovery of the ability of renin to bind to mesangial cells, the human renin/pro-renin receptor was cloned (627). The renin/prorenin receptor is a single-transmembrane 45-kDa protein consisting of 350 amino acids with no apparent homology to known receptors. This novel receptor can bind both pro-renin and renin (627). The binding of renin to its receptor resulted in a fivefold increase in the catalytic activity compared with renin in solution. The binding of pro-renin to the receptor increased its enzymatic activity from virtually zero to values comparable to those of active renin in solution (for details on renin/pro-renin receptorindependent activation of pro-renin, see sect. IIIA1). Although the exact mechanism of pro-renin activation upon receptor binding will require additional experimental efforts, it was suggested that pro-renin may employ its handle region to bind to the receptor, which would eventually expose the active site of the enzyme (627). Furthermore, the binding of renin to its receptor initiated the activation of the intracellular ERK1/ERK2 pathway independent of renin enzymatic activity (627). Thus the bind- 90 • APRIL 2010 • www.prv.org 646 CASTROP ET AL. ing of renin and/or pro-renin to its receptor boosts the locally defined renin activity and is accompanied by intracellular signaling of the receptor-expressing cell. These findings subsequently sparked interest in the evaluation of possible clinical consequences of the renin/ pro-renin receptor binding in vivo. To address this issue, a competitive inhibitor peptide was developed to prevent pro-renin/renin from binding to its receptor (755). The renin/pro-renin receptor inhibitor was used in rats during endotoxin-induced uveitis, since ANG II was shown to be crucially involved in the induction of ocular inflammation. The inflammatory parameters were markedly alleviated under the concomitant decoy peptide application (755). The therapeutic benefit of the renin/pro-renin receptor inhibitor appears to be substantial. The inhibition of renin/pro-renin receptor binding also markedly ameliorated the progression of cardiac fibrosis in stroke-prone SHR rats (368). In addition, the renin/pro-renin receptor inhibition was able to block MAPK activation and the development of glomerulosclerosis in a model of diabetic nephropathy (366). In a follow-up study in AT1a-deficient mice, the authors suggested an ANG II-independent effect that depended on pro-renin/renin receptor activation (416). In addition, ACE inhibition was shown to be substantially less efficient than the application of the prorenin/renin receptor antagonist in preventing the development of diabetic nephropathy (416). With respect to the effect of the renin/pro-renin receptor inhibitor on the progression of cardiac fibrosis in SHR rats, the aforementioned results could not be confirmed without restrictions. Thus the prolonged infusion of the renin/pro-renin receptor antagonist was shown in another study to somewhat reduce the left ventricular mass in SHR rats, but it did not affect fibrotic cardiac remodeling, coronary hemodynamics, or left ventricular function (835). Similarly, the beneficial effect of pro-renin/renin receptor inhibition on renal function could not be confirmed in high-renin models of hypertension like the two-kidney one-clip model (543). In view of the outstanding relevance of the initial findings regarding the beneficial effects of prorenin/renin receptor antagonism, future studies will be required to substantiate the initial reports and to confirm the results under modified experimental conditions. In summary, a novel receptor type that binds renin and pro-renin with equal affinity was discovered. Upon receptor binding, a dual effect is observed. First, the specific enzymatic activity of both renin and pro-renin is substantially enhanced and, due to local renin/pro-renin trapping, restricted to the local environment of the receptor-expressing cells. Therefore, the renin/pro-renin binding may provide a basis for the locally restricted renin activity that might well exceed the magnitude of systemic renin activity. Second, the binding of renin/pro-renin appears to initiate intracellular signaling in the receptorPhysiol Rev • VOL expressing cell, and this effect might be of relevance under pathophysiological conditions. C. The ANG-(1–7)-ACE2-Mas Axis New components and functions of the renin-angiotensin system are still being discovered. Within the last decade, it has been recognized that the smaller peptide fragments of the RAS, including ANG III, ANG IV, and ANG-(1–7), also possess biological activity, but that their plasma values are much lower than those of ANG II, with the exception of ANG-(1–7) (321, 680). The identification of the ACE homolog ACE2, an important enzyme for the generation of ANG-(1–7), and of the G protein-coupled receptor Mas, which is encoded by the Mas protooncogene, as a receptor for ANG-(1–7), broadened our knowledge of the RAS towards a system containing at least two cascades, with the ACE2-ANG-(1–7)-Mas axis likely acting as a counterregulatory portion of the classical RAS axis. The increasing interest in this area of research has been documented by several reviews, in which the interested reader can obtain a more comprehensive overview (177, 223, 224, 424, 449, 498, 713, 752, 907). 1. Function of Ang-(1–7) and expression of ACE2 in the cardiovascular system ANG-(1–7) can be formed from ANG I and ANG II by the action of ACE, ACE2, and several other enzymes, including neprilysin (NEP) and prolyl oligopeptidase (POP) (222), whereas ACE is the main enzyme responsible for the conversion of ANG I into ANG II. The carboxypeptidase ACE2 cleaves ANG II into ANG-(1–7) (912). Furthermore, ACE2 forms ANG-(1–9) from ANG I, which can then be converted into ANG-(1–7) by ACE (202). However, the preferable physiological substrate for ACE2 seems to be ANG II (878, 912). ANG-(1–7) is subsequently metabolized into the inactive fragment ANG(1–5) by the activity of ACE (16, 139, 186). In addition, ACE2 is also capable of metabolizing other peptides, such as apelin-13, des-Arg[9]-[bradykinin], -casomorphin-(1– 7), dynorphin A-(1–13), ghrelin, and neurotensin-(1–11) (202, 912). The half-life of the heptapeptide ANG-(1–7) is very short (several seconds) in rats (962), and ACE inhibitors, which inhibit the metabolism of ANG-(1–7) into ANG-(1–5), clearly increase the half-life of ANG-(1–7) (962). The 805-amino acid enzyme ACE2 is a monocarboxypeptidase with one active site domain that shares ⬃42% homology with the active site of ACE (202, 878, 912). ACE2 gene expression has been found in the heart, kidney, and testes and, to a lower extent, in the liver, lung, small intestine, and brain of humans and rodents (259, 303, 451). ACE2 is an ectoenzyme, although a soluble active form has also been described in plasma and urine (790, 939). 90 • APRIL 2010 • www.prv.org PHYSIOLOGY OF KIDNEY RENIN The first biological activities of ANG-(1–7) to be described were the demonstration of AVP release from the hypothalamo-neurohypophysial system and the decrease of arterial blood pressure after microinjection of low doses of ANG-(1–7) into the dorsal medulla of rats (111, 757). Further studies have demonstrated immunostaining of ANG-(1–7) in central regions related to the synthesis and release of vasopressin (79, 110, 467). Subsequent studies have shown that ANG-(1–7) is involved in the baroreflex control of the heart rate at the nucleus tractus solitarii (67, 112, 143, 645, 742), enhances the long-term potentiation in the hippocampus (330), and contributes to the maintenance of sympathetic nerve activity in the neurons of the paraventricular hypothalamic nucleus (805). In line with these observations, ACE2 expression has been reported in various cardiovascular regulatory brain areas (203, 742). ACE2 protein expression was found to be lower in the rostral ventrolateral medulla of SHR compared with normotensive Wistar-Kyoto rats (WKY), and vascular or central overexpression of ACE2 was found to decrease blood pressure in SHR (724, 965). With regard to the heart, it has been found that ANG-(1–7) evokes antiarrhythmogenic effects (226, 751), dilates the coronary arteries (87), increases coronary blood flow (123, 124), and affects the cardiac contractility (225, 750). The cardiac expression of ACE2 has been localized to endothelial cells and smooth muscle cells from the intramyocardial vessels and myocytes (104, 202, 878). ACE2-deficient mice show reduced cardiac contractility, a decrease in aortic and ventricular pressure, and a thinning of the left ventricular wall. These changes progressed with age and were more prominent in male mice. The hearts of ACE2 knockout mice exhibited enhanced ANG II levels and increased levels of hypoxia-inducible genes (170). However, these effects seem to be dependent on the background of the ACE2-deficient mice (288). Increased cardiac ACE2 expression and activity have been found in failing human and rat hearts following arterial ligation and AT1-receptor antagonism (104, 223, 378). In agreement with these findings, ANG-(1–7) exerts a protective role for the heart in several animal models by decreasing or preventing the development of cardiac hypertrophy and fibrosis (68, 281, 282, 533, 751). This effect seems to be independent of blood pressure reduction (281, 282). In addition, it has been shown that ANG-(1–7) initiates antifibrotic and antitrophic effects in cardiac fibroblasts and inhibits growth in rat cardiomyocytes (386, 847). Moreover, ANG-(1–7) has been reported to decrease cardiac ANG II levels (577) and to attenuate the diabetesinduced cardiovascular dysfunction (69), as well as hypertension-induced cardiac remodeling (580). Vascular endothelial cells can form ANG-(1–7) via the activity of ACE2 (104, 749) and are also an important target for the action of ANG-(1–7). The vasodilatory effect of ANG-(1–7) depends on an intact endothelium (503), Physiol Rev • VOL 647 where ANG-(1–7) can induce the release of NO via eNOS (744). In addition to its effect on NO release, ANG-(1–7) stimulates the release of prostanoids from endothelial and vascular smooth muscle cells (393, 394, 613). Moreover, ANG-(1–7) seems to potentiate the vasodilatory effect of bradykinin (87, 519, 675, 676) and attenuate the vasoconstrictor effect of ANG II (895). However, vasoconstriction in response to ANG-(1–7) has also been reported (1, 656). In addition, ANG-(1–7) also acts on vascular smooth muscle cells, where it inhibits cell growth (233, 846). More recently, ANG-(1–7) has been proposed to attenuate atherosclerotic plaques in animals (201, 540). The pulmonary ACE2 expression has been described for type I and type II alveolar epithelial cells, bronchiolar epithelial cells, endothelial cells, and arterial smooth muscle cells (303, 372, 471). ACE2 expression decreases with aging in rat lungs and is higher in old female rats compared with old male rats. The ACE2-deficient mice do not show any pulmonary abnormalities (170). However, ACE2 deletion worsens the severe acute lung injury induced by acid aspiration or sepsis, and the injection of recombinant human ACE2 attenuates the acute lung failure in the ACE2 knockout as well as wild-type mice (372). In addition, ACE2 is a well-described receptor for the severe acute respiratory syndrome coronavirus (SARS-CoV) and for the human coronavirus-NL63 (348, 515). The SARSCoV infection can be attenuated by soluble ACE2 molecules, ACE2 antibodies, and the genetic deletion of ACE2 (304, 472, 515). Several studies have demonstrated that the kidney is an additional target for the action of ANG-(1–7). ANG(1–7) injection has been shown to increase the sodium and water excretion, as well as the GFR, without affecting the renovascular resistance in isolated perfused rat kidneys and in anesthetized animals (189, 899). Subsequent studies have found that ANG-(1–7) modulates the activity of Na⫹-K⫹-ATPase in the basolateral membranes of kidney proximal tubules (121, 305). In addition, ANG-(1–7) has been reported to stimulate the arachidonic acid release from isolated proximal tubules (26), and the inhibition of prostaglandin release by COX inhibition attenuates the ANG-(1–7)-induced increase in urine flow and sodium excretion (336). ANG-(1–7) has also been shown to cause afferent arteriolar vasodilation and to antagonize the vasoconstrictor effects of ANG II (32). The intrarenal blockade of ANG-(1–7) causes a decrease in GFR, renal plasma flow, and sodium excretion in rats with increased activity of renal RAS (100). However, ANG-(1–7) also induces antidiuresis in water-loaded rodents (748) and increases renal tubular sodium reabsorption in rats (101). ACE2 gene expression has been described for glomeruli, vasa recta, and all nephron segments except the mTAL (514). Relatively high amounts of ACE2 were detected in the apical brush-border membrane of proximal tubule epithelia, where it colocalized with ACE (514). 90 • APRIL 2010 • www.prv.org 648 CASTROP ET AL. Within the glomerulus, ACE2 is localized in podocytes and, to a smaller extent, in the mesangial cells (974). The increased renal ANG-(1–7) levels and renal expression of ACE2 have been found during pregnancy (88, 410) and in response to ACE inhibition or ANG-II-AT1 receptor antagonism (404, 876). Renal ACE2 expression was found to be lower in adult SHR compared with age-matched WKY rats during lipopolysaccharide-induced acute renal failure and in several models of diabetic kidney injury (287, 875, 876, 974, 988). Several findings have suggested a protective role for the ACE2-ANG-(1–7)-Mas axis during diabetic nephropathy: 1) ACE2 gene deletion and pharmacological ACE2 inhibition with the ACE2 inhibitor MLN-4760 worsen the development of glomerular injury in diabetic mice (819, 957), 2) treatment with ANG-(1–7) or the Mas receptor agonist AVE0991 ameliorates diabetes-induced renal and cardiovascular dysfunction (69, 70, 210), 3) attenuation of diabetic renal injury by ACE inhibition is paralleled by the increased ACE2 expression (876), and 4) increased ACE2 expression in streptozotocin-induced diabetic mice has been reported (960). However, ACE2 polymorphisms and diabetic nephropathy do not seem to be associated in humans (242). A recent study reported that loss of the ACE2 gene leads to the development of ANG II-dependent glomerular injury in male mice, but not in female mice. Moreover, these authors found that the greater degree of glomerulosclerosis and proteinuria was attenuated by ANG II-AT1 receptor inhibition (661). In humans, the de novo expression of ACE2 in biopsies of patients with renal diseases and an increased ACE to ACE2 ratio in subjects with hypertension have been reported (508, 930). 2. Mas receptor The existence of a receptor for ANG-(1–7) was initially suggested by pharmacological studies using ANG(1–7) antagonists (848). The orphan heterotrimeric guanine nucleotide-binding protein-coupled receptor, i.e., the Mas receptor, was later suggested to be the functional binding partner for ANG-(1–7) (510). The Mas gene encodes for a G protein-coupled receptor and was originally detected in vivo by tumorigenic properties originating from the rearrangement of its 5⬘ region flanking a protooncogene, due to its tumorigenicity (708, 979). The genetic deletion of the Mas receptor has been shown to eliminate the binding of ANG-(1–7) to mouse renal cells, leading to impaired cardiac and renal function and to attenuation of the vasodilator effects of ANG-(1–7) (753) (Fig. 7). The Mas gene is expressed in the brain, testes, kidney, and heart (391, 581, 978). Within the central nervous system, the Mas receptor expression has been found in various regions, including the cardiovascular regulatory areas (58). In agreement with the activities described for ANG-(1–7), Mas-deficient mice exhibit increased Physiol Rev • VOL FIG. 7. Components of the renin-angiotensin I-Mas axis. For details, please see text. blood pressure, impaired endothelial function, decreased NO production, and decreased endothelial NO synthase expression (679, 961). In line with the cardioprotective effects of ANG-(1–7), the genetic deletion of the Mas receptor impairs heart function and changes the extracellular matrix to a profibrotic state (750). In addition, the genetic deletion of the Mas receptor leads to changes in glucose and lipid metabolism (754), erectile function (176), heart function (123, 750), and brain function (330). The intracellular signal transduction mechanisms that follow the activation of the Mas receptor are only poorly understood. ANG-(1–7) stimulates the phosphorylation of Janus kinase 2 (JAK2) and insulin receptor substrate (IRS)-1 in the rat heart in vivo (266). Mas receptor activation leads to an increase in the NO production via the phosphorylation of eNOS, which involves the activation of the phosphatidylinositol 3-kinase-dependent Akt phosphorylation (192, 744). Furthermore, the inhibition of MAPK phosphorylation upon activation of the Mas receptor has been described (830, 847). In addition, the Mas deficiency impairs Ca2⫹ management in cardiomyocytes (192). In the Mas-transfected CHO and COS cells, ANG(1–7) stimulated the release of arachidonic acid (753). It should be noted, however, that some studies have shown that ANG-(1–7) can also bind to the AT1 and AT2 receptors, although rather high concentrations of ANG(1–7) are necessary (552, 730, 931). In addition, the exis- 90 • APRIL 2010 • www.prv.org PHYSIOLOGY OF KIDNEY RENIN tence of another ANG-(1–7) receptor has been suggested (806), and an interaction between the different angiotensin receptors has been proposed (124, 460). VII. CONCLUSION Despite the long-standing awareness of renin production and secretion, our knowledge of its regulation and (patho)physiological relevance has steadily increased during the last few years. We have begun to understand the fundamental properties of renin gene regulation: in particular, its stimulation by the cAMP pathway and (mostly) inhibitory modulation by specific hormones and cytokines. We have improved our knowledge of the development, cell biology, and function of renin-producing cells in the kidney, commonly referred to as JG cells. The discovery of the functional relevance of gap junctions in JG cells has revealed novel and promising insights into the cell biology of renin-producing cells. Although the cAMP signaling pathway is known as a very powerful stimulator of renin secretion, the detailed mechanisms of renin release are still obscure and await further clarification. The mysterious “calcium paradox,” referring to the inhibitory effect of calcium on secretion in renin-producing cells, may have found at least a partial explanation in the existence of calcium-inhibitable adenylyl cyclases in JG cells. ATP, NO, and prostanoids have emerged as powerful signals that mediate the local control of renin secretion and synthesis. Furthermore, the list of hormones that act directly on JG cells has been substantially enlarged. ANG II, the classic biological effector of the RAS, has been shown to act through different receptors that mediate antagonistic effects. Moreover, ANG II is probably not the only biological mediator of the reninangiotensin system because (pro)renin and ANG-(1–7) were also found to exert direct and distinct biological effects. The knowledge about kidney renin is certainly not yet complete, although 100 years have passed since its discovery. We may therefore anticipate with interest the novel findings about kidney renin that are expected in the upcoming years. ACKNOWLEDGMENTS Address for reprint requests and other correspondence: A. Kurtz, Institute of Physiology, Univ. of Regensburg, D-93040 Regensburg, Germany (e-mail: armin.kurtz@vkl.uniregensburg.de). GRANTS The authors’ work was financially supported by Deutsche Forschungsgemeinschaft Grant SFB 699. REFERENCES 1. Abbas A, Gorelik G, Carbini LA, Scicli AG. Angiotensin-(1–7) induces bradykinin-mediated hypotensive responses in anesthetized rats. Hypertension 30: 217–221, 1997. Physiol Rev • VOL 649 2. Abdalla S, Lother H, Abdel-tawab AM, Quitterer U. The angiotensin II AT2 receptor is an AT1 receptor antagonist. J Biol Chem 276: 39721–39726, 2001. 3. Abel KJ, Gross KW. Close physical linkage of the murine Ren-1 and Ren-2 loci. Nucleic Acids Res 16: 2111–2126, 1988. 4. Ackermann M, Ritthaler T, Riegger G, Kurtz A, Kramer BK. Endothelin inhibits cAMP-induced renin release from isolated renal juxtaglomerular cells. J Cardiovasc Pharmacol 26 Suppl 3: S135– S137, 1995. 5. Adamczak M, Kokot F, Wiecek AW. Relationship between plasma renin profile and leptinaemia in patients with essential hypertension. J Hum Hypertens 14: 503–509, 2000. 6. Adams DJ, Beveridge DJ, van der Weyden L, Mangs H, Leedman PJ, Morris BJ. HADHB, HuR, and CP1 bind to the distal 3⬘-untranslated region of human renin mRNA and differentially modulate renin expression. J Biol Chem 278: 44894 – 44903, 2003. 7. Adams DJ, Head GA, Markus MA, Lovicu FJ, van der Weyden L, Kontgen F, Arends MJ, Thiru S, Mayorov DN, Morris BJ. Renin enhancer is critical for control of renin gene expression and cardiovascular function. J Biol Chem 281: 31753–31761, 2006. 8. Aguilera G. Role of angiotensin II receptor subtypes on the regulation of aldosterone secretion in the adrenal glomerulosa zone in the rat. Mol Cell Endocrinol 90: 53– 60, 1992. 9. Aguilera G, Kiss A, Sunar-Akbasak B. Hyperreninemic hypoaldosteronism after chronic stress in the rat. J Clin Invest 96: 1512– 1519, 1995. 10. Ahlborg G, Lundberg JM. Inhibitory effects of neuropeptide Y on splanchnic glycogenolysis and renin release in humans. Clin Physiol 14: 187–196, 1994. 11. Albinus M, Finkbeiner E, Sosath B, Osswald H. Isolated superfused juxtaglomerular cells from rat kidney: a model for study of renin secretion. Am J Physiol Renal Physiol 275: F991–F997, 1998. 12. Alcolea S, Jarry-Guichard T, de Bakker J, Gonzalez D, Lamers W, Coppen S, Barrio L, Jongsma H, Gros D, van Rijen H. Replacement of connexin40 by connexin45 in the mouse: impact on cardiac electrical conduction. Circ Res 94: 100 –109, 2004. 13. Aldred GP, Fu P, Crawford RJ, Fernley RT. The sequence and tissue expression of ovine renin. J Mol Endocrinol 8: 3–11, 1992. 14. Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edition. Br J Pharmacol 153 Suppl 2: S1– S209, 2008. 15. Allen AM, Dampney RA, Mendelsohn FA. Angiotensin receptor binding and pressor effects in cat subretrofacial nucleus. Am J Physiol Heart Circ Physiol 255: H1011–H1017, 1988. 16. Allred AJ, Diz DI, Ferrario CM, Chappell MC. Pathways for angiotensin-(1–7) metabolism in pulmonary and renal tissues. Am J Physiol Renal Physiol 279: F841–F850, 2000. 17. Alund M. Juxtaglomerular cell activity during hemorrhage and ischemia as revealed by quinacrine histofluorescence. Acta Physiol Scand 110: 113–121, 1980. 18. Amenta F, Barili P, Bronzetti E, Ricci A. Dopamine D1-like receptor subtypes in the rat kidney: a microanatomical study. Clin Exp Hypertens 21: 17–23, 1999. 19. Amenta F, Ricci A. Demonstration of dopamine DA-1 receptor sites in rat juxtaglomerular cells by light microscope autoradiography. Naunyn-Schmiedebergs Arch Pharmacol 342: 719 –721, 1990. 20. Amlal H, Sheriff S, Faroqui S, Ma L, Barone S, Petrovic S, Soleimani M. Regulation of acid-base transporters by vasopressin in the kidney collecting duct of Brattleboro rat. Am J Nephrol 26: 194 –205, 2006. 21. Andersen JL, Andersen LJ, Sandgaard NC, Bie P. Volume expansion natriuresis during servo control of systemic blood pressure in conscious dogs. Am J Physiol Regul Integr Comp Physiol 278: R19 –R27, 2000. 22. Andersen LJ, Andersen JL, Pump B, Bie P. Natriuresis induced by mild hypernatremia in humans. Am J Physiol Regul Integr Comp Physiol 282: R1754 –R1761, 2002. 23. Andersen LJ, Jensen TU, Bestle MH, Bie P. Isotonic and hypertonic sodium loading in supine humans. Acta Physiol Scand 166: 23–30, 1999. 24. Andersen LJ, Norsk P, Johansen LB, Christensen P, Engstrom T, Bie P. Osmoregulatory control of renal sodium excretion 90 • APRIL 2010 • www.prv.org 650 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. 38. 39. 40. 41. 42. 43. CASTROP ET AL. after sodium loading in humans. Am J Physiol Regul Integr Comp Physiol 275: R1833–R1842, 1998. Anderson EA, Sinkey CA, Lawton WJ, Mark AL. Elevated sympathetic nerve activity in borderline hypertensive humans. Evidence from direct intraneural recordings. Hypertension 14: 177– 183, 1989. Andreatta-van Leyen S, Romero MF, Khosla MC, Douglas JG. Modulation of phospholipase A2 activity and sodium transport by angiotensin-(1–7). Kidney Int 44: 932–936, 1993. Antonipillai I, Nadler JL, Robin EC, Horton R. The inhibitory role of 12- and 15-lipoxygenase products on renin release. Hypertension 10: 61– 66, 1987. Aoyagi T, Izumi Y, Hiroyama M, Matsuzaki T, Yasuoka Y, Sanbe A, Miyazaki H, Fujiwara Y, Nakayama Y, Kohda Y, Yamauchi J, Inoue T, Kawahara K, Saito H, Tomita K, Nonoguchi H, Tanoue A. Vasopressin regulates the renin-angiotensinaldosterone system via V1a receptors in macula densa cells. Am J Physiol Renal Physiol 295: F100 –F107, 2008. Aranda A, Pascual A. Nuclear hormone receptors and gene expression. Physiol Rev 81: 1269 –1304, 2001. Ardaillou R. Angiotensin II receptors. J Am Soc Nephrol 10 Suppl 11: S30 –S39, 1999. Arensbak B, Mikkelsen HB, Gustafsson F, Christensen T, Holstein-Rathlou NH. Expression of connexin 37, 40, and 43 mRNA and protein in renal preglomerular arterioles. Histochem Cell Biol 115: 479 – 487, 2001. Arima S. Role of angiotensin II and endogenous vasodilators in the control of glomerular hemodynamics. Clin Exp Nephrol 7: 172–178, 2003. Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 257: 79 – 83, 1999. Armando I, Jezova M, Juorio AV, Terron JA, Falcon-Neri A, Semino-Mora C, Imboden H, Saavedra JM. Estrogen upregulates renal angiotensin II AT(2) receptors. Am J Physiol Renal Physiol 283: F934 –F943, 2002. Arpin-Bott MP, Kaissling B, Waltisperger E, Rabhi M, Saussine P, Freund-Mercier MJ, Stoeckel ME. Historadioautographic localization of oxytocin and V1a vasopressin binding sites in the kidney of developing and adult rabbit, mouse and merione and of adult human. Exp Nephrol 10: 196 –208, 2002. Arpin-Bott MP, Waltisperger E, Freund-Mercier MJ, Stoeckel ME. Historadioautographic localization, pharmacology and ontogeny of V(1a) vasopressin binding sites in the rat kidney. Nephron 83: 74 – 84, 1999. Aubert JF, Burnier M, Waeber B, Nussberger J, Dipette DJ, Burris JF, Brunner HR. Effects of a nonpressor dose of neuropeptide Y on cardiac output, regional blood flow distribution and plasma renin, vasopressin and catecholamine levels. J Pharmacol Exp Ther 244: 1109 –1115, 1988. Aubert JF, Walker P, Grouzmann E, Nussberger J, Brunner HR, Waeber B. Inhibitory effect of neuropeptide Y on stimulated renin secretion of awake rats. Clin Exp Pharmacol Physiol 19: 223–228, 1992. Bachmann J, Feldmer M, Ganten U, Stock G, Ganten D. Sexual dimorphism of blood pressure: possible role of the renin-angiotensin system. J Steroid Biochem Mol Biol 40: 511–515, 1991. Bachmann S, Bosse HM, Mundel P. Topography of nitric oxide synthesis by localizing constitutive NO synthases in mammalian kidney. Am J Physiol Renal Fluid Electrolyte Physiol 268: F885– F898, 1995. Bachmann S, Mundel P. Nitric oxide in the kidney: synthesis, localization, and function. Am J Kidney Dis 24: 112–129, 1994. Bagby SP, LeBard LS, Luo Z, Ogden BE, Corless C, McPherson ED, Speth RC. ANG II AT(1) and AT(2) receptors in developing kidney of normal microswine. Am J Physiol Renal Physiol 283: F755–F764, 2002. Balakrishnan VS, Coles GA, Williams JD. A potential role for endogenous adenosine in control of human glomerular and tubular Physiol Rev • VOL 44. 45. 46. 47. 48. 49. 50. 51. 52. 53. 54. 55. 56. 57. 58. 59. 60. 61. 62. 63. 64. 65. 66. function. Am J Physiol Renal Fluid Electrolyte Physiol 265: F504 – F510, 1993. Ball SG. Vasopressin and disorders of water balance: the physiology and pathophysiology of vasopressin. Ann Clin Biochem 44: 417– 431, 2007. Baltatu O, Cayla C, Iliescu R, Andreev D, Bader M. Abolition of end-organ damage by antiandrogen treatment in female hypertensive transgenic rats. Hypertension 41: 830 – 833, 2003. Barajas L, Liu L, Powers K. Anatomy of the renal innervation: intrarenal aspects and ganglia of origin. Can J Physiol Pharmacol 70: 735–749, 1992. Bardoux P, Bichet DG, Martin H, Gallois Y, Marre M, Arthus MF, Lonergan M, Ruel N, Bouby N, Bankir L. Vasopressin increases urinary albumin excretion in rats and humans: involvement of V2 receptors and the renin-angiotensin system. Nephrol Dial Transplant 18: 497–506, 2003. Barrett CJ, Guild SJ, Ramchandra R, Malpas SC. Baroreceptor denervation prevents sympathoinhibition during angiotensin II-induced hypertension. Hypertension 46: 168 –172, 2005. Barrett CJ, Ramchandra R, Guild SJ, Lala A, Budgett DM, Malpas SC. What sets the long-term level of renal sympathetic nerve activity: a role for angiotensin II and baroreflexes? Circ Res 92: 1330 –1336, 2003. Barrett GL, Morgan TO, Alcorn D. Stimulation of renin synthesis in the hydronephrotic kidney during sodium depletion. Pflügers Arch 415: 774 –776, 1990. Barton JS, Hindmarsh PC, Preece MA, Brook CG. Blood pressure and the renin-angiotensin-aldosterone system in children receiving recombinant human growth hormone. Clin Endocrinol 38: 245–251, 1993. Baumann H, Wang Y, Richards CD, Jones CA, Black TA, Gross KW. Endotoxin-induced renal inflammatory response. Oncostatin M as a major mediator of suppressed renin expression. J Biol Chem 275: 22014 –22019, 2000. Baumbach L, Leyssac PP, Skinner SL. Studies on renin release from isolated superfused glomeruli: effects of temperature, urea, ouabain and ethacrynic acid. J Physiol 258: 243–256, 1976. Baumbach L, Skott O. Renin release from different parts of rat afferent arterioles in vitro. Am J Physiol Renal Fluid Electrolyte Physiol 251: F12–F16, 1986. Baylis C. Sexual dimorphism in the aging kidney: differences in the nitric oxide system. Nat Rev Nephrol 5: 384 –396, 2009. Baylis C, Handa RK, Sorkin M. Glucocorticoids and control of glomerular filtration rate. Semin Nephrol 10: 320 –329, 1990. Beavo JA. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol Rev 75: 725–748, 1995. Becker LK, Etelvino GM, Walther T, Santos RA, CampagnoleSantos MJ. Immunofluorescence localization of the receptor Mas in cardiovascular-related areas of the rat brain. Am J Physiol Heart Circ Physiol 293: H1416 –H1424, 2007. Beierwaltes WH. cGMP stimulates renin secretion in vivo by inhibiting phosphodiesterase-3. Am J Physiol Renal Physiol 290: F1376 –F1381, 2006. Beierwaltes WH. Macula densa stimulation of renin is reversed by selective inhibition of neuronal nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol 272: R1359 –R1364, 1997. Beierwaltes WH. Selective neuronal nitric oxide synthase inhibition blocks furosemide-stimulated renin secretion in vivo. Am J Physiol Renal Physiol 269: F134 –F139, 1995. Beierwaltes WH, Prada J, Carretero OA. Effect of glandular kallikrein on renin release in isolated rat glomeruli. Hypertension 7: 27–31, 1985. Beierwaltes WH, Prada J, Carretero OA. Kinin stimulation of renin release in isolated rat glomeruli. Am J Physiol Renal Fluid Electrolyte Physiol 248: F757–F761, 1985. Bell PD, Lapointe JY. Characteristics of membrane transport processes of macula densa cells. Clin Exp Pharmacol Physiol 24: 541–547, 1997. Bell PD, Lapointe JY, Cardinal J, Chang YS. Transport pathways in macula densa cells. Kidney Int Suppl 32: S59 –S64, 1991. Bell PD, Lapointe JY, Sabirov R, Hayashi S, Peti-Peterdi J, Manabe K, Kovacs G, Okada Y. Macula densa cell signaling 90 • APRIL 2010 • www.prv.org PHYSIOLOGY OF KIDNEY RENIN 67. 68. 69. 70. 71. 72. 73. 74. 75. 76. 77. 78. 79. 80. 81. 82. 83. 84. 85. 86. 87. 88. 89. involves ATP release through a maxi anion channel. Proc Natl Acad Sci USA 100: 4322– 4327, 2003. Benter IF, Diz DI, Ferrario CM. Pressor and reflex sensitivity is altered in spontaneously hypertensive rats treated with angiotensin-(1–7). Hypertension 26: 1138 –1144, 1995. Benter IF, Yousif MH, Anim JT, Cojocel C, Diz DI. Angiotensin(1–7) prevents development of severe hypertension and end-organ damage in spontaneously hypertensive rats treated with L-NAME. Am J Physiol Heart Circ Physiol 290: H684 –H691, 2006. Benter IF, Yousif MH, Cojocel C, Al-Maghrebi M, Diz DI. Angiotensin-(1–7) prevents diabetes-induced cardiovascular dysfunction. Am J Physiol Heart Circ Physiol 292: H666 –H672, 2007. Benter IF, Yousif MH, Dhaunsi GS, Kaur J, Chappell MC, Diz DI. Angiotensin-(1–7) prevents activation of NADPH oxidase and renal vascular dysfunction in diabetic hypertensive rats. Am J Nephrol 28: 25–33, 2008. Berger S, Bleich M, Schmid W, Greger R, Schutz G. Mineralocorticoid receptor knockout mice: lessons on Na⫹ metabolism. Kidney Int 57: 1295–1298, 2000. Bichet DG, Razi M, Lonergan M, Arthus MF, Papukna V, Kortas C, Barjon JN. Hemodynamic and coagulation responses to 1-desamino[8-D-arginine]vasopressin in patients with congenital nephrogenic diabetes insipidus. N Engl J Med 318: 881– 887, 1988. Bie P, Molstrom S, Wamberg S. Normotensive sodium loading in conscious dogs: regulation of renin secretion during beta-receptor blockade. Am J Physiol Regul Integr Comp Physiol 296: R428 – R435, 2009. Bie P, Sandgaard NC. Determinants of the natriuresis after acute, slow sodium loading in conscious dogs. Am J Physiol Regul Integr Comp Physiol 278: R1–R10, 2000. Bie P, Wamberg S, Kjolby M. Volume natriuresis vs. pressure natriuresis. Acta Physiol Scand 181: 495–503, 2004. Bing J, Poulsen K. Differences in renal and submaxillary renin release after stimulation with isoprenaline and noradrenaline. Acta Physiol Scand 105: 58 – 63, 1979. Bischoff A, Avramidis P, Erdbrugger W, Munter K, Michel MC. Receptor subtypes Y1 and Y5 are involved in the renal effects of neuropeptide Y. Br J Pharmacol 120: 1335–1343, 1997. Bischoff A, Michel MC. Renal effects of neuropeptide Y. Pflügers Arch 435: 443– 453, 1998. Block CH, Santos RA, Brosnihan KB, Ferrario CM. Immunocytochemical localization of angiotensin-(1–7) in the rat forebrain. Peptides 9: 1395–1401, 1988. Bodin P, Burnstock G. Evidence that release of adenosine triphosphate from endothelial cells during increased shear stress is vesicular. J Cardiovasc Pharmacol 38: 900 –908, 2001. Boivin V, Jahns R, Gambaryan S, Ness W, Boege F, Lohse MJ. Immunofluorescent imaging of beta 1- and beta 2-adrenergic receptors in rat kidney. Kidney Int 59: 515–531, 2001. Bonvalet JP. Regulation of sodium transport by steroid hormones. Kidney Int Suppl 65: S49 –S56, 1998. Borensztein P, Germain S, Fuchs S, Philippe J, Corvol P, Pinet F. Cis -regulatory elements and trans-acting factors directing basal and cAMP-stimulated human renin gene expression in chorionic cells. Circ Res 74: 764 –773, 1994. Bosse HM, Bohm R, Resch S, Bachmann S. Parallel regulation of constitutive NO synthase and renin at JGA of rat kidney under various stimuli. Am J Physiol Renal Physiol 269: F793–F805, 1995. Brechler V, Chu WN, Baxter JD, Thibault G, Reudelhuber TL. A protease processing site is essential for prorenin sorting to the regulated secretory pathway. J Biol Chem 271: 20636 –20640, 1996. Brink HS, Derkx FH, Boomsma F, Schalekamp MA. Effects of DDAVP on renal hemodynamics and renin secretion in subjects with essential hypertension. Clin Nephrol 42: 95–101, 1994. Brosnihan KB, Li P, Ferrario CM. Angiotensin-(1–7) dilates canine coronary arteries through kinins and nitric oxide. Hypertension 27: 523–528, 1996. Brosnihan KB, Neves LA, Joyner J, Averill DB, Chappell MC, Sarao R, Penninger J, Ferrario CM. Enhanced renal immunocytochemical expression of ANG-(1–7) and ACE2 during pregnancy. Hypertension 42: 749 –753, 2003. Brosnihan KB, Weddle D, Anthony MS, Heise C, Li P, Ferrario CM. Effects of chronic hormone replacement on the renin-angioPhysiol Rev • VOL 90. 91. 92. 93. 94. 95. 96. 97. 98. 99. 100. 101. 102. 103. 104. 105. 106. 107. 108. 651 tensin system in cynomolgus monkeys. J Hypertens 15: 719 –726, 1997. Brown R, Ollerstam A, Johansson B, Skott O, Gebre-Medhin S, Fredholm B, Persson AE. Abolished tubuloglomerular feedback and increased plasma renin in adenosine A1 receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol 281: R1362–R1367, 2001. Brown RD, Thoren P, Steege A, Mrowka R, Sallstrom J, Skott O, Fredholm BB, Persson AE. Influence of the adenosine A1 receptor on blood pressure regulation and renin release. Am J Physiol Regul Integr Comp Physiol 290: R1324 –R1329, 2006. Bucher M, Hobbhahn J, Taeger K, Kurtz A. Cytokine-mediated downregulation of vasopressin V(1A) receptors during acute endotoxemia in rats. Am J Physiol Regul Integr Comp Physiol 282: R979 –R984, 2002. Bucher M, Ittner KP, Hobbhahn J, Taeger K, Kurtz A. Downregulation of angiotensin II type 1 receptors during sepsis. Hypertension 38: 177–182, 2001. Bucher M, Kees F, Taeger K, Kurtz A. Cytokines down-regulate alpha1-adrenergic receptor expression during endotoxemia. Crit Care Med 31: 566 –571, 2003. Bührle CP, Nobiling R, Mannek E, Schneider D, Hackenthal E, Taugner R. The afferent glomerular arteriole: immunocytochemical and electrophysiological investigations. J Cardiovasc Pharmacol 6 Suppl 2: S383–S393, 1984. Bührle CP, Nobiling R, Taugner R. Intracellular recordings from renin-positive cells of the afferent glomerular arteriole. Am J Physiol Renal Fluid Electrolyte Physiol 249: F272–F281, 1985. Bührle CP, Scholz H, Hackenthal E, Nobiling R, Taugner R. Epithelioid cells: membrane potential changes induced by substances influencing renin secretion. Mol Cell Endocrinol 45: 37– 47, 1986. Bumke-Vogt C, Bahr V, Diederich S, Herrmann SM, Anagnostopoulos I, Oelkers W, Quinkler M. Expression of the progesterone receptor and progesterone-metabolising enzymes in the female and male human kidney. J Endocrinol 175: 349 –364, 2002. Bunag RD, Page IH, McCubbin JW. Inhibition of renin release by vasopressin and angiotensin. Cardiovasc Res 1: 67–73, 1967. Burgelova M, Kramer HJ, Teplan V, Thumova M, Cervenka L. Effects of angiotensin-(1–7) blockade on renal function in rats with enhanced intrarenal ANG II activity. Kidney Int 67: 1453–1461, 2005. Burgelova M, Kramer HJ, Teplan V, Velickova G, Vitko S, Heller J, Maly J, Cervenka L. Intrarenal infusion of angiotensin(1–7) modulates renal functional responses to exogenous angiotensin II in the rat. Kidney Blood Press Res 25: 202–210, 2002. Burnett JC Jr, Granger JP, Opgenorth TJ. Effects of synthetic atrial natriuretic factor on renal function and renin release. Am J Physiol Renal Fluid Electrolyte Physiol 247: F863–F866, 1984. Burnham CE, Hawelu-Johnson CL, Frank BM, Lynch KR. Molecular cloning of rat renin cDNA and its gene. Proc Natl Acad Sci USA 84: 5605–5609, 1987. Burrell LM, Risvanis J, Kubota E, Dean RG, MacDonald PS, Lu S, Tikellis C, Grant SL, Lew RA, Smith AI, Cooper ME, Johnston CI. Myocardial infarction increases ACE2 expression in rat and humans. Eur Heart J 26: 369 –375, 2005. Burson JM, Aguilera G, Gross KW, Sigmund CD. Differential expression of angiotensin receptor 1A and 1B in mouse. Am J Physiol Endocrinol Metab 267: E260 –E267, 1994. Burt DW, Nakamura N, Kelley P, Dzau VJ. Identification of negative and positive regulatory elements in the human renin gene. J Biol Chem 264: 7357–7362, 1989. Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988 –1991. Hypertension 25: 305–313, 1995. Butt E, Nolte C, Schulz S, Beltman J, Beavo JA, Jastorff B, Walter U. Analysis of the functional role of cGMP-dependent protein kinase in intact human platelets using a specific activator 8-parachlorophenylthio-cGMP. Biochem Pharmacol 43: 2591–2600, 1992. 90 • APRIL 2010 • www.prv.org 652 CASTROP ET AL. 109. Calam J, Dimaline R, Peart WS, Unwin R. Studies on the renin response to vasoactive intestinal polypeptide (VIP) in the conscious rabbit. Br J Pharmacol 80: 13–15, 1983. 110. Calka J, Block CH. Angiotensin-(1–7) and nitric oxide synthase in the hypothalamo-neurohypophysial system. Brain Res Bull 30: 677– 685, 1993. 111. Campagnole-Santos MJ, Diz DI, Santos RA, Khosla MC, Brosnihan KB, Ferrario CM. Cardiovascular effects of angiotensin-(1–7) injected into the dorsal medulla of rats. Am J Physiol Heart Circ Physiol 257: H324 –H329, 1989. 112. Campagnole-Santos MJ, Heringer SB, Batista EN, Khosla MC, Santos RA. Differential baroreceptor reflex modulation by centrally infused angiotensin peptides. Am J Physiol Regul Integr Comp Physiol 263: R89 –R94, 1992. 113. Campean V, Theilig F, Paliege A, Breyer M, Bachmann S. Key enzymes for renal prostaglandin synthesis: site-specific expression in rodent kidney (rat, mouse). Am J Physiol Renal Physiol 285: F19 –F32, 2003. 114. Cantin M, Araujo-Nascimento MD, Benchimol S, Desormeaux Y. Metaplasia of smooth muscle cells into juxtaglomerular cells in the juxtaglomerular apparatus, arteries, arterioles of the ischemic (endocrine) kidney. An ultrastructural-cytochemical and autoradiographic study. Am J Pathol 87: 581– 602, 1977. 115. Carbone GM, Sheikh AU, Rogers S, Brewer G, Rose JC. Developmental changes in renin gene expression in ovine kidney cortex. Am J Physiol Regul Integr Comp Physiol 264: R591–R596, 1993. 116. Carey RM, Howell NL, Jin XH, Siragy HM. Angiotensin type 2 receptor-mediated hypotension in angiotensin type-1 receptorblocked rats. Hypertension 38: 1272–1277, 2001. 117. Carey RM, McGrath HE, Pentz ES, Gomez RA, Barrett PQ. Biomechanical coupling in renin-releasing cells. J Clin Invest 100: 1566 –1574, 1997. 118. Carillo BA, Beutel A, Mirandola DA, Vidonho AF Jr, Furukawa LN, Casarini D, Campos RR, Dolnikoff MS, Heimann JC, Bergamaschi CT. Differential sympathetic and angiotensinergic responses in rats submitted to low- or high-salt diet. Regul Pept 140: 5–11, 2007. 119. Carlsson S, Skarphedinsson JO, Delle M, Hoffman P, Thoren P. Reflex changes in post- and preganglionic sympathetic adrenal nerve activity and postganglionic sympathetic renal nerve activity upon arterial baroreceptor activation and during severe haemorrhage in the rat. Acta Physiol Scand 144: 317–323, 1992. 120. Carlyle M, Jones OB, Kuo JJ, Hall JE. Chronic cardiovascular and renal actions of leptin: role of adrenergic activity. Hypertension 39: 496 –501, 2002. 121. Caruso-Neves C, Lara LS, Rangel LB, Grossi AL, Lopes AG. Angiotensin-(1–7) modulates the ouabain-insensitive Na⫹-ATPase activity from basolateral membrane of the proximal tubule. Biochim Biophys Acta 1467: 189 –197, 2000. 122. Casellas D, Dupont M, Kaskel FJ, Inagami T, Moore LC. Direct visualization of renin-cell distribution in preglomerular vascular trees dissected from rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 265: F151–F156, 1993. 123. Castro CH, Santos RA, Ferreira AJ, Bader M, Alenina N, Almeida AP. Effects of genetic deletion of angiotensin-(1–7) receptor Mas on cardiac function during ischemia/reperfusion in the isolated perfused mouse heart. Life Sci 80: 264 –268, 2006. 124. Castro CH, Santos RA, Ferreira AJ, Bader M, Alenina N, Almeida AP. Evidence for a functional interaction of the angiotensin-(1–7) receptor Mas with AT1 and AT2 receptors in the mouse heart. Hypertension 46: 937–942, 2005. 125. Castrop H, Huang Y, Hashimoto S, Mizel D, Hansen P, Theilig F, Bachmann S, Deng C, Briggs J, Schnermann J. Impairment of tubuloglomerular feedback regulation of GFR in ecto-5⬘-nucleotidase/CD73-deficient mice. J Clin Invest 114: 634 – 642, 2004. 126. Castrop H, Kammerl M, Mann B, Jensen BL, Kramer BK, Kurtz A. Cyclooxygenase 2 and neuronal nitric oxide synthase expression in the renal cortex are not interdependent in states of salt deficiency. Pflügers Arch 441: 235–240, 2000. 127. Castrop H, Klar J, Wagner C, Hocherl K, Kurtz A. General inhibition of renocortical cyclooxygenase-2 expression by the rePhysiol Rev • VOL 128. 130. 131. 132. 133. 134. 135. 136. 137. 138. 139. 140. 141. 142. 143. 144. 145. 146. 147. nin-angiotensin system. Am J Physiol Renal Physiol 284: F518 – F524, 2003. Castrop H, Lorenz JN, Hansen PB, Friis U, Mizel D, Oppermann M, Jensen BL, Briggs J, Skott O, Schnermann J. Contribution of the basolateral isoform of the Na-K-2Cl cotransporter (NKCC1/BSC2) to renin secretion. Am J Physiol Renal Physiol 289: F1185–F1192, 2005. Castrop H, Schweda F, Mizel D, Huang Y, Briggs J, Kurtz A, Schnermann J. Permissive role of nitric oxide in macula densa control of renin secretion. Am J Physiol Renal Physiol 286: F848 – F857, 2004. Castrop H, Schweda F, Schumacher K, Wolf K, Kurtz A. Role of renocortical cyclooxygenase-2 for renal vascular resistance and macula densa control of renin secretion. J Am Soc Nephrol 12: 867– 874, 2001. Catanzaro DF, Mesterovic N, Morris BJ. Studies of the regulation of mouse renin genes by measurement of renin messenger ribonucleic acid. Endocrinology 117: 872– 878, 1985. Celio MR, Groscurth P, Inagami T. Ontogeny of renin immunoreactive cells in the human kidney. Anat Embryol 173: 149 –155, 1985. Cervenka L, Harrison-Bernard LM, Dipp S, Primrose G, Imig JD, El-Dahr SS. Early onset salt-sensitive hypertension in bradykinin B(2) receptor null mice. Hypertension 34: 176 –180, 1999. Chang CP, Brocchieri L, Shen WF, Largman C, Cleary ML. Pbx modulation of Hox homeodomain amino-terminal arms establishes different DNA-binding specificities across the Hox locus. Mol Cell Biol 16: 1734 –1745, 1996. Chang CT, Sun CY, Pong CY, Chen YC, Lin GP, Chang TC, Wu MS. Interaction of estrogen and progesterone in the regulation of sodium channels in collecting tubular cells. Chang Gung Med J 30: 305–312, 2007. Chapman AB, Zamudio S, Woodmansee W, Merouani A, Osorio F, Johnson A, Moore LG, Dahms T, Coffin C, Abraham WT, Schrier RW. Systemic and renal hemodynamic changes in the luteal phase of the menstrual cycle mimic early pregnancy. Am J Physiol Renal Physiol 273: F777–F782, 1997. Chappell MC, Gallagher PE, Averill DB, Ferrario CM, Brosnihan KB. Estrogen or the AT1 antagonist olmesartan reverses the development of profound hypertension in the congenic mRen2 Lewis rat. Hypertension 42: 781–786, 2003. Chappell MC, Pirro NT, Sykes A, Ferrario CM. Metabolism of angiotensin-(1–7) by angiotensin-converting enzyme. Hypertension 31: 362–367, 1998. Charles CJ, Rademaker MT, Richards AM. Hemodynamic, hormonal, and renal actions of adrenomedullin-2 in normal conscious sheep. Endocrinology 147: 1871–1877, 2006. Charles CJ, Rademaker MT, Richards AM, Cooper GJ, Coy DH, Jing NY, Nicholls MG. Hemodynamic, hormonal, and renal effects of adrenomedullin in conscious sheep. Am J Physiol Regul Integr Comp Physiol 272: R2040 –R2047, 1997. Chatziantoniou C, Pauti MD, Pinet F, Promeneur D, Dussaule JC, Ardaillou R. Regulation of renin release is impaired after nitric oxide inhibition. Kidney Int 49: 626 – 633, 1996. Chaves GZ, Caligiorne SM, Santos RA, Khosla MC, Campagnole-Santos MJ. Modulation of the baroreflex control of heart rate by angiotensin-(1–7) at the nucleus tractus solitarii of normotensive and spontaneously hypertensive rats. J Hypertens 18: 1841– 1848, 2000. Chen K, Carey LC, Valego NK, Liu J, Rose JC. Thyroid hormone modulates renin and ANG II receptor expression in fetal sheep. Am J Physiol Regul Integr Comp Physiol 289: R1006 –R1014, 2005. Chen K, Carey LC, Valego NK, Rose JC. Thyroid hormone replacement normalizes renal renin and angiotensin receptor expression in thyroidectomized fetal sheep. Am J Physiol Regul Integr Comp Physiol 293: R701–R706, 2007. Chen L, Kim SM, Oppermann M, Faulhaber-Walter R, Huang Y, Mizel D, Chen M, Lopez ML, Weinstein LS, Gomez RA, Briggs JP, Schnermann J. Regulation of renin in mice with Cre recombinase-mediated deletion of G protein Gsalpha in juxtaglomerular cells. Am J Physiol Renal Physiol 292: F27–F37, 2007. Chen M, Schnermann J, Smart AM, Brosius FC, Killen PD, Briggs JP. cAMP selectively increases renin mRNA stability in 90 • APRIL 2010 • www.prv.org PHYSIOLOGY OF KIDNEY RENIN 148. 149. 150. 151. 152. 153. 154. 155. 156. 157. 158. 159. 160. 161. 162. 163. 164. 165. 166. 167. 168. 169. cultured juxtaglomerular granular cells. J Biol Chem 268: 24138 – 24144, 1993. Chen YF, Meng QC. Sexual dimorphism of blood pressure in spontaneously hypertensive rats is androgen dependent. Life Sci 48: 85–96, 1991. Chen YF, Naftilan AJ, Oparil S. Androgen-dependent angiotensinogen and renin messenger RNA expression in hypertensive rats. Hypertension 19: 456 – 463, 1992. Cheng HF, Becker BN, Burns KD, Harris RC. Angiotensin II upregulates type-1 angiotensin II receptors in renal proximal tubule. J Clin Invest 95: 2012–2019, 1995. Cheng HF, Wang JL, Zhang MZ, Miyazaki Y, Ichikawa I, McKanna JA, Harris RC. Angiotensin II attenuates renal cortical cyclooxygenase-2 expression. J Clin Invest 103: 953–961, 1999. Cheng HF, Wang JL, Zhang MZ, Wang SW, McKanna JA, Harris RC. Genetic deletion of COX-2 prevents increased renin expression in response to ACE inhibition. Am J Physiol Renal Physiol 280: F449 –F456, 2001. Chidambaram M, Duncan JA, Lai VS, Cattran DC, Floras JS, Scholey JW, Miller JA. Variation in the renin angiotensin system throughout the normal menstrual cycle. J Am Soc Nephrol 13: 446 – 452, 2002. Chiu N, Park I, Reid IA. Stimulation of renin secretion by the phosphodiesterase IV inhibitor rolipram. J Pharmacol Exp Ther 276: 1073–1077, 1996. Chiu N, Reid IA. Role of kinins in basal and furosemide-stimulated renin secretion. J Hypertens 15: 517–521, 1997. Chiu T, Reid IA. Role of cyclic GMP-inhibitable phosphodiesterase and nitric oxide in the beta adrenoceptor control of renin secretion. J Pharmacol Exp Ther 278: 793–799, 1996. Chiu YJ, Hu SH, Reid IA. Inhibition of phosphodiesterase III with milrinone increases renin secretion in human subjects. J Pharmacol Exp Ther 290: 16 –19, 1999. Churchill PC. Effect of D-600 on inhibition of in vitro renin release in the rat by high extracellular potassium and angiotensin II. J Physiol 304: 449 – 458, 1980. Churchill PC. Second messengers in renin secretion. Am J Physiol Renal Fluid Electrolyte Physiol 249: F175–F184, 1985. Churchill PC, Churchill MC. A1 and A2 adenosine receptor activation inhibits and stimulates renin secretion of rat renal cortical slices. J Pharmacol Exp Ther 232: 589 –594, 1985. Churchill PC, Churchill MC, McDonald FD. Evidence that beta 1-adrenoceptor activation mediates isoproterenol-stimulated renin secretion in the rat. Endocrinology 113: 687– 692, 1983. Churchill PC, Ellis VR. Purinergic P2y receptors stimulate renin secretion by rat renal cortical slices. J Pharmacol Exp Ther 266: 160 –163, 1993. Clark AF, Sharp MG, Morley SD, Fleming S, Peters J, Mullins JJ. Renin-1 is essential for normal renal juxtaglomerular cell granulation and macula densa morphology. J Biol Chem 272: 18185– 18190, 1997. Clauser E, Curnow KM, Davies E, Conchon S, Teutsch B, Vianello B, Monnot C, Corvol P. Angiotensin II receptors: protein and gene structures, expression and potential pathological involvements. Eur J Endocrinol 134: 403– 411, 1996. Cohen Y, Rahamimov R, Naveh-Many T, Silver J, Rahamimoff R. Where is the “inverting factor” in hormone secretion from parathyroid cells? Am J Physiol Endocrinol Metab 273: E631–E637, 1997. Conchon S, Barrault MB, Miserey S, Corvol P, Clauser E. The C-terminal third intracellular loop of the rat AT1A angiotensin receptor plays a key role in G protein coupling specificity and transduction of the mitogenic signal. J Biol Chem 272: 25566 –25572, 1997. Conrad KP, Gellai M, North WG, Valtin H. Influence of oxytocin on renal hemodynamics and sodium excretion. Ann NY Acad Sci 689: 346 –362, 1993. Corder R, Vallotton MB, Lowry PJ, Ramage AG. Neuropeptide Y lowers plasma renin activity in the anaesthetised cat. Neuropeptides 14: 111–114, 1989. Cowley AW Jr. Long-term control of arterial blood pressure. Physiol Rev 72: 231–300, 1992. Physiol Rev • VOL 653 170. Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, Oliveira-dos-Santos AJ, da Costa J, Zhang L, Pei Y, Scholey J, Ferrario CM, Manoukian AS, Chappell MC, Backx PH, Yagil Y, Penninger JM. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature 417: 822– 828, 2002. 171. Crofford LJ. COX-1 and COX-2 tissue expression: implications and predictions. J Rheumatol Suppl 49: 15–19, 1997. 172. Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci USA 103: 17985–17990, 2006. 173. Crowley SD, Gurley SB, Oliverio MI, Pazmino AK, Griffiths R, Flannery PJ, Spurney RF, Kim HS, Smithies O, Le TH, Coffman TM. Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. J Clin Invest 115: 1092–1099, 2005. 174. Cui T, Nakagami H, Iwai M, Takeda Y, Shiuchi T, Tamura K, Daviet L, Horiuchi M. ATRAP, novel AT1 receptor associated protein, enhances internalization of AT1 receptor and inhibits vascular smooth muscle cell growth. Biochem Biophys Res Commun 279: 938 –941, 2000. 175. Cuneo RC, Salomon F, Wilmshurst P, Byrne C, Wiles CM, Hesp R, Sonksen PH. Cardiovascular effects of growth hormone treatment in growth-hormone-deficient adults: stimulation of the renin-aldosterone system. Clin Sci 81: 587–592, 1991. 176. Da Costa Goncalves AC, Leite R, Fraga-Silva RA, Pinheiro SV, Reis AB, Reis FM, Touyz RM, Webb RC, Alenina N, Bader M, Santos RA. Evidence that the vasodilator angiotensin-(1–7)-Mas axis plays an important role in erectile function. Am J Physiol Heart Circ Physiol 293: H2588 –H2596, 2007. 177. Danilczyk U, Penninger JM. Angiotensin-converting enzyme II in the heart and the kidney. Circ Res 98: 463– 471, 2006. 178. Danser AH, Derkx FH, Schalekamp MA, Hense HW, Riegger GA, Schunkert H. Determinants of interindividual variation of renin and prorenin concentrations: evidence for a sexual dimorphism of (pro)renin levels in humans. J Hypertens 16: 853– 862, 1998. 179. Darnell JE Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 264: 1415–1421, 1994. 180. Daviet L, Lehtonen JY, Tamura K, Griese DP, Horiuchi M, Dzau VJ. Cloning and characterization of ATRAP, a novel protein that interacts with the angiotensin II type 1 receptor. J Biol Chem 274: 17058 –17062, 1999. 181. Davis JO, Freeman RH. Mechanisms regulating renin release. Physiol Rev 56: 1–56, 1976. 182. Davisson RL, Oliverio MI, Coffman TM, Sigmund CD. Divergent functions of angiotensin II receptor isoforms in the brain. J Clin Invest 106: 103–106, 2000. 183. De Matteo R, May CN. Glucocorticoid-induced renal vasodilatation is mediated by a direct renal action involving nitric oxide. Am J Physiol Regul Integr Comp Physiol 273: R1972–R1979, 1997. 184. De Vito E, Gordon SB, Cabrera RR, Fasciolo JC. Release of renin by rat kidney slices. Am J Physiol 219: 1036 –1041, 1970. 185. De Wit C, Roos F, Bolz SS, Pohl U. Lack of vascular connexin 40 is associated with hypertension and irregular arteriolar vasomotion. Physiol Genomics 13: 169 –177, 2003. 186. Deddish PA, Marcic B, Jackman HL, Wang HZ, Skidgel RA, Erdos EG. N-domain-specific substrate and C-domain inhibitors of angiotensin-converting enzyme: angiotensin-(1–7) and keto-ACE. Hypertension 31: 912–917, 1998. 187. Del Rio G, Velardo A, Zizzo G, Avogaro A, Cipolli C, Della Casa L, Marrama P, MacDonald IA. Effect of estradiol on the sympathoadrenal response to mental stress in normal men. J Clin Endocrinol Metab 79: 836 – 840, 1994. 188. Della Bruna R, Pinet F, Corvol P, Kurtz A. Opposite regulation of renin gene expression by cAMP and calcium in isolated mouse juxtaglomerular cells. Kidney Int 47: 1266 –1273, 1995. 189. DelliPizzi AM, Hilchey SD, Bell-Quilley CP. Natriuretic action of angiotensin(1–7). Br J Pharmacol 111: 1–3, 1994. 90 • APRIL 2010 • www.prv.org 654 CASTROP ET AL. 190. Deschepper CF, Mellon SH, Cumin F, Baxter JD, Ganong WF. Analysis by immunocytochemistry and in situ hybridization of renin and its mRNA in kidney, testis, adrenal, and pituitary of the rat. Proc Natl Acad Sci USA 83: 7552–7556, 1986. 191. Dhein S. Gap junction channels in the cardiovascular system: pharmacological and physiological modulation. Trends Pharmacol Sci 19: 229 –241, 1998. 192. Dias-Peixoto MF, Santos RA, Gomes ER, Alves MN, Almeida PW, Greco L, Rosa M, Fauler B, Bader M, Alenina N, Guatimosim S. Molecular mechanisms involved in the angiotensin-(1–7)/ Mas signaling pathway in cardiomyocytes. Hypertension 52: 542– 548, 2008. 193. DiBona GF. Nervous kidney. Interaction between renal sympathetic nerves and the renin-angiotensin system in the control of renal function. Hypertension 36: 1083–1088, 2000. 194. DiBona GF. Neural control of the kidney: functionally specific renal sympathetic nerve fibers. Am J Physiol Regul Integr Comp Physiol 279: R1517–R1524, 2000. 195. DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev 77: 75–197, 1997. 196. DiBona GF, Sawin LL. Renal nerve activity in conscious rats during volume expansion and depletion. Am J Physiol Renal Fluid Electrolyte Physiol 248: F15–F23, 1985. 197. Dickinson DP, Gross KW, Piccini N, Wilson CM. Evolution and variation of renin genes in mice. Genetics 108: 651– 667, 1984. 198. Dimaline R, Peart WS, Unwin RJ. Effects of vasoactive intestinal polypeptide (VIP) on renal function and plasma renin activity in the conscious rabbit. J Physiol 344: 379 –388, 1983. 199. Dinchuk JE, Car BD, Focht RJ, Johnston JJ, Jaffee BD, Covington MB, Contel NR, Eng VM, Collins RJ, Czerniak PM. Renal abnormalities and an altered inflammatory response in mice lacking cyclooxygenase II. Nature 378: 406 – 409, 1995. 200. Dodge AH. Sites of renin production in fetal, neonatal, and postnatal Syrian hamster kidneys. Anat Rec 235: 144 –150, 1993. 201. Dong B, Zhang C, Feng JB, Zhao YX, Li SY, Yang YP, Dong QL, Deng BP, Zhu L, Yu QT, Liu CX, Liu B, Pan CM, Song HD, Zhang MX, Zhang Y. Overexpression of ACE2 enhances plaque stability in a rabbit model of atherosclerosis. Arterioscler Thromb Vasc Biol 28: 1270 –1276, 2008. 202. Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S. A novel angiotensin-converting enzymerelated carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res 87: E1–9, 2000. 203. Doobay MF, Talman LS, Obr TD, Tian X, Davisson RL, Lazartigues E. Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol 292: R373–R381, 2007. 204. Drukker A, Donoso VS, Linshaw MA, Bailie MD. Intrarenal distribution of renin in the developing rabbit. Pediatr Res 17: 762–765, 1983. 205. Du G, Huang P, Liang BT, Frohman MA. Phospholipase D2 localizes to the plasma membrane and regulates angiotensin II receptor endocytosis. Mol Biol Cell 15: 1024 –1030, 2004. 206. Dzau VJ. Theodore Cooper Lecture: tissue angiotensin and pathobiology of vascular disease: a unifying hypothesis. Hypertension 37: 1047–1052, 2001. 207. Dzau VJ, Herrmann HC. Hormonal control of angiotensinogen production. Life Sci 30: 577–584, 1982. 208. Dzau VJ, Ingelfinger J, Pratt RE, Ellison KE. Identification of renin and angiotensinogen messenger RNA sequences in mouse and rat brains. Hypertension 8: 544 –548, 1986. 209. Ebara T, Miura K, Okumura M, Matsuura T, Kim S, Yukimura T, Iwao H. Effect of adrenomedullin on renal hemodynamics and functions in dogs. Eur J Pharmacol 263: 69 –73, 1994. 210. Ebermann L, Spillmann F, Sidiropoulos M, Escher F, Heringer-Walther S, Schultheiss HP, Tschope C, Walther T. The angiotensin-(1–7) receptor agonist AVE0991 is cardioprotective in diabetic rats. Eur J Pharmacol 590: 276 –280, 2008. 211. Ecelbarger CA, Terris J, Hoyer JR, Nielsen S, Wade JB, Knepper MA. Localization and regulation of the rat renal Na⫹-K⫹-2Cl⫺ cotransporter, BSC-1. Am J Physiol Renal Fluid Electrolyte Physiol 271: F619 –F628, 1996. Physiol Rev • VOL 212. Egecioglu E, Andersson IJ, Bollano E, Palsdottir V, Gabrielsson BG, Kopchick JJ, Skott O, Bie P, Isgaard J, Bohlooly YM, Bergstrom G, Wickman A. Growth hormone receptor deficiency in mice results in reduced systolic blood pressure and plasma renin, increased aortic eNOS expression, and altered cardiovascular structure and function. Am J Physiol Endocrinol Metab 292: E1418 –E1425, 2007. 213. Ehmke H, Persson PB, Just A, Nafz B, Seyfarth M, Hackenthal E, Kirchheim HR. Physiological concentrations of ANP exert a dual regulatory influence on renin release in conscious dogs. Am J Physiol Regul Integr Comp Physiol 263: R529 –R536, 1992. 214. Ekker M, Tronik D, Rougeon F. Extra-renal transcription of the renin genes in multiple tissues of mice and rats. Proc Natl Acad Sci USA 86: 5155–5158, 1989. 215. El-Dahr SS, Yosipiv IV, Lewis L, Mitchell KD. Role of bradykinin B2 receptors in the developmental changes of renal hemodynamics in the neonatal rat. Am J Physiol Renal Fluid Electrolyte Physiol 269: F786 –F792, 1995. 216. Elias LA, Wang DD, Kriegstein AR. Gap junction adhesion is necessary for radial migration in the neocortex. Nature 448: 901– 907, 2007. 217. Ellison KE, Ingelfinger JR, Pivor M, Dzau VJ. Androgen regulation of rat renal angiotensinogen messenger RNA expression. J Clin Invest 83: 1941–1945, 1989. 218. Emori T, Hirata Y, Kanno K, Ohta K, Eguchi S, Imai T, Shichiri M, Marumo F. Endothelin-3 stimulates production of endothelium-derived nitric oxide via phosphoinositide breakdown. Biochem Biophys Res Commun 174: 228 –235, 1991. 219. Endemann D, Schweda F, Stubanus M, Ittner KP, Fischereder M, Kammerl MC, Kramer BK. Naftidrofuryl exerts antiserotonergic but no endothelin-receptor blocking effects in AS4.1 cells, juxtaglomerular cells and isolated perfused rat kidneys. J Cardiovasc Pharmacol 39: 1– 8, 2002. 220. Esther CR Jr, Howard TE, Marino EM, Goddard JM, Capecchi MR, Bernstein KE. Mice lacking angiotensin-converting enzyme have low blood pressure, renal pathology, and reduced male fertility. Lab Invest 74: 953–965, 1996. 221. Evequoz D, Aubert JF, Nussberger J, Biollaz J, Diezi J, Brunner HR, Waeber B. Effects of neuropeptide Y on intrarenal hemodynamics, plasma renin activity and urinary sodium excretion in rats. Nephron 73: 467– 472, 1996. 222. Ferrario CM, Chappell MC, Dean RH, Iyer SN. Novel angiotensin peptides regulate blood pressure, endothelial function, and natriuresis. J Am Soc Nephrol 9: 1716 –1722, 1998. 223. Ferrario CM, Trask AJ, Jessup JA. Advances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1–7) in regulation of cardiovascular function. Am J Physiol Heart Circ Physiol 289: H2281–H2290, 2005. 224. Ferreira AJ, Santos RA. Cardiovascular actions of angiotensin(1–7). Braz J Med Biol Res 38: 499 –507, 2005. 225. Ferreira AJ, Santos RA, Almeida AP. Angiotensin-(1–7) improves the post-ischemic function in isolated perfused rat hearts. Braz J Med Biol Res 35: 1083–1090, 2002. 226. Ferreira AJ, Santos RA, Almeida AP. Angiotensin-(1–7): cardioprotective effect in myocardial ischemia/reperfusion. Hypertension 38: 665– 668, 2001. 227. Ferrier CP, Kurtz A, Lehner P, Shaw SG, Pusterla C, Saxenhofer H, Weidmann P. Stimulation of renin secretion by potassium-channel activation with cromakalim. Eur J Clin Pharmacol 36: 443– 447, 1989. 228. Field LJ, Gross KW. Ren-1 and Ren-2 loci are expressed in mouse kidney. Proc Natl Acad Sci USA 82: 6196 – 6200, 1985. 229. Field LJ, McGowan RA, Dickinson DP, Gross KW. Tissue and gene specificity of mouse renin expression. Hypertension 6: 597– 603, 1984. 230. Fitzsimons JT. Angiotensin, thirst, and sodium appetite. Physiol Rev 78: 583– 686, 1998. 231. Flordal PA, Ljungstrom KG. Renal effects of high dose desmopressin and dextran. A study in normal subjects and in patients undergoing total hip replacement. Acta Anaesthesiol Scand 37: 1– 6, 1993. 90 • APRIL 2010 • www.prv.org PHYSIOLOGY OF KIDNEY RENIN 231a.Forhead AJ, Broughton Pipkin A, Fowden AL. Effect of cortisol on blood pressure and the renin-angiotensin system in fetal sheep during late gestation. J Physiol 526: 167–176, 2000. 232. Freedman DS, Khan LK, Serdula MK, Galuska DA, Dietz WH. Trends and correlates of class 3 obesity in the United States from 1990 through 2000. JAMA 288: 1758 –1761, 2002. 233. Freeman EJ, Chisolm GM, Ferrario CM, Tallant EA. Angiotensin-(1–7) inhibits vascular smooth muscle cell growth. Hypertension 28: 104 –108, 1996. 234. Fregly MJ, Thrasher TN. Response of heart rate to acute administration of isoproterenol in rats treated chronically with norethynodrel, ethinyl estradiol, and both combined. Endocrinology 100: 148 –154, 1977. 235. Friis UG, Jensen BL, Aas JK, Skott O. Direct demonstration of exocytosis and endocytosis in single mouse juxtaglomerular cells. Circ Res 84: 929 –936, 1999. 236. Friis UG, Jensen BL, Jorgensen F, Andreasen D, Skott O. Electrophysiology of the renin-producing juxtaglomerular cells. Nephrol Dial Transplant 20: 1287–1290, 2005. 237. Friis UG, Jensen BL, Sethi S, Andreasen D, Hansen PB, Skott O. Control of renin secretion from rat juxtaglomerular cells by cAMP-specific phosphodiesterases. Circ Res 90: 996 –1003, 2002. 238. Friis UG, Jorgensen F, Andreasen D, Jensen BL, Skott O. Membrane potential and cation channels in rat juxtaglomerular cells. Acta Physiol Scand 181: 391–396, 2004. 239. Friis UG, Jorgensen F, Andreasen D, Jensen BL, Skott O. Molecular and functional identification of cAMP-sensitive BKCa potassium channels (ZERO variant) and L-type voltage-dependent calcium channels in single rat juxtaglomerular cells. Circ Res 93: 213–220, 2003. 240. Friis UG, Madsen K, Svenningsen P, Hansen PB, Gulaveerasingam A, Jorgensen F, Aalkjaer C, Skott O, Jensen BL. Hypotonicity-induced renin exocytosis from juxtaglomerular cells requires aquaporin-1 and cyclooxygenase-2. J Am Soc Nephrol 20: 2154 –2161, 2009. 241. Friis UG, Stubbe J, Uhrenholt TR, Svenningsen P, Nusing RM, Skott O, Jensen BL. Prostaglandin E2 EP2 and EP4 receptor activation mediates cAMP-dependent hyperpolarization and exocytosis of renin in juxtaglomerular cells. Am J Physiol Renal Physiol 289: F989 –F997, 2005. 242. Frojdo S, Sjolind L, Parkkonen M, Makinen VP, Kilpikari R, Pettersson-Fernholm K, Forsblom C, Fagerudd J, Tikellis C, Cooper ME, Wessman M, Groop PH. Polymorphisms in the gene encoding angiotensin I converting enzyme 2 and diabetic nephropathy. Diabetologia 48: 2278 –2281, 2005. 243. Fuchs S, Philippe J, Corvol P, Pinet F. Implication of Ref-1 in the repression of renin gene transcription by intracellular calcium. J Hypertens 21: 327–335, 2003. 244. Fujino T, Nakagawa N, Yuhki K, Hara A, Yamada T, Takayama K, Kuriyama S, Hosoki Y, Takahata O, Taniguchi T, Fukuzawa J, Hasebe N, Kikuchi K, Narumiya S, Ushikubi F. Decreased susceptibility to renovascular hypertension in mice lacking the prostaglandin I2 receptor IP. J Clin Invest 114: 805– 812, 2004. 245. Fukamizu A, Nishi K, Cho T, Saitoh M, Nakayama K, Ohkubo H, Nakanishi S, Murakami K. Structure of the rat renin gene. J Mol Biol 201: 443– 450, 1988. 246. Fukamizu A, Seo MS, Hatae T, Yokoyama M, Nomura T, Katsuki M, Murakami K. Tissue-specific expression of the human renin gene in transgenic mice. Biochem Biophys Res Commun 165: 826 – 832, 1989. 247. Fukuhara M, Tsuchihashi T, Abe I, Fujishima M. Cardiovascular and neurohormonal effects of intravenous adrenomedullin in conscious rabbits. Am J Physiol Regul Integr Comp Physiol 269: R1289 –R1293, 1995. 248. Furchgott RF. Role of endothelium in responses of vascular smooth muscle. Circ Res 53: 557–573, 1983. 249. Furuhashi M, Ura N, Higashiura K, Murakami H, Tanaka M, Moniwa N, Yoshida D, Shimamoto K. Blockade of the reninangiotensin system increases adiponectin concentrations in patients with essential hypertension. Hypertension 42: 76 – 81, 2003. 250. Fuson AL, Komlosi P, Unlap TM, Bell PD, Peti-Peterdi J. Immunolocalization of a microsomal prostaglandin E synthase in rabbit kidney. Am J Physiol Renal Physiol 285: F558 –F564, 2003. Physiol Rev • VOL 655 251. Gainer JV, Morrow JD, Loveland A, King DJ, Brown NJ. Effect of bradykinin-receptor blockade on the response to angiotensinconverting-enzyme inhibitor in normotensive and hypertensive subjects. N Engl J Med 339: 1285–1292, 1998. 252. Gallagher PE, Li P, Lenhart JR, Chappell MC, Brosnihan KB. Estrogen regulation of angiotensin-converting enzyme mRNA. Hypertension 33: 323–328, 1999. 253. Gambaryan S, Hausler C, Markert T, Pohler D, Jarchau T, Walter U, Haase W, Kurtz A, Lohmann SM. Expression of type II cGMP-dependent protein kinase in rat kidney is regulated by dehydration and correlated with renin gene expression. J Clin Invest 98: 662– 670, 1996. 254. Gambaryan S, Wagner C, Smolenski A, Walter U, Poller W, Haase W, Kurtz A, Lohmann SM. Endogenous or overexpressed cGMP-dependent protein kinases inhibit cAMP-dependent renin release from rat isolated perfused kidney, microdissected glomeruli, and isolated juxtaglomerular cells. Proc Natl Acad Sci USA 95: 9003–9008, 1998. 255. Ganten D, Minnich JL, Granger P, Hayduk K, Brecht HM, Barbeau A, Boucher R, Genest J. Angiotensin-forming enzyme in brain tissue. Science 173: 64 – 65, 1971. 256. Ganten U, Schroder G, Witt M, Zimmermann F, Ganten D, Stock G. Sexual dimorphism of blood pressure in spontaneously hypertensive rats: effects of anti-androgen treatment. J Hypertens 7: 721–726, 1989. 257. Garcia-Suarez O, Gonzalez-Martinez T, Germana A, Monjil DF, Torrecilla JR, Laura R, Silos-Santiago I, Guate JL, Vega JA. Expression of TrkB in the murine kidney. Microsc Res Tech 69: 1014 –1020, 2006. 258. Gasc JM, Shanmugam S, Sibony M, Corvol P. Tissue-specific expression of type 1 angiotensin II receptor subtypes. An in situ hybridization study. Hypertension 24: 531–537, 1994. 259. Gembardt F, Sterner-Kock A, Imboden H, Spalteholz M, Reibitz F, Schultheiss HP, Siems WE, Walther T. Organ-specific distribution of ACE2 mRNA and correlating peptidase activity in rodents. Peptides 26: 1270 –1277, 2005. 260. Gerber JG, Nies AS. The role of histamine receptors in the release of renin. Br J Pharmacol 79: 57– 61, 1983. 261. Germain A, Buysse DJ, Shear MK, Fayyad R, Austin C. Clinical correlates of poor sleep quality in posttraumatic stress disorder. J Trauma Stress 17: 477– 484, 2004. 262. Germain S, Bonnet F, Fuchs S, Philippe J, Corvol P, Pinet F. Dissection of silencer elements in first intron controlling the human renin gene. J Hypertens 17: 899 –905, 1999. 263. Germain S, Bonnet F, Philippe J, Fuchs S, Corvol P, Pinet F. A novel distal enhancer confers chorionic expression on the human renin gene. J Biol Chem 273: 25292–25300, 1998. 264. Germain S, Konoshita T, Fuchs S, Philippe J, Corvol P, Pinet F. Regulation of human renin gene transcription by cAMP. Clin Exp Hypertens 19: 543–550, 1997. 265. Germain S, Konoshita T, Philippe J, Corvol P, Pinet F. Transcriptional induction of the human renin gene by cAMP requires cAMP response element-binding protein (CREB) and a factor binding a pituitary-specific trans -acting factor (Pit-1) motif. Biochem J 316: 107–113, 1996. 266. Giani JF, Gironacci MM, Munoz MC, Pena C, Turyn D, Dominici FP. Angiotensin-(1–7) stimulates the phosphorylation of JAK2, IRS-1 and Akt in rat heart in vivo: role of the AT1 and Mas receptors. Am J Physiol Heart Circ Physiol 293: H1154 –H1163, 2007. 267. Gibson RE, Thorpe HH, Cartwright ME, Frank JD, Schorn TW, Bunting PB, Siegl PK. Angiotensin II receptor subtypes in renal cortex of rats and rhesus monkeys. Am J Physiol Renal Fluid Electrolyte Physiol 261: F512–F518, 1991. 268. Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev 81: 629 – 683, 2001. 269. Ginesi LM, Munday KA, Noble AR. Secretion control for active and inactive renin: effects of calcium and potassium on rabbit kidney cortex slices. J Physiol 344: 453– 463, 1983. 269a.Glenn ST, Jones CA, Pan L, Gross KW. In vivo analysis of key elements within the renin regulatory region. Physiol Genomics 35: 243–253, 2008. 90 • APRIL 2010 • www.prv.org 656 CASTROP ET AL. 270. Glorioso N, Atlas SA, Laragh JH, Jewelewicz R, Sealey JE. Prorenin in high concentrations in human ovarian follicular fluid. Science 233: 1422–1424, 1986. 271. Golin R, Pieruzzi F, Munforti C, Busca G, Di Blasio A, Zanchetti A. Role of the renal nerves in the control of renin synthesis during different sodium intakes in the rat. J Hypertens 19: 1271–1277, 2001. 272. Gomez RA, Lynch KR, Chevalier RL, Everett AD, Johns DW, Wilfong N, Peach MJ, Carey RM. Renin and angiotensinogen gene expression and intrarenal renin distribution during ACE inhibition. Am J Physiol Renal Fluid Electrolyte Physiol 254: F900 – F906, 1988. 273. Gomez RA, Lynch KR, Sturgill BC, Elwood JP, Chevalier RL, Carey RM, Peach MJ. Distribution of renin mRNA and its protein in the developing kidney. Am J Physiol Renal Fluid Electrolyte Physiol 257: F850 –F858, 1989. 274. Gonzalez CB, Figueroa CD. Vasopressin and bradykinin receptors in the kidney: implications for tubular function. Biol Res 32: 63–76, 1999. 275. Graham PC, Kingdom JC, Raweily EA, Gibson AA, Lindop GB. Distribution of renin-containing cells in the developing human kidney: an immunocytochemical study. Br J Obstet Gynaecol 99: 765–769, 1992. 276. Greenberg SG, He XR, Schnermann JB, Briggs JP. Effect of nitric oxide on renin secretion. I. Studies in isolated juxtaglomerular granular cells. Am J Physiol Renal Fluid Electrolyte Physiol 268: F948 –F952, 1995. 277. Greenberg SG, Lorenz JN, He XR, Schnermann JB, Briggs JP. Effect of prostaglandin synthesis inhibition on macula densa-stimulated renin secretion. Am J Physiol Renal Physiol 265: F578 – F583, 1993. 278. Gregory LC, Reid IA. Effect of renal denervation on the suppression of renin secretion by vasopressin in conscious dogs. Am J Physiol Renal Fluid Electrolyte Physiol 247: F881–F887, 1984. 279. Griendling KK, Delafontaine P, Rittenhouse SE, Gimbrone MA Jr, Alexander RW. Correlation of receptor sequestration with sustained diacylglycerol accumulation in angiotensin II-stimulated cultured vascular smooth muscle cells. J Biol Chem 262: 14555– 14562, 1987. 280. Griendling KK, Lassegue B, Alexander RW. Angiotensin receptors and their therapeutic implications. Annu Rev Pharmacol Toxicol 36: 281–306, 1996. 281. Grobe JL, Mecca AP, Lingis M, Shenoy V, Bolton TA, Machado JM, Speth RC, Raizada MK, Katovich MJ. Prevention of angiotensin II-induced cardiac remodeling by angiotensin-(1–7). Am J Physiol Heart Circ Physiol 292: H736 –H742, 2007. 282. Grobe JL, Mecca AP, Mao H, Katovich MJ. Chronic angiotensin(1–7) prevents cardiac fibrosis in DOCA-salt model of hypertension. Am J Physiol Heart Circ Physiol 290: H2417–H2423, 2006. 283. Grünberger C, Obermayer B, Klar J, Kurtz A, Schweda F. The calcium paradoxon of renin release: calcium suppresses renin exocytosis by inhibition of calcium-dependent adenylate cyclases AC5 and AC6. Circ Res 99: 1197–1206, 2006. 284. Gunther S, Gimbrone MA Jr, Alexander RW. Regulation by angiotensin II of its receptors in resistance blood vessels. Nature 287: 230 –232, 1980. 285. Guo DF, Chenier I, Lavoie JL, Chan JS, Hamet P, Tremblay J, Chen XM, Wang DH, Inagami T. Development of hypertension and kidney hypertrophy in transgenic mice overexpressing ARAP1 gene in the kidney. Hypertension 48: 453– 459, 2006. 286. Guo DF, Tardif V, Ghelima K, Chan JS, Ingelfinger JR, Chen X, Chenier I. A novel angiotensin II type 1 receptor-associated protein induces cellular hypertrophy in rat vascular smooth muscle and renal proximal tubular cells. J Biol Chem 279: 21109 –21120, 2004. 287. Gupta A, Rhodes GJ, Berg DT, Gerlitz B, Molitoris BA, Grinnell BW. Activated protein C ameliorates LPS-induced acute kidney injury and downregulates renal INOS and angiotensin 2. Am J Physiol Renal Physiol 293: F245–F254, 2007. 288. Gurley SB, Allred A, Le TH, Griffiths R, Mao L, Philip N, Haystead TA, Donoghue M, Breitbart RE, Acton SL, Rockman HA, Coffman TM. Altered blood pressure responses and normal Physiol Rev • VOL 289. 290. 291. 292. 293. 294. 295. 296. 297. 298. 299. 300. 301. 302. 303. 304. 305. 306. 307. 308. 309. 310. cardiac phenotype in ACE2-null mice. J Clin Invest 116: 2218 –2225, 2006. Gurley SB, Clare SE, Snow KP, Hu A, Meyer TW, Coffman TM. Impact of genetic background on nephropathy in diabetic mice. Am J Physiol Renal Physiol 290: F214 –F222, 2006. Gustafsson F, Mikkelsen HB, Arensbak B, Thuneberg L, Neve S, Jensen LJ, Holstein-Rathlou NH. Expression of connexin 37, 40 and 43 in rat mesenteric arterioles and resistance arteries. Histochem Cell Biol 119: 139 –148, 2003. Guyton AC. Blood pressure control: special role of the kidneys and body fluids. Science 252: 1813–1816, 1991. Hackenthal E, Aktories K, Jakobs KH, Lang RE. Neuropeptide Y inhibits renin release by a pertussis toxin-sensitive mechanism. Am J Physiol Renal Fluid Electrolyte Physiol 252: F543–F550, 1987. Hackenthal E, Paul M, Ganten D, Taugner R. Morphology, physiology, and molecular biology of renin secretion. Physiol Rev 70: 1067–1116, 1990. Hadengue A, Gadano A, Moreau R, Giostra E, Durand F, Valla D, Erlinger S, Lebrec D. Beneficial effects of the 2-day administration of terlipressin in patients with cirrhosis and hepatorenal syndrome. J Hepatol 29: 565–570, 1998. Haefliger JA, Krattinger N, Martin D, Pedrazzini T, Capponi A, Doring B, Plum A, Charollais A, Willecke K, Meda P. Connexin43-dependent mechanism modulates renin secretion and hypertension. J Clin Invest 116: 405– 413, 2006. Haefliger JA, Nicod P, Meda P. Contribution of connexins to the function of the vascular wall. Cardiovasc Res 62: 345–356, 2004. Hagner S, Stahl U, Knoblauch B, McGregor GP, Lang RE. Calcitonin receptor-like receptor: identification and distribution in human peripheral tissues. Cell Tissue Res 310: 41–50, 2002. Hainsworth R. Reflexes from the heart. Physiol Rev 71: 617– 658, 1991. Hajj-Ali AF, Wong PC. Beta adrenoceptor blockade in rabbit inhibits the renin-releasing effect of AT1 receptor antagonist losartan. J Pharmacol Exp Ther 267: 1423–1427, 1993. Hall JE. Control of sodium excretion by angiotensin II: intrarenal mechanisms and blood pressure regulation. Am J Physiol Regul Integr Comp Physiol 250: R960 –R972, 1986. Hall JE. The kidney, hypertension, and obesity. Hypertension 41: 625– 633, 2003. Hall JE, Brands MW, Henegar JR. Angiotensin II and long-term arterial pressure regulation: the overriding dominance of the kidney. J Am Soc Nephrol 10 Suppl 12: S258 –265, 1999. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 203: 631– 637, 2004. Han DP, Penn-Nicholson A, Cho MW. Identification of critical determinants on ACE2 for SARS-CoV entry and development of a potent entry inhibitor. Virology 350: 15–25, 2006. Handa RK, Ferrario CM, Strandhoy JW. Renal actions of angiotensin-(1–7): in vivo and in vitro studies. Am J Physiol Renal Fluid Electrolyte Physiol 270: F141–F147, 1996. Hanner F, Chambrey R, Bourgeois S, Meer E, Mucsi I, Rosivall L, Shull GE, Lorenz JN, Eladari D, Peti-Peterdi J. Increased renal renin content in mice lacking the Na⫹/H⫹ exchanger NHE2. Am J Physiol Renal Physiol 294: F937–F944, 2008. Hano T, Shiotani M, Baba A, Nishio I, Masuyama Y. DA1 receptor-mediated renin release from isolated rat glomeruli. Hypertens Res 18 Suppl 1: S141–143, 1995. Hansen PB, Castrop H, Briggs J, Schnermann J. Adenosine induces vasoconstriction through Gi-dependent activation of phospholipase C in isolated perfused afferent arterioles of mice. J Am Soc Nephrol 14: 2457–2465, 2003. Hansen PB, Yang T, Huang Y, Mizel D, Briggs J, Schnermann J. Plasma renin in mice with one or two renin genes. Acta Physiol Scand 181: 431– 437, 2004. Hansen TK, Moller J, Thomsen K, Frandsen E, Dall R, Jorgensen JO, Christiansen JS. Effects of growth hormone on renal tubular handling of sodium in healthy humans. Am J Physiol Endocrinol Metab 281: E1326 –E1332, 2001. 90 • APRIL 2010 • www.prv.org PHYSIOLOGY OF KIDNEY RENIN 311. Harding P, Carretero OA, Beierwaltes WH. Chronic cyclooxygenase-2 inhibition blunts low sodium-stimulated renin without changing renal haemodynamics. J Hypertens 18: 1107–1113, 2000. 312. Harding P, Sigmon DH, Alfie ME, Huang PL, Fishman MC, Beierwaltes WH, Carretero OA. Cyclooxygenase-2 mediates increased renal renin content induced by low- sodium diet. Hypertension 29: 297–302, 1997. 313. Hardman JA, Hort YJ, Catanzaro DF, Tellam JT, Baxter JD, Morris BJ, Shine J. Primary structure of the human renin gene. DNA 3: 457– 468, 1984. 314. Harris PJ, Thomas D, Morgan TO. Atrial natriuretic peptide inhibits angiotensin-stimulated proximal tubular sodium and water reabsorption. Nature 326: 697– 698, 1987. 315. Harris RC, McKanna JA, Akai Y, Jacobson HR, Dubois RN, Breyer MD. Cyclooxygenase-2 is associated with the macula densa of rat kidney and increases with salt restriction. J Clin Invest 94: 2504 –2510, 1994. 316. Harrison-Bernard LM, Navar LG, Ho MM, Vinson GP, el-Dahr SS. Immunohistochemical localization of ANG II AT1 receptor in adult rat kidney using a monoclonal antibody. Am J Physiol Renal Physiol 273: F170 –F177, 1997. 317. Harshfield GA, Alpert BS, Pulliam DA, Somes GW, Wilson DK. Ambulatory blood pressure recordings in children and adolescents. Pediatrics 94: 180 –184, 1994. 318. Hartner A, Cordasic N, Goppelt-Struebe M, Veelken R, Hilgers KF. Role of macula densa cyclooxygenase-2 in renovascular hypertension. Am J Physiol Renal Physiol 284: F498 –F502, 2003. 319. Hasunuma K, Yamada K, Tamura Y, Yoshida S. Cardiovascular and renin responses to desmopressin in humans: role of prostacyclin and beta-adrenergic systems. Am J Physiol Heart Circ Physiol 260: H1031–H1036, 1991. 320. Hauger-Klevene JH, De Vito E, Fasciolo JC. The effect of thyroid hormone on renin production and release by rat kidney slices. Acta Physiol Lat Am 27: 37– 41, 1977. 321. Haulica I, Bild W, Serban DN. Angiotensin peptides and their pleiotropic actions. J Renin Angiotensin Aldosterone Syst 6: 121– 131, 2005. 322. Hautmann M, Friis UG, Desch M, Todorov V, Castrop H, Segerer F, Otto C, Schutz G, Schweda F. Pituitary adenylate cyclase-activating polypeptide stimulates renin secretion via activation of PAC1 receptors. J Am Soc Nephrol 18: 1150 –1156, 2007. 323. Haynes WG, Sivitz WI, Morgan DA, Walsh SA, Mark AL. Sympathetic and cardiorenal actions of leptin. Hypertension 30: 619 – 623, 1997. 324. He W, Miao FJ, Lin DC, Schwandner RT, Wang Z, Gao J, Chen JL, Tian H, Ling L. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature 429: 188 –193, 2004. 325. He XR, Greenberg SG, Briggs JP, Schnermann J. Effects of furosemide and verapamil on the NaCl dependency of macula densa-mediated renin secretion. Hypertension 26: 137–142, 1995. 326. He XR, Greenberg SG, Briggs JP, Schnermann JB. Effect of nitric oxide on renin secretion. II. Studies in the perfused juxtaglomerular apparatus. Am J Physiol Renal Fluid Electrolyte Physiol 268: F953–F959, 1995. 327. Healy DP, Munzel PA, Insel PA. Localization of beta 1- and beta 2-adrenergic receptors in rat kidney by autoradiography. Circ Res 57: 278 –284, 1985. 328. Hein L, Barsh GS, Pratt RE, Dzau VJ, Kobilka BK. Behavioural and cardiovascular effects of disrupting the angiotensin II type-2 receptor in mice. Nature 377: 744 –747, 1995. 329. Hein L, Meinel L, Pratt RE, Dzau VJ, Kobilka BK. Intracellular trafficking of angiotensin II and its AT1 and AT2 receptors: evidence for selective sorting of receptor and ligand. Mol Endocrinol 11: 1266 –1277, 1997. 330. Hellner K, Walther T, Schubert M, Albrecht D. Angiotensin(1–7) enhances LTP in the hippocampus through the G-proteincoupled receptor Mas. Mol Cell Neurosci 29: 427– 435, 2005. 331. Henrich WL, McAlister EA, Smith PB, Lipton J, Campbell WB. Direct inhibitory effect of atriopeptin III on renin release in primate kidney. Life Sci 41: 259 –264, 1987. 332. Henrich WL, McAllister EA, Smith PB, Campbell WB. Guanosine 3⬘,5⬘-cyclic monophosphate as a mediator of inhibition Physiol Rev • VOL 333. 334. 335. 336. 337. 338. 339. 340. 341. 342. 343. 344. 345. 346. 347. 348. 349. 350. 351. 657 of renin release. Am J Physiol Renal Fluid Electrolyte Physiol 255: F474 –F478, 1988. Herlitz H, Jonsson O, Bengtsson BA. Effect of recombinant human growth hormone on cellular sodium metabolism. Clin Sci 86: 233–237, 1994. Hesse B, Nielsen I. Suppression of plasma renin activity by intravenous infusion of antidiuretic hormone in man. Clin Sci Mol Med 52: 357–360, 1977. Hesse IF, Johns EJ. The role of alpha-adrenoceptors in the regulation of renal tubular sodium reabsorption and renin secretion in the rabbit. Br J Pharmacol 84: 715–724, 1985. Hilchey SD, Bell-Quilley CP. Association between the natriuretic action of angiotensin-(1–7) and selective stimulation of renal prostaglandin I2 release. Hypertension 25: 1238 –1244, 1995. Hirata Y, Emori T, Eguchi S, Kanno K, Imai T, Ohta K, Marumo F. Endothelin receptor subtype B mediates synthesis of nitric oxide by cultured bovine endothelial cells. J Clin Invest 91: 1367–1373, 1993. Hirooka Y, Potts PD, Dampney RA. Role of angiotensin II receptor subtypes in mediating the sympathoexcitatory effects of exogenous and endogenous angiotensin peptides in the rostral ventrolateral medulla of the rabbit. Brain Res 772: 107–114, 1997. Hirota N, Ichihara A, Koura Y, Hayashi M, Saruta T. Phospholipase D contributes to transmural pressure control of prorenin processing in juxtaglomerular cell. Hypertension 39: 363–367, 2002. Ho KY, Weissberger AJ. The antinatriuretic action of biosynthetic human growth hormone in man involves activation of the renin-angiotensin system. Metabolism 39: 133–137, 1990. Hobart PM, Fogliano M, O’Connor BA, Schaefer IM, Chirgwin JM. Human renin gene: structure and sequence analysis. Proc Natl Acad Sci USA 81: 5026 –5030, 1984. Hocherl K, Kammerl M, Kees F, Kramer BK, Grobecker HF, Kurtz A. Role of renal nerves in stimulation of renin, COX-2, nNOS in rat renal cortex during salt deficiency. Am J Physiol Renal Physiol 282: F478 –F484, 2002. Hocherl K, Kammerl MC, Schumacher K, Endemann D, Grobecker HF, Kurtz A. Role of prostanoids in regulation of the renin-angiotensin-aldosterone system by salt intake. Am J Physiol Renal Physiol 283: F294 –F301, 2002. Hocherl K, Kees F, Kramer BK, Kurtz A. Cyclosporine A attenuates the natriuretic action of loop diuretics by inhibition of renal COX-2 expression. Kidney Int 65: 2071–2080, 2004. Hocherl K, Schmidt C, Kurt B, Bucher M. Activation of the PGI(2)/IP system contributes to the development of circulatory failure in a rat model of endotoxic shock. Hypertension 52: 330 – 335, 2008. Hocherl K, Wolf K, Castrop H, Ittner KP, Bucher M, Kees F, Grobecker HF, Kurtz A. Renocortical expression of renin and of cyclooxygenase-2 in response to angiotensin II AT1 receptor blockade is closely coordinated but not causally linked. Pflügers Arch 442: 821– 827, 2001. Hoffman DM, Crampton L, Sernia C, Nguyen TV, Ho KK. Short-term growth hormone (GH) treatment of GH-deficient adults increases body sodium and extracellular water, but not blood pressure. J Clin Endocrinol Metab 81: 1123–1128, 1996. Hofmann H, Pyrc K, van der Hoek L, Geier M, Berkhout B, Pohlmann S. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proc Natl Acad Sci USA 102: 7988 –7993, 2005. Holdaas H, DiBona GF, Kiil F. Effect of low-level renal nerve stimulation on renin release from nonfiltering kidneys. Am J Physiol Renal Fluid Electrolyte Physiol 241: F156 –F161, 1981. Holdaas H, Langaard O, Eide I, Kiil F. Conditions for enhancement of renin release by isoproterenol, dopamine, and glucagon. Am J Physiol Renal Fluid Electrolyte Physiol 242: F267–F273, 1982. Holla VR, Adas F, Imig JD, Zhao X, Price E Jr, Olsen N, Kovacs WJ, Magnuson MA, Keeney DS, Breyer MD, Falck JR, Waterman MR, Capdevila JH. Alterations in the regulation of androgen-sensitive Cyp 4a monooxygenases cause hypertension. Proc Natl Acad Sci USA 98: 5211–5216, 2001. 90 • APRIL 2010 • www.prv.org 658 CASTROP ET AL. 352. Holm I, Ollo R, Panthier JJ, Rougeon F. Evolution of aspartyl proteases by gene duplication: the mouse renin gene is organized in two homologous clusters of four exons. EMBO J 3: 557–562, 1984. 353. Holmer S, Eckardt KU, LeHir M, Schricker K, Riegger G, Kurtz A. Influence of dietary NaCl intake on renin gene expression in the kidneys and adrenal glands of rats. Pflügers Arch 425: 62– 67, 1993. 354. Holmer S, Rinne B, Eckardt KU, Le Hir M, Schricker K, Kaissling B, Riegger G, Kurtz A. Role of renal nerves for the expression of renin in adult rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 266: F738 –F745, 1994. 355. Holmer SR, Kaissling B, Putnik K, Pfeifer M, Kramer BK, Riegger GA, Kurtz A. Beta-adrenergic stimulation of renin expression in vivo. J Hypertens 15: 1471–1479, 1997. 356. Horiuchi M, Nakamura N, Tang SS, Barrett G, Dzau VJ. Molecular mechanism of tissue-specific regulation of mouse renin gene expression by cAMP. Identification of an inhibitory protein that binds nuclear transcriptional factor. J Biol Chem 266: 16247– 16254, 1991. 357. Horiuchi M, Pratt RE, Nakamura N, Dzau VJ. Distinct nuclear proteins competing for an overlapping sequence of cyclic adenosine monophosphate and negative regulatory elements regulate tissue-specific mouse renin gene expression. J Clin Invest 92: 1805–1811, 1993. 358. Houseley J, Tollervey D. The many pathways of RNA degradation. Cell 136: 763–776, 2009. 359. Huang A, Kaley G. Gender-specific regulation of cardiovascular function: estrogen as key player. Microcirculation 11: 9 –38, 2004. 360. Huang W, Sjoquist M, Skott O, Stricker EM, Sved AF. Oxytocin-induced renin secretion in conscious rats. Am J Physiol Regul Integr Comp Physiol 278: R226 –R230, 2000. 361. Huang W, Sjoquist M, Skott O, Stricker EM, Sved AF. Oxytocin antagonist disrupts hypotension-evoked renin secretion and other responses in conscious rats. Am J Physiol Regul Integr Comp Physiol 280: R760 –R765, 2001. 362. Huang Y, Wongamorntham S, Kasting J, McQuillan D, Owens RT, Yu L, Noble NA, Border W. Renin increases mesangial cell transforming growth factor-beta1 and matrix proteins through receptor-mediated, angiotensin II-independent mechanisms. Kidney Int 69: 105–113, 2006. 363. Hutchison FN, Cui X, Webster SK. The antiproteinuric action of angiotensin-converting enzyme is dependent on kinin. J Am Soc Nephrol 6: 1216 –1222, 1995. 364. Hwan Seul K, Beyer EC. Heterogeneous localization of connexin40 in the renal vasculature. Microvasc Res 59: 140 –148, 2000. 365. Ichihara A, Hayashi M, Hirota N, Okada H, Koura Y, Tada Y, Kaneshiro Y, Tsuganezawa H, Saruta T. Angiotensin II type 2 receptor inhibits prorenin processing in juxtaglomerular cells. Hypertens Res 26: 915–921, 2003. 366. Ichihara A, Hayashi M, Kaneshiro Y, Suzuki F, Nakagawa T, Tada Y, Koura Y, Nishiyama A, Okada H, Uddin MN, Nabi AH, Ishida Y, Inagami T, Saruta T. Inhibition of diabetic nephropathy by a decoy peptide corresponding to the “handle” region for nonproteolytic activation of prorenin. J Clin Invest 114: 1128 –1135, 2004. 367. Ichihara A, Kobori H, Miyashita Y, Hayashi M, Saruta T. Differential effects of thyroid hormone on renin secretion, content, and mRNA in juxtaglomerular cells. Am J Physiol Endocrinol Metab 274: E224 –E231, 1998. 368. Ichihara A, Suzuki F, Nakagawa T, Kaneshiro Y, Takemitsu T, Sakoda M, Nabi AH, Nishiyama A, Sugaya T, Hayashi M, Inagami T. Prorenin receptor blockade inhibits development of glomerulosclerosis in diabetic angiotensin II type 1a receptor-deficient mice. J Am Soc Nephrol 17: 1950 –1961, 2006. 369. Ichihara A, Suzuki H, Murakami M, Naitoh M, Matsumoto A, Saruta T. Interactions between angiotensin II and norepinephrine on renin release by juxtaglomerular cells. Eur J Endocrinol 133: 569 –577, 1995. 370. Ichiki T, Labosky PA, Shiota C, Okuyama S, Imagawa Y, Fogo A, Niimura F, Ichikawa I, Hogan BL, Inagami T. Effects on blood pressure and exploratory behaviour of mice lacking angiotensin II type-2 receptor. Nature 377: 748 –750, 1995. Physiol Rev • VOL 371. Ichiki T, Usui M, Kato M, Funakoshi Y, Ito K, Egashira K, Takeshita A. Downregulation of angiotensin II type 1 receptor gene transcription by nitric oxide. Hypertension 31: 342–348, 1998. 372. Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, Crackower MA, Fukamizu A, Hui CC, Hein L, Uhlig S, Slutsky AS, Jiang C, Penninger JM. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 436: 112–116, 2005. 373. Imbs JL, Schmidt M, Schwartz J. Effect of dopamine on renin secretion in the anesthetized dog. Eur J Pharmacol 33: 151–157, 1975. 374. Imig JD, Zhao X, Orengo SR, Dipp S, El-Dahr SS. The Bradykinin B2 receptor is required for full expression of renal COX-2 and renin. Peptides 24: 1141–1147, 2003. 375. Inscho EW. P2 receptors in regulation of renal microvascular function. Am J Physiol Renal Physiol 280: F927–F944, 2001. 376. Inscho EW, Cook AK, Navar LG. Pressure-mediated vasoconstriction of juxtamedullary afferent arterioles involves P2-purinoceptor activation. Am J Physiol Renal Fluid Electrolyte Physiol 271: F1077–F1085, 1996. 377. Ishimitsu T, Hirata Y, Matsuoka H, Ishii M, Sugimoto T, Kangawa K, Matsuo H. In vivo and in vitro effects of atrial natriuretic peptide on renin release. Clin Exp Pharmacol Physiol 19: 711–716, 1992. 378. Ishiyama Y, Gallagher PE, Averill DB, Tallant EA, Brosnihan KB, Ferrario CM. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension 43: 970 –976, 2004. 379. Itani HA, Liu X, Pratt JH, Sigmund CD. Functional characterization of polymorphisms in the kidney enhancer of the human renin gene. Endocrinology 148: 1424 –1430, 2007. 380. Ito M, Oliverio MI, Mannon PJ, Best CF, Maeda N, Smithies O, Coffman TM. Regulation of blood pressure by the type 1A angiotensin II receptor gene. Proc Natl Acad Sci USA 92: 3521– 3525, 1995. 381. Itoh S, Carretero OA. Role of the macula densa in renin release. Hypertension 7: I49 –54, 1985. 382. Itoh S, Carretero OA, Murray RD. Possible role of adenosine in the macula densa mechanism of renin release in rabbits. J Clin Invest 76: 1412–1417, 1985. 383. Iwai N, Inagami T, Ohmichi N, Kinoshita M. Renin is expressed in rat macrophage/monocyte cells. Hypertension 27: 399 – 403, 1996. 384. Iwai N, Yamano Y, Chaki S, Konishi F, Bardhan S, Tibbetts C, Sasaki K, Hasegawa M, Matsuda Y, Inagami T. Rat angiotensin II receptor: cDNA sequence and regulation of the gene expression. Biochem Biophys Res Commun 177: 299 –304, 1991. 385. Iwashima Y, Katsuya T, Ishikawa K, Ouchi N, Ohishi M, Sugimoto K, Fu Y, Motone M, Yamamoto K, Matsuo A, Ohashi K, Kihara S, Funahashi T, Rakugi H, Matsuzawa Y, Ogihara T. Hypoadiponectinemia is an independent risk factor for hypertension. Hypertension 43: 1318 –1323, 2004. 386. Iwata M, Cowling RT, Gurantz D, Moore C, Zhang S, Yuan JX, Greenberg BH. Angiotensin-(1–7) binds to specific receptors on cardiac fibroblasts to initiate antifibrotic and antitrophic effects. Am J Physiol Heart Circ Physiol 289: H2356 –H2363, 2005. 387. Iwata T, Hiwada K, Kokubu T. Effects of renal denervation on renin release induced by intracerebroventricular administration of biological active peptides in conscious rats. Neuropeptides 6: 437– 443, 1985. 388. Jackson EK. Adenosine: a physiological brake on renin release. Annu Rev Pharmacol Toxicol 31: 1–35, 1991. 389. Jackson EK, Li P. Human leptin has natriuretic activity in the rat. Am J Physiol Renal Fluid Electrolyte Physiol 272: F333–F338, 1997. 390. Jackson EK, Zhu C, Tofovic SP. Expression of adenosine receptors in the preglomerular microcirculation. Am J Physiol Renal Physiol 283: F41–F51, 2002. 391. Jackson TR, Blair LA, Marshall J, Goedert M, Hanley MR. The mas oncogene encodes an angiotensin receptor. Nature 335: 437– 440, 1988. 90 • APRIL 2010 • www.prv.org PHYSIOLOGY OF KIDNEY RENIN 392. Jaffa AA, Vio C, Velarde V, LeRoith D, Mayfield RK. Induction of renal kallikrein and renin gene expression by insulin and IGF-I in the diabetic rat. Diabetes 46: 2049 –2056, 1997. 393. Jaiswal N, Diz DI, Chappell MC, Khosla MC, Ferrario CM. Stimulation of endothelial cell prostaglandin production by angiotensin peptides. Characterization of receptors. Hypertension 19: II49 –55, 1992. 394. Jaiswal N, Jaiswal RK, Tallant EA, Diz DI, Ferrario CM. Alterations in prostaglandin production in spontaneously hypertensive rat smooth muscle cells. Hypertension 21: 900 –905, 1993. 395. James GD, Sealey JE, Muller F, Alderman M, Madhavan S, Laragh JH. Renin relationship to sex, race and age in a normotensive population. J Hypertens Suppl 4: S387–S389, 1986. 396. Jensen BL, Gambaryan S, Scholz H, Kurtz A. KATP channels are not essential for pressure-dependent control of renin secretion. Pflügers Arch 435: 670 – 677, 1998. 397. Jensen BL, Kramer BK, Kurtz A. Adrenomedullin stimulates renin release and renin mRNA in mouse juxtaglomerular granular cells. Hypertension 29: 1148 –1155, 1997. 398. Jensen BL, Kurtz A. Differential regulation of renal cyclooxygenase mRNA by dietary salt intake. Kidney Int 52: 1242–1249, 1997. 399. Jensen BL, Lehle U, Muller M, Wagner C, Kurtz A. Interleukin-1 inhibits renin gene expression in As4.1 cells but not in native juxtaglomerular cells. Pflügers Arch 436: 673– 678, 1998. 400. Jensen BL, Rasch R, Nyengaard JR, Skott O. Giant renin secretory granules in beige mouse renal afferent arterioles. Cell Tissue Res 288: 399 – 406, 1997. 401. Jensen BL, Schmid C, Kurtz A. Prostaglandins stimulate renin secretion and renin mRNA in mouse renal juxtaglomerular cells. Am J Physiol Renal Physiol 271: F659 –F669, 1996. 403. Jensen BL, Skott O. Blockade of chloride channels by DIDS stimulates renin release and inhibits contraction of afferent arterioles. Am J Physiol Renal Fluid Electrolyte Physiol 270: F718 – F727, 1996. 404. Jessup JA, Gallagher PE, Averill DB, Brosnihan KB, Tallant EA, Chappell MC, Ferrario CM. Effect of angiotensin II blockade on a new congenic model of hypertension derived from transgenic Ren-2 rats. Am J Physiol Heart Circ Physiol 291: H2166 – H2172, 2006. 405. Johannessen A, Nielsen AH, Poulsen K. Measurement of inactive renin in rat plasma: effect of nephrectomy and sialoadenectomy on the plasma concentration. J Hypertens 8: 345–349, 1990. 406. Johns EJ. Angiotensin II in the brain and the autonomic control of the kidney. Exp Physiol 90: 163–168, 2005. 407. Johnson RA, Freeman RH. Renin release in rats during blockade of nitric oxide synthesis. Am J Physiol Regul Integr Comp Physiol 266: R1723–R1729, 1994. 408. Jost-Vu E, Horton R, Antonipillai I. Altered regulation of renin secretion by insulinlike growth factors and angiotensin II in diabetic rats. Diabetes 41: 1100 –1105, 1992. 409. Jougasaki M, Wei CM, Aarhus LL, Heublein DM, Sandberg SM, Burnett JC Jr. Renal localization and actions of adrenomedullin: a natriuretic peptide. Am J Physiol Renal Fluid Electrolyte Physiol 268: F657–F663, 1995. 410. Joyner J, Neves LA, Granger JP, Alexander BT, Merrill DC, Chappell MC, Ferrario CM, Davis WP, Brosnihan KB. Temporal-spatial expression of ANG-(1–7) and angiotensin-converting enzyme 2 in the kidney of normal and hypertensive pregnant rats. Am J Physiol Regul Integr Comp Physiol 293: R169 –R177, 2007. 411. Kageyama S, Brown J. Effect of atrial natriuretic peptide on renin release in rat isolated glomeruli. Biochem Biophys Res Commun 168: 37– 42, 1990. 412. Kai H, Griendling KK, Lassegue B, Ollerenshaw JD, Runge MS, Alexander RW. Agonist-induced phosphorylation of the vascular type 1 angiotensin II receptor. Hypertension 24: 523–527, 1994. 413. Kammerl MC, Grimm D, Nabel C, Schweda F, Bach M, Fredersdorf S, Piehler H, Holmer SR, Riegger GA, Kromer EP, Kramer BK. Effects of growth hormone on renal renin gene expression in normal rats and rats with myocardial infarction. Nephrol Dial Transplant 15: 786 –790, 2000. Physiol Rev • VOL 659 414. Kammerl MC, Nusing RM, Richthammer W, Kramer BK, Kurtz A. Inhibition of COX-2 counteracts the effects of diuretics in rats. Kidney Int 60: 1684 –1691, 2001. 415. Kammerl MC, Nusing RM, Schweda F, Endemann D, Stubanus M, Kees F, Lackner KJ, Fischereder M, Kramer BK. Low sodium and furosemide-induced stimulation of the renin system in man is mediated by cyclooxygenase 2. Clin Pharmacol Ther 70: 468 – 474, 2001. 416. Kaneshiro Y, Ichihara A, Takemitsu T, Sakoda M, Suzuki F, Nakagawa T, Hayashi M, Inagami T. Increased expression of cyclooxygenase-2 in the renal cortex of human prorenin receptor gene-transgenic rats. Kidney Int 70: 641– 646, 2006. 417. Kang JJ, Toma I, Sipos A, Meer EJ, Vargas SL, Peti-Peterdi J. The collecting duct is the major source of prorenin in diabetes. Hypertension 51: 1597–1604, 2008. 418. Kaplan MR, Plotkin MD, Brown D, Hebert SC, Delpire E. Expression of the mouse Na-K-2Cl cotransporter, mBSC2, in the terminal inner medullary collecting duct, the glomerular and extraglomerular mesangium, and the glomerular afferent arteriole. J Clin Invest 98: 723–730, 1996. 419. Karginova EA, Pentz ES, Kazakova IG, Norwood VF, Carey RM, Gomez RA. Zis: a developmentally regulated gene expressed in juxtaglomerular cells. Am J Physiol Renal Physiol 273: F731– F738, 1997. 420. Karlsson C, Lindell K, Ottosson M, Sjostrom L, Carlsson B, Carlsson LM. Human adipose tissue expresses angiotensinogen and enzymes required for its conversion to angiotensin II. J Clin Endocrinol Metab 83: 3925–3929, 1998. 421. Kassab S, Kato T, Wilkins FC, Chen R, Hall JE, Granger JP. Renal denervation attenuates the sodium retention and hypertension associated with obesity. Hypertension 25: 893– 897, 1995. 422. Kato J, Kobayashi K, Etoh T, Tanaka M, Kitamura K, Imamura T, Koiwaya Y, Kangawa K, Eto T. Plasma adrenomedullin concentration in patients with heart failure. J Clin Endocrinol Metab 81: 180 –183, 1996. 423. Keeton TK, Campbell WB. The pharmacologic alteration of renin release. Pharmacol Rev 32: 81–227, 1980. 424. Keidar S, Kaplan M, Gamliel-Lazarovich A. ACE2 of the heart: from angiotensin I to angiotensin (1–7). Cardiovasc Res 73: 463– 469, 2007. 425. Keller-Wood M, Silbiger J, Wood CE. Progesterone-cortisol interaction in control of renin activity but not aldosterone. Am J Physiol Regul Integr Comp Physiol 259: R350 –R356, 1990. 426. Kennelly PJ, Krebs EG. Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J Biol Chem 266: 15555–15558, 1991. 427. Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 89: 2548 –2556, 2004. 428. Khan AI, Kato J, Ishiyama Y, Kitamura K, Kangawa K, Eto T. Effect of chronically infused adrenomedullin in two-kidney, oneclip hypertensive rats. Eur J Pharmacol 333: 187–190, 1997. 429. Khan KN, Venturini CM, Bunch RT, Brassard JA, Koki AT, Morris DL, Trump BF, Maziasz TJ, Alden CL. Interspecies differences in renal localization of cyclooxygenase isoforms: implications in nonsteroidal anti-inflammatory drug-related nephrotoxicity. Toxicol Pathol 26: 612– 620, 1998. 430. Khoury S, Yarows SA, O’Brien TK, Sowers JR. Ambulatory blood pressure monitoring in a nonacademic setting. Effects of age and sex. Am J Hypertens 5: 616 – 623, 1992. 431. Kienitz T, Quinkler M. Testosterone and blood pressure regulation. Kidney Blood Press Res 31: 71–79, 2008. 432. Kim HS, Krege JH, Kluckman KD, Hagaman JR, Hodgin JB, Best CF, Jennette JC, Coffman TM, Maeda N, Smithies O. Genetic control of blood pressure and the angiotensinogen locus. Proc Natl Acad Sci USA 92: 2735–2739, 1995. 433. Kim S, Shinjo M, Fukamizu A, Miyazaki H, Usuki S, Murakami K. Identification of renin and renin messenger RNA sequence in rat ovary and uterus. Biochem Biophys Res Commun 142: 169 –175, 1987. 434. Kim SM, Chen L, Faulhaber-Walter R, Oppermann M, Huang Y, Mizel D, Briggs JP, Schnermann J. Regulation of renin secretion and expression in mice deficient in beta1- and beta2-adrenergic receptors. Hypertension 50: 103–109, 2007. 90 • APRIL 2010 • www.prv.org 660 CASTROP ET AL. 435. Kim SM, Chen L, Mizel D, Huang YG, Briggs JP, Schnermann J. Low plasma renin and reduced renin secretory responses to acute stimuli in conscious COX-2-deficient mice. Am J Physiol Renal Physiol 292: F415–F422, 2007. 436. Kim SM, Mizel D, Huang YG, Briggs JP, Schnermann J. Adenosine as a mediator of macula densa-dependent inhibition of renin secretion. Am J Physiol Renal Physiol 290: F1016 –F1023, 2006. 437. Kimura K, Inokuchi S, Sugaya T, Suzuki N, Yoneda H, Shirato I, Mise N, Oba S, Miyashita K, Tojo A, Hirata Y, Goto A, Sakai T, Murakami K, Omata M. Location and action of angiotensin II type 1 receptor in the renal microcirculation. Kidney Int Suppl 63: S201–S204, 1997. 438. Kinoshita H, Fujimoto S, Kitamura K, Yokota N, Kawamoto M, Tokura T, Hisanaga S, Eto T. Plasma and urine levels of adrenomedullin and proadrenomedullin N-terminal 20 peptide in chronic glomerulonephritis. Am J Kidney Dis 34: 114 –119, 1999. 439. Kirchheim H, Ehmke H, Persson P. Physiology of the renal baroreceptor mechanism of renin release and its role in congestive heart failure. Am J Cardiol 62: 68E–71E, 1988. 440. Kirchner KA, Kotchen TA, Galla JH, Luke RG. Importance of chloride for acute inhibition of renin by sodium chloride. Am J Physiol Renal Fluid Electrolyte Physiol 235: F444 –F450, 1978. 441. Kjolby M, Bie P. Chronic activation of plasma renin is log-linearly related to dietary sodium and eliminates natriuresis in response to a pulse change in total body sodium. Am J Physiol Regul Integr Comp Physiol 294: R17–R25, 2008. 442. Klar J, Sandner P, Muller MW, Kurtz A. cAMP stimulates renin gene transcription in juxtaglomerular cells. Pflügers Arch 444: 335– 344, 2002. 443. Klar J, Sigl M, Obermayer B, Schweda F, Kramer BK, Kurtz A. Calcium inhibits renin gene expression by transcriptional and posttranscriptional mechanisms. Hypertension 46: 1340 –1346, 2005. 444. Klar J, Vitzthum H, Kurtz A. Aldosterone enhances renin gene expression in juxtaglomerular cells. Am J Physiol Renal Physiol 286: F349 –F355, 2004. 445. Kobori H, Hayashi M, Saruta T. Thyroid hormone stimulates renin gene expression through the thyroid hormone response element. Hypertension 37: 99 –104, 2001. 446. Kobori H, Ichihara A, Miyashita Y, Hayashi M, Saruta T. Mechanism of hyperthyroidism-induced renal hypertrophy in rats. J Endocrinol 159: 9 –14, 1998. 447. Kobori H, Ichihara A, Suzuki H, Miyashita Y, Hayashi M, Saruta T. Thyroid hormone stimulates renin synthesis in rats without involving the sympathetic nervous system. Am J Physiol Endocrinol Metab 272: E227–E232, 1997. 448. Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 59: 251–287, 2007. 449. Koitka A, Cooper ME, Thomas MC, Tikellis C. Angiotensin converting enzyme 2 in the kidney. Clin Exp Pharmacol Physiol 35: 420 – 425, 2008. 450. Kojima H, Tsujimoto T, Uemura M, Takaya A, Okamoto S, Ueda S, Nishio K, Miyamoto S, Kubo A, Minamino N, Kangawa K, Matsuo H, Fukui H. Significance of increased plasma adrenomedullin concentration in patients with cirrhosis. J Hepatol 28: 840 – 846, 1998. 451. Komatsu T, Suzuki Y, Imai J, Sugano S, Hida M, Tanigami A, Muroi S, Yamada Y, Hanaoka K. Molecular cloning, mRNA expression and chromosomal localization of mouse angiotensin-converting enzyme-related carboxypeptidase (mACE2). DNA Seq 13: 217–220, 2002. 452. Komhoff M, Grone HJ, Klein T, Seyberth HW, Nusing RM. Localization of cyclooxygenase-1 and -2 in adult and fetal human kidney: implication for renal function. Am J Physiol Renal Physiol 272: F460 –F468, 1997. 453. Komhoff M, Jeck ND, Seyberth HW, Grone HJ, Nusing RM, Breyer MD. Cyclooxygenase-2 expression is associated with the renal macula densa of patients with Bartter-like syndrome. Kidney Int 58: 2420 –2424, 2000. 454. Komhoff M, Reinalter SC, Grone HJ, Seyberth HW. Induction of microsomal prostaglandin E2 synthase in the macula densa in children with hypokalemic salt-losing tubulopathies. Pediatr Res 55: 261–266, 2004. Physiol Rev • VOL 455. Komlosi P, Peti-Peterdi J, Fuson AL, Fintha A, Rosivall L, Bell PD. Macula densa basolateral ATP release is regulated by luminal [NaCl] and dietary salt intake. Am J Physiol Renal Physiol 286: F1054 –F1058, 2004. 456. Kompanowska-Jezierska E, Wolff H, Kuczeriszka M, Gramsbergen JB, Walkowska A, Johns EJ, Bie P. Renal nerves and nNOS: roles in natriuresis of acute isovolumetric sodium loading in conscious rats. Am J Physiol Regul Integr Comp Physiol 294: R1130 –R1139, 2008. 457. Kon Y. Comparative study of renin-containing cells. Histological approaches. J Vet Med Sci 61: 1075–1086, 1999. 458. Kon Y, Alcorn D, Murakami K, Sugimura M, Ryan GB. Immunohistochemical studies of renin-containing cells in the developing sheep kidney. Anat Rec 239: 191–197, 1994. 459. Koshimizu TA, Nasa Y, Tanoue A, Oikawa R, Kawahara Y, Kiyono Y, Adachi T, Tanaka T, Kuwaki T, Mori T, Takeo S, Okamura H, Tsujimoto G. V1a vasopressin receptors maintain normal blood pressure by regulating circulating blood volume and baroreflex sensitivity. Proc Natl Acad Sci USA 103: 7807–7812, 2006. 460. Kostenis E, Milligan G, Christopoulos A, Sanchez-Ferrer CF, Heringer-Walther S, Sexton PM, Gembardt F, Kellett E, Martini L, Vanderheyden P, Schultheiss HP, Walther T. G-proteincoupled receptor Mas is a physiological antagonist of the angiotensin II type 1 receptor. Circulation 111: 1806 –1813, 2005. 461. Kostreva DR, Castaner A, Kampine JP. Reflex effects of hepatic baroreceptors on renal and cardiac sympathetic nerve activity. Am J Physiol Regul Integr Comp Physiol 238: R390 –R394, 1980. 462. Kramer BK, Ritthaler T, Ackermann M, Holmer S, Schricker K, Riegger GA, Kurtz A. Endothelium-mediated regulation of renin secretion. Kidney Int 46: 1577–1579, 1994. 463. Kramer BK, Schricker K, Scholz H, Clozel M, Riegger GA, Kurtz A. Role of endothelins for renin regulation. Kidney Int Suppl 55: S119 –S121, 1996. 464. Krattinger N, Alonso F, Capponi A, Mazzolai L, Nicod P, Meda P, Haefliger JA. Increased expression of renal cyclooxygenase-2 and neuronal nitric oxide synthase in hypertensive Cx40-deficient mice. J Vasc Res 46: 188 –198, 2008. 465. Krattinger N, Capponi A, Mazzolai L, Aubert JF, Caille D, Nicod P, Waeber G, Meda P, Haefliger JA. Connexin40 regulates renin production and blood pressure. Kidney Int 72: 814 – 822, 2007. 466. Krege JH, Kim HS, Moyer JS, Jennette JC, Peng L, Hiller SK, Smithies O. Angiotensin-converting enzyme gene mutations, blood pressures, and cardiovascular homeostasis. Hypertension 29: 150 –157, 1997. 467. Krob HA, Vinsant SL, Ferrario CM, Friedman DP. Angiotensin(1–7) immunoreactivity in the hypothalamus of the (mRen-2d)27 transgenic rat. Brain Res 798: 36 – 45, 1998. 468. Krogsgaard Thomsen M, Friis C, Sehested Hansen B, Johansen P, Eschen C, Nowak J, Poulsen K. Studies on the renal kinetics of growth hormone (GH) and on the GH receptor and related effects in animals. J Pediatr Endocrinol 7: 93–105, 1994. 469. Kuan CJ, Wells JN, Jackson EK. Endogenous adenosine restrains renin release during sodium restriction. J Pharmacol Exp Ther 249: 110 –116, 1989. 470. Kuan CJ, Wells JN, Jackson EK. Endogenous adenosine restrains renin release in conscious rats. Circ Res 66: 637– 646, 1990. 471. Kuba K, Imai Y, Penninger JM. Angiotensin-converting enzyme 2 in lung diseases. Curr Opin Pharmacol 6: 271–276, 2006. 472. Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 11: 875– 879, 2005. 473. Kurtz A. Cellular control of renin secretion. Rev Physiol Biochem Pharmacol 113: 1– 40, 1989. 474. Kurtz A, Della Bruna R, Pfeilschifter J, Bauer C. Role of cGMP as second messenger of adenosine in the inhibition of renin release. Kidney Int 33: 798 – 803, 1988. 475. Kurtz A, Della Bruna R, Pfeilschifter J, Taugner R, Bauer C. Atrial natriuretic peptide inhibits renin release from juxtaglomer- 90 • APRIL 2010 • www.prv.org PHYSIOLOGY OF KIDNEY RENIN 476. 477. 479. 480. 481. 482. 483. 484. 485. 486. 487. 488. 489. 490. 492. 493. 494. 495. 496. 497. 498. 499. ular cells by a cGMP-mediated process. Proc Natl Acad Sci USA 83: 4769 – 4773, 1986. Kurtz A, Della Bruna R, Pratz J, Cavero I. Rat juxtaglomerular cells are endowed with DA-1 dopamine receptors mediating renin release. J Cardiovasc Pharmacol 12: 658 – 663, 1988. Kurtz A, Gotz KH, Hamann M, Kieninger M, Wagner C. Stimulation of renin secretion by NO donors is related to the cAMP pathway. Am J Physiol Renal Physiol 274: F709 –F717, 1998. Kurtz A, Gotz KH, Hamann M, Wagner C. Stimulation of renin secretion by nitric oxide is mediated by phosphodiesterase 3. Proc Natl Acad Sci USA 95: 4743– 4747, 1998. Kurtz A, Hamann M, Götz K. Role of potassium channels in the control of renin secretion from isolated perfused rat kidneys. Pflügers Arch 440: 889 – 895, 2000. Kurtz A, Kaissling B, Busse R, Baier W. Endothelial cells modulate renin secretion from isolated mouse juxtaglomerular cells. J Clin Invest 88: 1147–1154, 1991. Kurtz A, Muff R, Born W, Lundberg JM, Millberg BI, Gnadinger MP, Uehlinger DE, Weidmann P, Hokfelt T, Fischer JA. Calcitonin gene-related peptide is a stimulator of renin secretion. J Clin Invest 82: 538 –543, 1988. Kurtz A, Penner R. Angiotensin II induces oscillations of intracellular calcium and blocks anomalous inward rectifying potassium current in mouse renal juxtaglomerular cells. Proc Natl Acad Sci USA 86: 3423–3427, 1989. Kurtz A, Pfeilschifter J, Bauer C. Is renin secretion governed by the calcium permeability of the juxtaglomerular cell membrane? Biochem Biophys Res Commun 124: 359 –366, 1984. Kurtz A, Pfeilschifter J, Hutter A, Buhrle C, Nobiling R, Taugner R, Hackenthal E, Bauer C. Role of protein kinase C in inhibition of renin release caused by vasoconstrictors. Am J Physiol Cell Physiol 250: C563–C571, 1986. Kurtz A, Schweda F. Osmolarity-induced renin secretion from kidneys: evidence for readily releasable renin pools. Am J Physiol Renal Physiol 290: F797–F805, 2006. Kurtz A, Skott O, Chegini S, Penner R. Lack of direct evidence for a functional role of voltage-operated calcium channels in juxtaglomerular cells. Pflügers Arch 416: 281–287, 1990. Kurtz A, Wagner C. Cellular control of renin secretion. J Exp Biol 202: 219 –225, 1999. Kurtz A, Wagner C. Role of nitric oxide in the control of renin secretion. Am J Physiol Renal Physiol 275: F849 –F862, 1998. Kurtz L, Gerl M, Kriz W, Wagner C, Kurtz A. Replacement of connexin 40 by connexin 45 causes ectopic localization of reninproducing cells in the kidney but maintains in vivo control of renin gene expression. Am J Physiol Renal Physiol 297: F403–F409, 2009. Kurtz L, Janssen-Bienhold U, Kurtz A, Wagner C. Connexin expression in renin-producing cells. J Am Soc Nephrol 20: 506 –512, 2009. Kurtz L, Schweda F, de Wit C, Kriz W, Witzgall R, Warth R, Sauter A, Kurtz A, Wagner C. Lack of connexin 40 causes displacement of renin-producing cells from afferent arterioles to the extraglomerular mesangium. J Am Soc Nephrol 18: 1103–1111, 2007. Lai EC. Notch signaling: control of cell communication and cell fate. Development 131: 965–973, 2004. Lainchbury JG, Nicholls MG, Espiner EA, Yandle TG, Lewis LK, Richards AM. Bioactivity and interactions of adrenomedullin and brain natriuretic peptide in patients with heart failure. Hypertension 34: 70 –75, 1999. Lainchbury JG, Troughton RW, Lewis LK, Yandle TG, Richards AM, Nicholls MG. Hemodynamic, hormonal, and renal effects of short-term adrenomedullin infusion in healthy volunteers. J Clin Endocrinol Metab 85: 1016 –1020, 2000. Lalli E, Sassone-Corsi P. Signal transduction and gene regulation: the nuclear response to cAMP. J Biol Chem 269: 17359 –17362, 1994. Lambert DW, Hooper NM, Turner AJ. Angiotensin-converting enzyme 2 and new insights into the renin-angiotensin system. Biochem Pharmacol 75: 781–786, 2008. Lang JA, Ying LH, Morris BJ, Sigmund CD. Transcriptional and posttranscriptional mechanisms regulate human renin gene expresPhysiol Rev • VOL 500. 501. 502. 503. 504. 505. 506. 507. 508. 509. 510. 511. 512. 513. 514. 515. 516. 517. 518. 519. 661 sion in Calu-6 cells. Am J Physiol Renal Fluid Electrolyte Physiol 271: F94 –F100, 1996. Lapointe JY, Bell PD, Cardinal J. Direct evidence for apical Na⫹:2Cl⫺:K⫹ cotransport in macula densa cells. Am J Physiol Renal Fluid Electrolyte Physiol 258: F1466 –F1469, 1990. Lassegue B, Alexander RW, Nickenig G, Clark M, Murphy TJ, Griendling KK. Angiotensin II down-regulates the vascular smooth muscle AT1 receptor by transcriptional and post-transcriptional mechanisms: evidence for homologous and heterologous regulation. Mol Pharmacol 48: 601– 609, 1995. Le Fevre ME, Guild SJ, Ramchandra R, Barrett CJ, Malpas SC. Role of angiotensin II in the neural control of renal function. Hypertension 41: 583–591, 2003. Le Tran Y, Forster C. Angiotensin-(1–7) and the rat aorta: modulation by the endothelium. J Cardiovasc Pharmacol 30: 676 – 682, 1997. LeFebvre J, Shintani A, Gebretsadik T, Petro JR, Murphey LJ, Brown NJ. Bradykinin B(2) receptor does not contribute to blood pressure lowering during AT(1) receptor blockade. J Pharmacol Exp Ther 320: 1261–1267, 2007. Lefkowitz RJ. G protein-coupled receptor kinases. Cell 74: 409 – 412, 1993. Lehoux JG, Bird IM, Rainey WE, Tremblay A, Ducharme L. Both low sodium and high potassium intake increase the level of adrenal angiotensin-II receptor type 1, but not that of adrenocorticotropin receptor. Endocrinology 134: 776 –782, 1994. Leichtle A, Rauch U, Albinus M, Benohr P, Kalbacher H, Mack AF, Veh RW, Quast U, Russ U. Electrophysiological and molecular characterization of the inward rectifier in juxtaglomerular cells from rat kidney. J Physiol 560: 365–376, 2004. Lely AT, Hamming I, van Goor H, Navis GJ. Renal ACE2 expression in human kidney disease. J Pathol 204: 587–593, 2004. Lely AT, Krikken JA, Bakker SJ, Boomsma F, Dullaart RP, Wolffenbuttel BH, Navis G. Low dietary sodium and exogenous angiotensin II infusion decrease plasma adiponectin concentrations in healthy men. J Clin Endocrinol Metab 92: 1821–1826, 2007. Lemos VS, Silva DM, Walther T, Alenina N, Bader M, Santos RA. The endothelium-dependent vasodilator effect of the nonpeptide Ang(1–7) mimic AVE 0991 is abolished in the aorta of masknockout mice. J Cardiovasc Pharmacol 46: 274 –279, 2005. Leonard BL, Evans RG, Navakatikyan MA, Malpas SC. Differential neural control of intrarenal blood flow. Am J Physiol Regul Integr Comp Physiol 279: R907–R916, 2000. Leung PS. The physiology of a local renin-angiotensin system in the pancreas. J Physiol 580: 31–37, 2007. Lew R, Summers RJ. The distribution of beta-adrenoceptors in dog kidney: an autoradiographic analysis. Eur J Pharmacol 140: 1–11, 1987. Li N, Zimpelmann J, Cheng K, Wilkins JA, Burns KD. The role of angiotensin converting enzyme 2 in the generation of angiotensin 1–7 by rat proximal tubules. Am J Physiol Renal Physiol 288: F353–F362, 2005. Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426: 450 – 454, 2003. Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D3 is a negative endocrine regulator of the reninangiotensin system. J Clin Invest 110: 229 –238, 2002. Li Z, Wang ZG, Chen X, Chen XD. Inhibitory effect of angiotensin II type 1 receptor-associated protein on vascular smooth muscle cell growth and neointimal formation. Chin Med Sci J 22: 22–26, 2007. Liguori A, Casini A, Di Loreto M, Andreini I, Napoli C. Loop diuretics enhance the secretion of prostacyclin in vitro, in healthy persons, and in patients with chronic heart failure. Eur J Clin Pharmacol 55: 117–124, 1999. Lima CV, Paula RD, Resende FL, Khosla MC, Santos RA. Potentiation of the hypotensive effect of bradykinin by short-term infusion of angiotensin-(1–7) in normotensive and hypertensive rats. Hypertension 30: 542–548, 1997. 90 • APRIL 2010 • www.prv.org 662 CASTROP ET AL. 520. Liu A, Ballermann BJ. TGF-beta type II receptor in rat renal vascular development: localization to juxtaglomerular cells. Kidney Int 53: 716 –725, 1998. 521. Liu X, Huang X, Sigmund CD. Identification of a nuclear orphan receptor (Ear2) as a negative regulator of renin gene transcription. Circ Res 92: 1033–1040, 2003. 522. Liu X, Shi Q, Sigmund CD. Interleukin-1beta attenuates renin gene expression via a mitogen-activated protein kinase kinaseextracellular signal-regulated kinase and signal transducer and activator of transcription 3-dependent mechanism in As4.1 cells. Endocrinology 147: 6011– 6018, 2006. 523. Lloyd T, Weisz J. Direct inhibition of tyrosine hydroxylase activity by catechol estrogens. J Biol Chem 253: 4841– 4843, 1978. 524. Lohmeier TE, Hildebrandt DA, Dwyer TM, Barrett AM, Irwin ED, Rossing MA, Kieval RS. Renal denervation does not abolish sustained baroreflex-mediated reductions in arterial pressure. Hypertension 49: 373–379, 2007. 525. Lohmeier TE, Hildebrandt DA, Hood WA. Renal nerves promote sodium excretion during long-term increases in salt intake. Hypertension 33: 487– 492, 1999. 526. Lohmeier TE, Irwin ED, Rossing MA, Serdar DJ, Kieval RS. Prolonged activation of the baroreflex produces sustained hypotension. Hypertension 43: 306 –311, 2004. 527. Lohmeier TE, Lohmeier JR, Haque A, Hildebrandt DA. Baroreflexes prevent neurally induced sodium retention in angiotensin hypertension. Am J Physiol Regul Integr Comp Physiol 279: R1437–R1448, 2000. 528. Lohmeier TE, Lohmeier JR, Reckelhoff JF, Hildebrandt DA. Sustained influence of the renal nerves to attenuate sodium retention in angiotensin hypertension. Am J Physiol Regul Integr Comp Physiol 281: R434 –R443, 2001. 529. Lohse MJ. Molecular mechanisms of membrane receptor desensitization. Biochim Biophys Acta 1179: 171–188, 1993. 530. Loichot C, Grima M, De Jong W, Helwig JJ, Imbs JL, Barthelmebs M. Oxytocin-induced renin secretion by denervated kidney in anaesthetized rat. Eur J Pharmacol 454: 241–247, 2002. 531. Lolait SJ, O’Carroll AM, Mahan LC, Felder CC, Button DC, Young WS 3rd, Mezey E, Brownstein MJ. Extrapituitary expression of the rat V1b vasopressin receptor gene. Proc Natl Acad Sci USA 92: 6783– 6787, 1995. 532. Lonnqvist F, Nordfors L, Schalling M. Leptin and its potential role in human obesity. J Intern Med 245: 643– 652, 1999. 533. Loot AE, Roks AJ, Henning RH, Tio RA, Suurmeijer AJ, Boomsma F, van Gilst WH. Angiotensin-(1–7) attenuates the development of heart failure after myocardial infarction in rats. Circulation 105: 1548 –1550, 2002. 534. Lopez-Ilasaca M, Liu X, Tamura K, Dzau VJ. The angiotensin II type I receptor-associated protein, ATRAP, is a transmembrane protein and a modulator of angiotensin II signaling. Mol Biol Cell 14: 5038 –5050, 2003. 535. Lopez GA, Romano FD, Aletich VA, Lissuzzo LM. Cyclic adenosine 3⬘:5-monophosphate mediation of the effect of dopamine on renin release by renal cortical slices from sodium-deficient rats: modification by dopaminergic and beta-adrenergic receptor blockade. Proc Soc Exp Biol Med 162: 471– 479, 1979. 536. Lopez J, Cuesta N, Martinez A, Montuenga L, Cuttitta F. Proadrenomedullin N-terminal 20 peptide (PAMP) immunoreactivity in vertebrate juxtaglomerular granular cells identified by both light and electron microscopy. Gen Comp Endocrinol 116: 192–203, 1999. 537. Lorenz JN, Kotchen TA, Ott CE. Effect of Na and Cl infusion on loop function and plasma renin activity in rats. Am J Physiol Renal Fluid Electrolyte Physiol 258: F1328 –F1335, 1990. 538. Lorenz JN, Weihprecht H, Schnermann J, Skott O, Briggs JP. Renin release from isolated juxtaglomerular apparatus depends on macula densa chloride transport. Am J Physiol Renal Fluid Electrolyte Physiol 260: F486 –F493, 1991. 539. Loutzenhiser R, Chilton L, Trottier G. Membrane potential measurements in renal afferent and efferent arterioles: actions of angiotensin II. Am J Physiol Renal Physiol 273: F307–F314, 1997. 540. Lovren F, Pan Y, Quan A, Teoh H, Wang G, Shukla PC, Levitt KS, Oudit GY, Al-Omran M, Stewart DJ, Slutsky AS, Peterson MD, Backx PH, Penninger JM, Verma S. Angiotensin converting Physiol Rev • VOL 541. 542. 543. 544. 545. 546. 547. 548. 549. 550. 551. 552. 553. 554. 555. 556. 557. 558. 559. 560. 561. enzyme-2 confers endothelial protection and attenuates atherosclerosis. Am J Physiol Heart Circ Physiol 295: H1377–H1384, 2008. Lucas FV, Skrinska VA, Chisolm GM, Hesse BL. Stability of prostacyclin in human and rabbit whole blood and plasma. Thromb Res 43: 379 –387, 1986. Luetscher JA, Kraemer FB, Wilson DM, Schwartz HC, BryerAsh M. Increased plasma inactive renin in diabetes mellitus A marker of microvascular complications. N Engl J Med 312: 1412– 1417, 1985. Luft FC. Renin and its putative receptor remain enigmas. J Am Soc Nephrol 18: 1989 –1992, 2007. Luft FC, Weinberger MH. Antihypertensive therapy with aliskiren. Kidney Int 73: 679 – 683, 2008. Luo M, Faure R, Tong YA, Dussault JH. Immunocytochemical localization of the nuclear 3,5,3⬘-triiodothyronine receptor in the adult rat: liver, kidney, heart, lung and spleen. Acta Endocrinol 120: 451– 458, 1989. Madeddu P, Anania V, Parpaglia PP, Demontis MP, Varoni MV, Fattaccio MC, Glorioso N. Chronic kinin receptor blockade induces hypertension in deoxycorticosterone-treated rats. Br J Pharmacol 108: 651– 657, 1993. Madeddu P, Emanueli C, Song Q, Varoni MV, Demontis MP, Anania V, Glorioso N, Chao J. Regulation of bradykinin B2receptor expression by oestrogen. Br J Pharmacol 121: 1763–1769, 1997. Madeddu P, Varoni MV, Palomba D, Emanueli C, Demontis MP, Glorioso N, Dessi-Fulgheri P, Sarzani R, Anania V. Cardiovascular phenotype of a mouse strain with disruption of bradykinin B2-receptor gene. Circulation 96: 3570 –3578, 1997. Madsen K, Stubbe J, Yang T, Skott O, Bachmann S, Jensen BL. Low endogenous glucocorticoid allows induction of kidney cortical cyclooxygenase-2 during postnatal rat development. Am J Physiol Renal Physiol 286: F26 –F37, 2004. Magness RR, Parker CR Jr, Rosenfeld CR. Systemic and uterine responses to chronic infusion of estradiol-17 beta. Am J Physiol Endocrinol Metab 265: E690 –E698, 1993. Magness RR, Rosenfeld CR. Local and systemic estradiol-17 beta: effects on uterine and systemic vasodilation. Am J Physiol Endocrinol Metab 256: E536 –E542, 1989. Mahon JM, Carr RD, Nicol AK, Henderson IW. Angiotensin(1–7) is an antagonist at the type 1 angiotensin II receptor. J Hypertens 12: 1377–1381, 1994. Maillard MP, Tedjani A, Perregaux C, Burnier M. Calciumsensing receptors modulate renin release in vivo and in vitro in the rat. J Hypertens 27: 1980 –1987, 2009. Majid DS, Inscho EW, Navar LG. P2 purinoceptor saturation by adenosine triphosphate impairs renal autoregulation in dogs. J Am Soc Nephrol 10: 492– 498, 1999. Makhanova N, Lee G, Takahashi N, Sequeira Lopez ML, Gomez RA, Kim HS, Smithies O. Kidney function in mice lacking aldosterone. Am J Physiol Renal Physiol 290: F61–F69, 2006. Malayan SA, Ramsay DJ, Keil LC, Reid IA. Effects of increases in plasma vasopressin concentration on plasma renin activity, blood pressure, heart rate, and plasma corticosteroid concentration in conscious dogs. Endocrinology 107: 1899 –1904, 1980. Malpas SC, Hore TA, Navakatikyan M, Lukoshkova EV, Nguang SK, Austin PC. Resonance in the renal vasculature evoked by activation of the sympathetic nerves. Am J Physiol Regul Integr Comp Physiol 276: R1311–R1319, 1999. Mangat H, Peterson LN, Burns KD. Hypercalcemia stimulates expression of intrarenal prostaglandin A2 and prostaglandin H synthase-2 in rats. J Clin Invest 100: 1941–1950, 1997. Mann RS, Affolter M. Hox proteins meet more partners. Curr Opin Genet Dev 8: 423– 429, 1998. Marchant C, Brown L, Sernia C. Renin-angiotensin system in thyroid dysfunction in rats. J Cardiovasc Pharmacol 22: 449 – 455, 1993. Mark AL, Agassandian K, Morgan DA, Liu X, Cassell MD, Rahmouni K. Leptin signaling in the nucleus tractus solitarii increases sympathetic nerve activity to the kidney. Hypertension 53: 375–380, 2009. 90 • APRIL 2010 • www.prv.org PHYSIOLOGY OF KIDNEY RENIN 562. Markus MA, Goy C, Adams DJ, Lovicu FJ, Morris BJ. Renin enhancer is crucial for full response in renin expression to an in vivo stimulus. Hypertension 50: 933–938, 2007. 563. Marsh AC, Gibson KJ, Wu J, Owens PC, Owens JA, Lumbers ER. Chronic effect of insulin-like growth factor I on renin synthesis, secretion, and renal function in fetal sheep. Am J Physiol Regul Integr Comp Physiol 281: R318 –R326, 2001. 564. Marsh AC, Gibson KJ, Wu J, Owens PC, Owens JA, Lumbers ER. Insulin-like growth factor I alters renal function and stimulates renin secretion in late gestation fetal sheep. J Physiol 530: 253–262, 2001. 565. Martins D, Nelson K, Pan D, Tareen N, Norris K. The effect of gender on age-related blood pressure changes and the prevalence of isolated systolic hypertension among older adults: data from NHANES III. J Gend Specif Med 4: 10 –13, 20, 2001. 566. Matsumura K, Averill DB, Ferrario CM. Angiotensin II acts at AT1 receptors in the nucleus of the solitary tract to attenuate the baroreceptor reflex. Am J Physiol Regul Integr Comp Physiol 275: R1611–R1619, 1998. 567. Matsumura Y, Miyawaki N, Sasaki Y, Morimoto S. Inhibitory effects of norepinephrine, methoxamine and phenylephrine on renin release from rat kidney cortical slices. J Pharmacol Exp Ther 233: 782–787, 1985. 568. Matsusaka T, Nishimura H, Utsunomiya H, Kakuchi J, Niimura F, Inagami T, Fogo A, Ichikawa I. Chimeric mice carrying “regional” targeted deletion of the angiotensin type 1A receptor gene. Evidence against the role for local angiotensin in the in vivo feedback regulation of renin synthesis in juxtaglomerular cells. J Clin Invest 98: 1867–1877, 1996. 569. Matzdorf C, Kurtz A, Hocherl K. COX-2 activity determines the level of renin expression but is dispensable for acute upregulation of renin expression in rat kidneys. Am J Physiol Renal Physiol 292: F1782–F1790, 2007. 570. McBryde FD, Guild SJ, Barrett CJ, Osborn JW, Malpas SC. Angiotensin II-based hypertension and the sympathetic nervous system: the role of dose and increased dietary salt in rabbits. Exp Physiol 92: 831– 840, 2007. 571. McBryde FD, Malpas SC, Guild SJ, Barrett CJ. A high-salt diet does not influence renal sympathetic nerve activity: a direct telemetric investigation. Am J Physiol Regul Integr Comp Physiol 297: R396 –R402, 2009. 572. McGregor DO, Troughton RW, Frampton C, Lynn KL, Yandle T, Richards AM, Nicholls MG. Hypotensive and natriuretic actions of adrenomedullin in subjects with chronic renal impairment. Hypertension 37: 1279 –1284, 2001. 573. McKinley MJ, Evered M, Mathai M, Coghlan JP. Effects of central losartan on plasma renin and centrally mediated natriuresis. Kidney Int 46: 1479 –1482, 1994. 574. McKinley MJ, McBurnie MI, Mathai ML. Neural mechanisms subserving central angiotensinergic influences on plasma renin in sheep. Hypertension 37: 1375–1381, 2001. 575. Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol 292: C82–C97, 2007. 576. Melo LG, Veress AT, Chong CK, Pang SC, Flynn TG, Sonnenberg H. Salt-sensitive hypertension in ANP knockout mice: potential role of abnormal plasma renin activity. Am J Physiol Regul Integr Comp Physiol 274: R255–R261, 1998. 577. Mendes AC, Ferreira AJ, Pinheiro SV, Santos RA. Chronic infusion of angiotensin-(1–7) reduces heart angiotensin II levels in rats. Regul Pept 125: 29 –34, 2005. 578. Meneton P, Ichikawa I, Inagami T, Schnermann J. Renal physiology of the mouse. Am J Physiol Renal Physiol 278: F339 –F351, 2000. 579. Mercure C, Jutras I, Day R, Seidah NG, Reudelhuber TL. Prohormone convertase PC5 is a candidate processing enzyme for prorenin in the human adrenal cortex. Hypertension 28: 840 – 846, 1996. 580. Mercure C, Yogi A, Callera GE, Aranha AB, Bader M, Ferreira AJ, Santos RA, Walther T, Touyz RM, Reudelhuber TL. Angiotensin(1–7) blunts hypertensive cardiac remodeling by a direct effect on the heart. Circ Res 103: 1319 –1326, 2008. Physiol Rev • VOL 663 581. Metzger R, Bader M, Ludwig T, Berberich C, Bunnemann B, Ganten D. Expression of the mouse and rat mas proto-oncogene in the brain and peripheral tissues. FEBS Lett 357: 27–32, 1995. 582. Milner P, Kirkpatrick KA, Ralevic V, Toothill V, Pearson J, Burnstock G. Endothelial cells cultured from human umbilical vein release ATP, substance P and acetylcholine in response to increased flow. Proc R Soc Lond B Biol Sci 241: 245–248, 1990. 583. Mink D, Schiller A, Kriz W, Taugner R. Interendothelial junctions in kidney vessels. Cell Tissue Res 236: 567–576, 1984. 584. Minuth M, Hackenthal E, Poulsen K, Rix E, Taugner R. Renin immunocytochemistry of the differentiating juxtaglomerular apparatus. Anat Embryol 162: 173–181, 1981. 585. Misra A, Garg A. Leptin, its receptor and obesity. J Invest Med 44: 540 –548, 1996. 586. Mizoguchi H, Dzau VJ, Siwek LG, Barger AC. Effect of intrarenal administration of dopamine on renin release in conscious dogs. Am J Physiol Heart Circ Physiol 244: H39 –H45, 1983. 587. Moe O, Tejedor A, Campbell WB, Alpern RJ, Henrich WL. Effects of endothelin on in vitro renin secretion. Am J Physiol Endocrinol Metab 260: E521–E525, 1991. 588. Moller J, Fisker S, Rosenfalck AM, Frandsen E, Jorgensen JO, Hilsted J, Christiansen JS. Long-term effects of growth hormone (GH) on body fluid distribution in GH deficient adults: a four months double blind placebo controlled trial. Eur J Endocrinol 140: 11–16, 1999. 589. Moller J, Frandsen E, Fisker S, Jorgensen JO, Christiansen JS. Decreased plasma and extracellular volume in growth hormone deficient adults and the acute and prolonged effects of GH administration: a controlled experimental study. Clin Endocrinol 44: 533–539, 1996. 590. Moller J, Jorgensen JO, Frandsen E, Laursen T, Christiansen JS. Body fluids, circadian blood pressure and plasma renin during growth hormone administration: a placebo-controlled study with two growth hormone doses in healthy adults. Scand J Clin Lab Invest 55: 663– 669, 1995. 591. Moller J, Jorgensen JO, Marqversen J, Frandsen E, Christiansen JS. Insulin-like growth factor I administration induces fluid and sodium retention in healthy adults: possible involvement of renin and atrial natriuretic factor. Clin Endocrinol 52: 181–186, 2000. 592. Moller J, Moller N, Frandsen E, Wolthers T, Jorgensen JO, Christiansen JS. Blockade of the renin-angiotensin-aldosterone system prevents growth hormone-induced fluid retention in humans. Am J Physiol Endocrinol Metab 272: E803–E808, 1997. 593. Molstrom S, Larsen NH, Simonsen JA, Washington R, Bie P. Normotensive sodium loading in normal man: regulation of renin secretion during beta-receptor blockade. Am J Physiol Regul Integr Comp Physiol 296: R436 –R445, 2009. 594. Moncada S. Biology and therapeutic potential of prostacyclin. Stroke 14: 157–168, 1983. 595. Monstein HJ, Truedsson M, Ryberg A, Ohlsson B. Vasopressin receptor mRNA expression in the human gastrointestinal tract. Eur Surg Res 40: 34 – 40, 2008. 596. Montminy M. Transcriptional regulation by cAMP. Annu Rev Biochem 66: 807– 822, 1997. 597. Moore PK, Bland-Ward PA. 7-Nitroindazole: an inhibitor of nitric oxide synthase. Methods Enzymol 268: 393–398, 1996. 598. Moosavi SM, Johns EJ. The effect of isoprenaline infusion on renal renin and angiotensinogen gene expression in the anaesthetised rat. Exp Physiol 88: 221–227, 2003. 599. Morello F, de Boer RA, Steffensen KR, Gnecchi M, Chisholm JW, Boomsma F, Anderson LM, Lawn RM, Gustafsson JA, Lopez-Ilasaca M, Pratt RE, Dzau VJ. Liver X receptors alpha and beta regulate renin expression in vivo. J Clin Invest 115: 1913– 1922, 2005. 600. Morham SG, Langenbach R, Loftin CD, Tiano HF, Vouloumanos N, Jennette JC, Mahler JF, Kluckman KD, Ledford A, Lee CA, Smithies O. Prostaglandin synthase 2 gene disruption causes severe renal pathology in the mouse. Cell 83: 473– 482, 1995. 601. Morris BJ. Fluorescence activated cell sorting of transiently transfected As4.1 cells shows renin enhancer directs on/off switching of renin promoter in vitro. Clin Exp Pharmacol Physiol 35: 367–371, 2008. 90 • APRIL 2010 • www.prv.org 664 CASTROP ET AL. 602. Morris BJ, Adams DJ, Beveridge DJ, van der Weyden L, Mangs H, Leedman PJ. cAMP controls human renin mRNA stability via specific RNA-binding proteins. Acta Physiol Scand 181: 369 –373, 2004. 603. Morris BJ, Reid IA, Ganong WF. Inhibition by alpha-adrenoceptor agonists of renin release in vitro. Eur J Pharmacol 59: 37– 45, 1979. 604. Mukoyama M, Nakajima M, Horiuchi M, Sasamura H, Pratt RE, Dzau VJ. Expression cloning of type 2 angiotensin II receptor reveals a unique class of seven-transmembrane receptors. J Biol Chem 268: 24539 –24542, 1993. 605. Mulkay JP, Louis H, Donckier V, Bourgeois N, Adler M, Deviere J, Le Moine O. Long-term terlipressin administration improves renal function in cirrhotic patients with type 1 hepatorenal syndrome: a pilot study. Acta Gastroenterol Belg 64: 15–19, 2001. 606. Muller MW, Todorov V, Kramer BK, Kurtz A. Angiotensin II inhibits renin gene transcription via the protein kinase C pathway. Pflügers Arch 444: 499 –505, 2002. 607. Mullins LJ, Payne CM, Kotelevtseva N, Brooker G, Fleming S, Harris S, Mullins JJ. Granulation rescue and developmental marking of juxtaglomerular cells using “piggy-BAC” recombination of the mouse ren locus. J Biol Chem 275: 40378 – 40384, 2000. 608. Mundel P, Bachmann S, Bader M, Fischer A, Kummer W, Mayer B, Kriz W. Expression of nitric oxide synthase in kidney macula densa cells. Kidney Int 42: 1017–1019, 1992. 609. Munter K, Hackenthal E. The effects of endothelin on renovascular resistance and renin release. J Hypertens Suppl 7: S276 – S277, 1989. 610. Munter K, Hackenthal E. The participation of the endothelium in the control of renin release. J Hypertens Suppl 9: S236 –S237, 1991. 611. Murakami K, Tsuchiya K, Naruse M, Naruse K, Demura H, Arai J, Nihei H. Nitric oxide synthase I immunoreactivity in the macula densa of the kidney is angiotensin II dependent. Kidney Int Suppl 63: S208 –S210, 1997. 612. Murphey LJ, Kumar S, Brown NJ. Endogenous bradykinin and the renin and pressor responses to furosemide in humans. J Pharmacol Exp Ther 295: 644 – 648, 2000. 613. Muthalif MM, Benter IF, Uddin MR, Harper JL, Malik KU. Signal transduction mechanisms involved in angiotensin-(1–7)stimulated arachidonic acid release and prostanoid synthesis in rabbit aortic smooth muscle cells. J Pharmacol Exp Ther 284: 388 –398, 1998. 614. Mutig K, Paliege A, Kahl T, Jons T, Muller-Esterl W, Bachmann S. Vasopressin V2 receptor expression along rat, mouse, and human renal epithelia with focus on TAL. Am J Physiol Renal Physiol 293: F1166 –F1177, 2007. 615. Nabel C, Schweda F, Riegger GA, Kramer BK, Kurtz A. Chloride channel blockers attenuate the inhibition of renin secretion by angiotensin II. Pflügers Arch 438: 694 – 699, 1999. 616. Naftilan AJ, Oparil S. Inhibition of renin release from rat kidney slices by the angiotensins. Am J Physiol Renal Fluid Electrolyte Physiol 235: F62–F68, 1978. 617. Nafz B, Berthold H, Ehmke H, Hackenthal E, Kirchheim HR, Persson PB. Flow versus pressure in the control of renin release in conscious dogs. Am J Physiol Renal Physiol 273: F200 –F205, 1997. 618. Nagaya N, Nishikimi T, Uematsu M, Satoh T, Oya H, Kyotani S, Sakamaki F, Ueno K, Nakanishi N, Miyatake K, Kangawa K. Haemodynamic and hormonal effects of adrenomedullin in patients with pulmonary hypertension. Heart 84: 653– 658, 2000. 619. Naitoh M, Suzuki H, Murakami M, Matsumoto A, Ichihara A, Nakamoto H, Yamamura Y, Saruta T. Arginine vasopressin produces renal vasodilation via V2 receptors in conscious dogs. Am J Physiol Regul Integr Comp Physiol 265: R934 –R942, 1993. 620. Nakamura N, Burt DW, Paul M, Dzau VJ. Negative control elements and cAMP responsive sequences in the tissue-specific expression of mouse renin genes. Proc Natl Acad Sci USA 86: 56 –59, 1989. 621. Nantel F, Meadows E, Denis D, Connolly B, Metters KM, Giaid A. Immunolocalization of cyclooxygenase-2 in the macula densa of human elderly. FEBS Lett 457: 475– 477, 1999. 622. Naruse M, Nitta K, Sanaka T, Naruse K, Demura H, Inagami T, Shizume K, Sugino N. Immunohistochemical localization of atrial Physiol Rev • VOL 623. 624. 625. 626. 627. 628. 629. 630. 631. 632. 633. 634. 635. 636. 637. 638. 639. 640. 641. natriuretic peptide binding sites in juxtaglomerular cells and vascular walls of rat kidney. Acta Endocrinol 119: 235–239, 1988. Nausch LW, Ledoux J, Bonev AD, Nelson MT, Dostmann WR. Differential patterning of cGMP in vascular smooth muscle cells revealed by single GFP-linked biosensors. Proc Natl Acad Sci USA 105: 365–370, 2008. Neubauer B, Machura K, Chen M, Weinstein LS, Oppermann M, Sequeria-Lopez ML, Gomez RA, Schnermann J, Castrop H, Kurtz A, Wagner C. Development of renin expression in the kidney critically depends on the cyclic pathway. Am J Physiol Renal Physiol 296: F1006 –F1012, 2009. Neves FA, Duncan KG, Baxter JD. Cathepsin B is a prorenin processing enzyme. Hypertension 27: 514 –517, 1996. Nguyen G, Delarue F, Berrou J, Rondeau E, Sraer JD. Specific receptor binding of renin on human mesangial cells in culture increases plasminogen activator inhibitor-1 antigen. Kidney Int 50: 1897–1903, 1996. Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest 109: 1417–1427, 2002. Nickenig G, Baumer AT, Grohe C, Kahlert S, Strehlow K, Rosenkranz S, Stablein A, Beckers F, Smits JF, Daemen MJ, Vetter H, Bohm M. Estrogen modulates AT1 receptor gene expression in vitro and in vivo. Circulation 97: 2197–2201, 1998. Nickenig G, Bohm M. Regulation of the angiotensin AT1 receptor expression by hypercholesterolemia. Eur J Med Res 2: 285–289, 1997. Nickenig G, Strehlow K, Wassmann S, Baumer AT, Albory K, Sauer H, Bohm M. Differential effects of estrogen and progesterone on AT(1) receptor gene expression in vascular smooth muscle cells. Circulation 102: 1828 –1833, 2000. Nishiyama A, Majid DS, Taher KA, Miyatake A, Navar LG. Relation between renal interstitial ATP concentrations and autoregulation-mediated changes in renal vascular resistance. Circ Res 86: 656 – 662, 2000. Nishiyama A, Majid DS, Walker M 3rd, Miyatake A, Navar LG. Renal interstitial ATP responses to changes in arterial pressure during alterations in tubuloglomerular feedback activity. Hypertension 37: 753–759, 2001. Noble AR, Abu-Kishk RA, D’Aloia MA, Williams BC, Lush DJ. Cyclic GMP-linked pathway for renin secretion. Kidney Int 46: 1588 –1590, 1994. Norregaard R, Uhrenholt TR, Bistrup C, Skott O, Jensen BL. Stimulation of 11-beta-hydroxysteroid dehydrogenase type 2 in rat colon but not in kidney by low dietary NaCl intake. Am J Physiol Renal Physiol 285: F348 –F358, 2003. Nusing RM, Treude A, Weissenberger C, Jensen B, Bek M, Wagner C, Narumiya S, Seyberth HW. Dominant role of prostaglandin E2 EP4 receptor in furosemide-induced salt-losing tubulopathy: a model for hyperprostaglandin E syndrome/antenatal Bartter syndrome. J Am Soc Nephrol 16: 2354 –2362, 2005. O’Connell DP, Botkin SJ, Ramos SI, Sibley DR, Ariano MA, Felder RA, Carey RM. Localization of dopamine D1A receptor protein in rat kidneys. Am J Physiol Renal Fluid Electrolyte Physiol 268: F1185–F1197, 1995. O’Shaughnessy KM, Karet FE. Salt handling and hypertension. J Clin Invest 113: 1075–1081, 2004. O’Tierney PF, Komolova M, Tse MY, Adams MA, Pang SC. Altered regulation of renal interstitial hydrostatic pressure and the renal renin-angiotensin system in the absence of atrial natriuretic peptide. J Hypertens 26: 303–311, 2008. Obana K, Naruse M, Naruse K, Sakurai H, Demura H, Inagami T, Shizume K. Synthetic rat atrial natriuretic factor inhibits in vitro and in vivo renin secretion in rats. Endocrinology 117: 1282–1284, 1985. Obermuller N, Kunchaparty S, Ellison DH, Bachmann S. Expression of the Na-K-2Cl cotransporter by macula densa and thick ascending limb cells of rat and rabbit nephron. J Clin Invest 98: 635– 640, 1996. Oelkers W, Berger V, Bolik A, Bahr V, Hazard B, Beier S, Elger W, Heithecker A. Dihydrospirorenone, a new progestogen with antimineralocorticoid activity: effects on ovulation, electrolyte ex- 90 • APRIL 2010 • www.prv.org PHYSIOLOGY OF KIDNEY RENIN 642. 643. 644. 645. 646. 647. 648. 649. 650. 651. 652. 653. 654. 655. 656. 657. 658. 659. 660. cretion, and the renin-aldosterone system in normal women. J Clin Endocrinol Metab 73: 837– 842, 1991. Oelkers WK. Effects of estrogens and progestogens on the reninaldosterone system and blood pressure. Steroids 61: 166 –171, 1996. Ohashi K, Kihara S, Ouchi N, Kumada M, Fujita K, Hiuge A, Hibuse T, Ryo M, Nishizawa H, Maeda N, Maeda K, Shibata R, Walsh K, Funahashi T, Shimomura I. Adiponectin replenishment ameliorates obesity-related hypertension. Hypertension 47: 1108 – 1116, 2006. Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, Ohashi K, Sakai N, Shimomura I, Kobayashi H, Terasaka N, Inaba T, Funahashi T, Matsuzawa Y. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation 106: 2767–2770, 2002. Oliveira DR, Santos RA, Santos GF, Khosla M, CampagnoleSantos MJ. Changes in the baroreflex control of heart rate produced by central infusion of selective angiotensin antagonists in hypertensive rats. Hypertension 27: 1284 –1290, 1996. Oliverio MI, Best CF, Smithies O, Coffman TM. Regulation of sodium balance and blood pressure by the AT(1A) receptor for angiotensin II. Hypertension 35: 550 –554, 2000. Oliverio MI, Madsen K, Best CF, Ito M, Maeda N, Smithies O, Coffman TM. Renal growth and development in mice lacking AT1A receptors for angiotensin II. Am J Physiol Renal Physiol 274: F43–F50, 1998. Onda T, Hashimoto Y, Nagai M, Kuramochi H, Saito S, Yamazaki H, Toya Y, Sakai I, Homcy CJ, Nishikawa K, Ishikawa Y. Type-specific regulation of adenylyl cyclase. Selective pharmacological stimulation and inhibition of adenylyl cyclase isoforms. J Biol Chem 276: 47785– 47793, 2001. Oppermann M, Freedman NJ, Alexander RW, Lefkowitz RJ. Phosphorylation of the type 1A angiotensin II receptor by G protein-coupled receptor kinases and protein kinase C. J Biol Chem 271: 13266 –13272, 1996. Oppermann M, Friedman DJ, Faulhaber-Walter R, Mizel D, Castrop H, Enjyoji K, Robson SC, Schnermann JB. Tubuloglomerular feedback and renin secretion in NTPDase1/CD39-deficient mice. Am J Physiol Renal Physiol 294: F965–F970, 2008. Oppermann M, Mizel D, Huang G, Li C, Deng C, Theilig F, Bachmann S, Briggs J, Schnermann J, Castrop H. Macula densa control of renin secretion and preglomerular resistance in mice with selective deletion of the B isoform of the Na,K,2Cl co-transporter. J Am Soc Nephrol 17: 2143–2152, 2006. Oppermann M, Mizel D, Kim SM, Chen L, Faulhaber-Walter R, Huang Y, Li C, Deng C, Briggs J, Schnermann J, Castrop H. Renal function in mice with targeted disruption of the A isoform of the Na-K-2Cl co-transporter. J Am Soc Nephrol 18: 440 – 448, 2007. Ortiz-Capisano MC, Ortiz PA, Garvin JL, Harding P, Beierwaltes WH. Expression and function of the calcium-sensing receptor in juxtaglomerular cells. Hypertension 50: 737–743, 2007. Ortiz-Capisano MC, Ortiz PA, Harding P, Garvin JL, Beierwaltes WH. Adenylyl cyclase isoform V mediates renin release from juxtaglomerular cells. Hypertension 49: 618 – 624, 2007. Ortiz-Capisano MC, Ortiz PA, Harding P, Garvin JL, Beierwaltes WH. Decreased intracellular calcium stimulates renin release via calcium-inhibitable adenylyl cyclase. Hypertension 49: 162–169, 2007. Osei SY, Ahima RS, Minkes RK, Weaver JP, Khosla MC, Kadowitz PJ. Differential responses to angiotensin-(1–7) in the feline mesenteric and hindquarters vascular beds. Eur J Pharmacol 234: 35– 42, 1993. Oshita A, Iwai M, Chen R, Ide A, Okumura M, Fukunaga S, Yoshii T, Mogi M, Higaki J, Horiuchi M. Attenuation of inflammatory vascular remodeling by angiotensin II type 1 receptorassociated protein. Hypertension 48: 671– 676, 2006. Ostrowski NL, Lolait SJ. Oxytocin receptor gene expression in female rat kidney. The effect of estrogen. Adv Exp Med Biol 395: 329 –340, 1995. Ostrowski NL, Young WS 3rd, Lolait SJ. Estrogen increases renal oxytocin receptor gene expression. Endocrinology 136: 1801– 1804, 1995. Otto JC, Smith WL. Prostaglandin endoperoxide synthases-1 and -2. J Lipid Mediat Cell Signal 12: 139 –156, 1995. Physiol Rev • VOL 665 661. Oudit GY, Herzenberg AM, Kassiri Z, Wong D, Reich H, Khokha R, Crackower MA, Backx PH, Penninger JM, Scholey JW. Loss of angiotensin-converting enzyme-2 leads to the late development of angiotensin II-dependent glomerulosclerosis. Am J Pathol 168: 1808 –1820, 2006. 662. Pals DT, Couch SJ. Renin release induced by losartan (DuP 753), an angiotensin II receptor antagonist. Clin Exp Hypertens 15: 1–13, 1993. 663. Pan L, Black TA, Shi Q, Jones CA, Petrovic N, Loudon J, Kane C, Sigmund CD, Gross KW. Critical roles of a cAMP responsive element and an E-box in regulation of mouse renin gene expression. J Biol Chem 276: 45530 – 45538, 2001. 664. Pan L, Glenn ST, Jones CA, Gronostajski RM, Gross KW. Regulation of renin enhancer activity by nuclear factor I and Sp1/ Sp3. Biochim Biophys Acta 1625: 280 –290, 2003. 665. Pan L, Glenn ST, Jones CA, Gross KW. Activation of the rat renin promoter by HOXD10. PBX1b PREP1, Ets-1, and the intracellular domain of notch. J Biol Chem 280: 20860 –20866, 2005. 666. Pan L, Gross KW. Transcriptional regulation of renin: an update. Hypertension 45: 3– 8, 2005. 667. Pan L, Jones CA, Glenn ST, Gross KW. Identification of a novel region in the proximal promoter of the mouse renin gene critical for expression. Am J Physiol Renal Physiol 286: F1107–F1115, 2004. 668. Pan L, Wang Y, Jones CA, Glenn ST, Baumann H, Gross KW. Enhancer-dependent inhibition of mouse renin transcription by inflammatory cytokines. Am J Physiol Renal Physiol 288: F117– F124, 2005. 669. Pan L, Xie Y, Black TA, Jones CA, Pruitt SC, Gross KW. An Abd-B class HOX. PBX recognition sequence is required for expression from the mouse Ren-1c gene. J Biol Chem 276: 32489 –32494, 2001. 670. Pandey KN, Maki M, Inagami T. Detection of renin mRNA in mouse testis by hybridization with renin cDNA probe. Biochem Biophys Res Commun 125: 662– 667, 1984. 671. Panthier JJ, Foote S, Chambraud B, Strosberg AD, Corvol P, Rougeon F. Complete amino acid sequence and maturation of the mouse submaxillary gland renin precursor. Nature 298: 90 –92, 1982. 672. Park F, Mattson DL, Skelton MM, Cowley AW Jr. Localization of the vasopressin V1a and V2 receptors within the renal cortical and medullary circulation. Am J Physiol Regul Integr Comp Physiol 273: R243–R251, 1997. 673. Parkes DG, May CN. ACTH-suppressive and vasodilator actions of adrenomedullin in conscious sheep. J Neuroendocrinol 7: 923– 929, 1995. 674. Paul M, Poyan Mehr A, Kreutz R. Physiology of local reninangiotensin systems. Physiol Rev 86: 747– 803, 2006. 675. Paula RD, Lima CV, Britto RR, Campagnole-Santos MJ, Khosla MC, Santos RA. Potentiation of the hypotensive effect of bradykinin by angiotensin-(1–7)-related peptides. Peptides 20: 493– 500, 1999. 676. Paula RD, Lima CV, Khosla MC, Santos RA. Angiotensin-(1–7) potentiates the hypotensive effect of bradykinin in conscious rats. Hypertension 26: 1154 –1159, 1995. 677. Paxton WG, Runge M, Horaist C, Cohen C, Alexander RW, Bernstein KE. Immunohistochemical localization of rat angiotensin II AT1 receptor. Am J Physiol Renal Fluid Electrolyte Physiol 264: F989 –F995, 1993. 678. Pearson JD, Gordon JL. Vascular endothelial and smooth muscle cells in culture selectively release adenine nucleotides. Nature 281: 384 –386, 1979. 679. Peiro C, Vallejo S, Gembardt F, Azcutia V, Heringer-Walther S, Rodriguez-Manas L, Schultheiss HP, Sanchez-Ferrer CF, Walther T. Endothelial dysfunction through genetic deletion or inhibition of the G protein-coupled receptor Mas: a new target to improve endothelial function. J Hypertens 25: 2421–2425, 2007. 680. Pendergrass KD, Averill DB, Ferrario CM, Diz DI, Chappell MC. Differential expression of nuclear AT1 receptors and angiotensin II within the kidney of the male congenic mRen2 Lewis rat. Am J Physiol Renal Physiol 290: F1497–F1506, 2006. 681. Pentz ES, Lopez ML, Cordaillat M, Gomez RA. Identity of the renin cell is mediated by cAMP and chromatin remodeling: an 90 • APRIL 2010 • www.prv.org 666 682. 683. 684. 685. 686. 687. 688. 689. 690. 691. 692. 693. 694. 695. 696. 697. 698. 699. 700. 701. CASTROP ET AL. in vitro model for studying cell recruitment and plasticity. Am J Physiol Heart Circ Physiol 294: H699 –H707, 2008. Pentz ES, Moyano MA, Thornhill BA, Sequeira Lopez ML, Gomez RA. Ablation of renin-expressing juxtaglomerular cells results in a distinct kidney phenotype. Am J Physiol Regul Integr Comp Physiol 286: R474 –R483, 2004. Persson PB, Baumann JE, Ehmke H, Hackenthal E, Kirchheim HR, Nafz B. Endothelium-derived NO stimulates pressure-dependent renin release in conscious dogs. Am J Physiol Renal Fluid Electrolyte Physiol 264: F943–F947, 1993. Persson PB, Ehmke H, Nafz B, Lang R, Hackenthal E, Nobiling R, Dietrich MS, Kirchheim HR. Effects of neuropeptide-Y on renal function and its interaction with sympathetic stimulation in conscious dogs. J Physiol 444: 289 –302, 1991. Peters J, Farrenkopf R, Clausmeyer S, Zimmer J, Kantachuvesiri S, Sharp MG, Mullins JJ. Functional significance of prorenin internalization in the rat heart. Circ Res 90: 1135–1141, 2002. Peters TK, Kaczmarczyk G. Plasma renin activity during hypotensive responses to electrical stimulation carotid sinus nerves in conscious dogs. Clin Exp Pharmacol Physiol 21: 1– 8, 1994. Peti-Peterdi J, Fintha A, Fuson AL, Tousson A, Chow RH. Real-time imaging of renin release in vitro. Am J Physiol Renal Physiol 287: F329 –F335, 2004. Peti-Peterdi J, Kang JJ, Toma I. Activation of the renal reninangiotensin system in diabetes–new concepts. Nephrol Dial Transplant 23: 3047–3049, 2008. Peti-Peterdi J, Komlosi P, Fuson AL, Guan Y, Schneider A, Qi Z, Redha R, Rosivall L, Breyer MD, Bell PD. Luminal NaCl delivery regulates basolateral PGE2 release from macula densa cells. J Clin Invest 112: 76 – 82, 2003. Petrovic N, Black TA, Fabian JR, Kane C, Jones CA, Loudon JA, Abonia JP, Sigmund CD, Gross KW. Role of proximal promoter elements in regulation of renin gene transcription. J Biol Chem 271: 22499 –22505, 1996. Petrovic N, Kane CM, Sigmund CD, Gross KW. Downregulation of renin gene expression by interleukin-1. Hypertension 30: 230 – 235, 1997. Pfeilschifter J, Kurtz A, Bauer C. Inhibition of renin secretion by platelet activating factor (acetylglyceryl ether phosphorylcholine) in cultured rat renal juxtaglomerular cells. Biochem Biophys Res Commun 127: 903–910, 1985. Pfister A, Waeber B, Nussberger J, Brunner HR. Neuropeptide Y normalizes renin secretion in adrenalectomized rats without changing blood pressure. Life Sci 39: 2161–2167, 1986. Phat VN, Camilleri JP, Bariety J, Galtier M, Baviera E, Corvol P, Menard J. Immunohistochemical characterization of renin-containing cells in the human juxtaglomerular apparatus during embryonal and fetal development. Lab Invest 45: 387–390, 1981. Piggott LA, Hassell KA, Berkova Z, Morris AP, Silberbach M, Rich TC. Natriuretic peptides and nitric oxide stimulate cGMP synthesis in different cellular compartments. J Gen Physiol 128: 3–14, 2006. Pinet F, Mizrahi J, Laboulandine I, Menard J, Corvol P. Regulation of prorenin secretion in cultured human transfected juxtaglomerular cells. J Clin Invest 80: 724 –731, 1987. Porter JP, Said SI, Ganong WF. Vasoactive intestinal peptide stimulates renin secretion in vitro: evidence for a direct action of the peptide on the renal juxtaglomerular cells. Neuroendocrinology 36: 404 – 408, 1983. Potier M, Elliot SJ, Tack I, Lenz O, Striker GE, Striker LJ, Karl M. Expression and regulation of estrogen receptors in mesangial cells: influence on matrix metalloproteinase-9. J Am Soc Nephrol 12: 241–251, 2001. Pratt RE, Carleton JE, Richie JP, Heusser C, Dzau VJ. Human renin biosynthesis and secretion in normal and ischemic kidneys. Proc Natl Acad Sci USA 84: 7837–7840, 1987. Pratt RE, Dzau VJ, Ouellette AJ. Influence of androgen on translatable renin mRNA in the mouse submandibular gland. Hypertension 6: 605– 613, 1984. Predel HG, Kuklinski P, Kramer HJ. Intranasal application of atrial natriuretic peptide and 1-deamino-D-arginine vasopressin in healthy volunteers. Hemodynamic, hormonal, and renal excretory effects. Am J Hypertens 5: 657– 660, 1992. Physiol Rev • VOL 702. Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, Ozawa Y, Hering-Smith KS, Hamm LL, Navar LG. Enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Hypertension 44: 223–229, 2004. 703. Pupilli C, Gomez RA, Tuttle JB, Peach MJ, Carey RM. Spatial association of renin-containing cells and nerve fibers in developing rat kidney. Pediatr Nephrol 5: 690 – 695, 1991. 704. Quail AW, Woods RL, Korner PI. Cardiac and arterial baroreceptor influences in release of vasopressin and renin during hemorrhage. Am J Physiol Heart Circ Physiol 252: H1120 –H1126, 1987. 705. Quan A, Chakravarty S, Chen JK, Chen JC, Loleh S, Saini N, Harris RC, Capdevila J, Quigley R. Androgens augment proximal tubule transport. Am J Physiol Renal Physiol 287: F452–F459, 2004. 706. Quinkler M, Bumke-Vogt C, Meyer B, Bahr V, Oelkers W, Diederich S. The human kidney is a progesterone-metabolizing and androgen-producing organ. J Clin Endocrinol Metab 88: 2803– 2809, 2003. 707. Quinkler M, Diederich S, Bahr V, Oelkers W. The role of progesterone metabolism and androgen synthesis in renal blood pressure regulation. Horm Metab Res 36: 381–386, 2004. 708. Rabin M, Birnbaum D, Young D, Birchmeier C, Wigler M, Ruddle FH. Human ros1 and mas1 oncogenes located in regions of chromosome 6 associated with tumor-specific rearrangements. Oncogene Res 1: 169 –178, 1987. 709. Radke KJ, Willis LR, Zimmerman GW, Weinberger MH, Selkurt EE. Effects of histamine-receptor antagonists on histamine-stimulated renin secretion. Eur J Pharmacol 123: 421– 426, 1986. 710. Rahmouni K, Correia ML, Haynes WG, Mark AL. Obesityassociated hypertension: new insights into mechanisms. Hypertension 45: 9 –14, 2005. 711. Rahmouni K, Morgan DA. Hypothalamic arcuate nucleus mediates the sympathetic and arterial pressure responses to leptin. Hypertension 49: 647– 652, 2007. 712. Rahmouni K, Morgan DA, Morgan GM, Mark AL, Haynes WG. Role of selective leptin resistance in diet-induced obesity hypertension. Diabetes 54: 2012–2018, 2005. 713. Raizada MK, Ferreira AJ. ACE2: a new target for cardiovascular disease therapeutics. J Cardiovasc Pharmacol 50: 112–119, 2007. 714. Rakugi H, Nakamaru M, Saito H, Higaki J, Ogihara T. Endothelin inhibits renin release from isolated rat glomeruli. Biochem Biophys Res Commun 155: 1244 –1247, 1988. 715. Rasch R, Jensen BL, Nyengaard JR, Skott O. Quantitative changes in rat renin secretory granules after acute and chronic stimulation of the renin system. Cell Tissue Res 292: 563–571, 1998. 716. Rasmussen MS, Simonsen JA, Sandgaard NC, HoilundCarlsen PF, Bie P. Effects of oxytocin in normal man during low and high sodium diets. Acta Physiol Scand 181: 247–257, 2004. 717. Rasmussen MS, Simonsen JA, Sandgaard NC, HoilundCarlsen PF, Bie P. Mechanisms of acute natriuresis in normal humans on low sodium diet. J Physiol 546: 591– 603, 2003. 718. Razga Z, Nyengaard JR. Estimation of the number of angiotensin II AT1 receptors in rat kidney afferent and efferent arterioles. Anal Quant Cytol Histol 29: 208 –216, 2007. 719. Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension 37: 1199 –1208, 2001. 720. Reddi V, Zaglul A, Pentz ES, Gomez RA. Renin-expressing cells are associated with branching of the developing kidney vasculature. J Am Soc Nephrol 9: 63–71, 1998. 721. Regoli D, Nsa Allogho S, Rizzi A, Gobeil FJ. Bradykinin receptors and their antagonists. Eur J Pharmacol 348: 1–10, 1998. 722. Reid IA, Chou L. Effect of blockade of nitric oxide synthesis on the renin secretory response to furosemide in conscious rabbits. Clin Sci 88: 657– 663, 1995. 723. Reinalter SC, Jeck N, Brochhausen C, Watzer B, Nusing RM, Seyberth HW, Komhoff M. Role of cyclooxygenase-2 in hyperprostaglandin E syndrome/antenatal Bartter syndrome. Kidney Int 62: 253–260, 2002. 724. Rentzsch B, Todiras M, Iliescu R, Popova E, Campos LA, Oliveira ML, Baltatu OC, Santos RA, Bader M. Transgenic angiotensin-converting enzyme 2 overexpression in vessels of 90 • APRIL 2010 • www.prv.org PHYSIOLOGY OF KIDNEY RENIN 725. 726. 727. 728. 729. 730. 731. 732. 733. 734. 735. 736. 737. 738. 739. 740. 741. 742. 743. 744. SHRSP rats reduces blood pressure and improves endothelial function. Hypertension 52: 967–973, 2008. Rettig R, Grisk O. The kidney as a determinant of genetic hypertension: evidence from renal transplantation studies. Hypertension 46: 463– 468, 2005. Richoux JP, Amsaguine S, Grignon G, Bouhnik J, Menard J, Corvol P. Earliest renin containing cell differentiation during ontogenesis in the rat. An immunocytochemical study. Histochemistry 88: 41– 46, 1987. Ritthaler T, Della Bruna R, Kramer BK, Kurtz A. Endothelins inhibit cyclic-AMP induced renin gene expression in cultured mouse juxtaglomerular cells. Kidney Int 50: 108 –115, 1996. Ritthaler T, Scholz H, Ackermann M, Riegger G, Kurtz A, Kramer BK. Effects of endothelins on renin secretion from isolated mouse renal juxtaglomerular cells. Am J Physiol Renal Fluid Electrolyte Physiol 268: F39 –F45, 1995. Rohrwasser A, Morgan T, Dillon HF, Zhao L, Callaway CW, Hillas E, Zhang S, Cheng T, Inagami T, Ward K, Terreros DA, Lalouel JM. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension 34: 1265–1274, 1999. Rowe BP, Saylor DL, Speth RC, Absher DR. Angiotensin-(1–7) binding at angiotensin II receptors in the rat brain. Regul Pept 56: 139 –146, 1995. Ruan X, Wagner C, Chatziantoniou C, Kurtz A, Arendshorst WJ. Regulation of angiotensin II receptor AT1 subtypes in renal afferent arterioles during chronic changes in sodium diet. J Clin Invest 99: 1072–1081, 1997. Russ U, Rauch U, Quast U. Pharmacological evidence for a KATP channel in renin-secreting cells from rat kidney. J Physiol 517: 781–790, 1999. Russo D, Minutolo R, Clienti C, De Nicola L, Iodice C, Savino FA, Andreucci VE. Endothelin-1 released by vascular smooth muscle cells enhances vascular responsiveness of rat mesenteric arterial bed exposed to high perfusion flow. Am J Hypertens 12: 1119 –1123, 1999. Ryan GB, Alcorn D, Coghlan JP, Hill PA, Jacobs R. Ultrastructural morphology of granule release from juxtaglomerular myoepithelioid and peripolar cells. Kidney Int Suppl 12: S3–S8, 1982. Ryan JW. Renin-like enzyme in the adrenal gland. Science 158: 1589 –1590, 1967. Ryan MJ, Black TA, Gross KW, Hajduczok G. Cyclic mechanical distension regulates renin gene transcription in As4.1 cells. Am J Physiol Endocrinol Metab 279: E830 –E837, 2000. Ryan MJ, Black TA, Millard SL, Gross KW, Hajduczok G. Endothelin-1 increases calcium and attenuates renin gene expression in As4.1 cells. Am J Physiol Heart Circ Physiol 283: H2458 – H2465, 2002. Ryan MJ, Gross KW, Hajduczok G. Calcium-dependent activation of phospholipase C by mechanical distension in renin-expressing As4.1 cells. Am J Physiol Endocrinol Metab 279: E823–E829, 2000. Sabolic I, Asif AR, Budach WE, Wanke C, Bahn A, Burckhardt G. Gender differences in kidney function. Pflügers Arch 455: 397– 429, 2007. Sadagopan N, Li W, Roberds SL, Major T, Preston GM, Yu Y, Tones MA. Circulating succinate is elevated in rodent models of hypertension and metabolic disease. Am J Hypertens 20: 1209 – 1215, 2007. Saigusa T, Iriki M, Arita J. Brain angiotensin II tonically modulates sympathetic baroreflex in rabbit ventrolateral medulla. Am J Physiol Heart Circ Physiol 271: H1015–H1021, 1996. Sakima A, Averill DB, Gallagher PE, Kasper SO, Tommasi EN, Ferrario CM, Diz DI. Impaired heart rate baroreflex in older rats: role of endogenous angiotensin-(1–7) at the nucleus tractus solitarii. Hypertension 46: 333–340, 2005. Salazar FJ, Fiksen-Olsen MJ, Opgenorth TJ, Granger JP, Burnett JC Jr, Romero JC. Renal effects of ANP without changes in glomerular filtration rate and blood pressure. Am J Physiol Renal Fluid Electrolyte Physiol 251: F532–F536, 1986. Sampaio WO, Souza dos Santos RA, Faria-Silva R, da Mata Machado LT, Schiffrin EL, Touyz RM. Angiotensin-(1–7) through receptor Mas mediates endothelial nitric oxide synthase Physiol Rev • VOL 745. 746. 747. 748. 749. 750. 751. 752. 753. 754. 755. 756. 757. 758. 759. 760. 761. 762. 667 activation via Akt-dependent pathways. Hypertension 49: 185–192, 2007. Sanada H, Yao L, Jose PA, Carey RM, Felder RA. Dopamine D3 receptors in rat juxtaglomerular cells. Clin Exp Hypertens 19: 93–105, 1997. Sandgaard NC, Andersen JL, Bie P. Hormonal regulation of renal sodium and water excretion during normotensive sodium loading in conscious dogs. Am J Physiol Regul Integr Comp Physiol 278: R11–R18, 2000. Sandgaard NC, Andersen JL, Holstein-Rathlou NH, Bie P. Saline-induced natriuresis and renal blood flow in conscious dogs: effects of sodium infusion rate and concentration. Acta Physiol Scand 185: 237–250, 2005. Santos RA, Baracho NC. Angiotensin-(1–7) is a potent antidiuretic peptide in rats. Braz J Med Biol Res 25: 651– 654, 1992. Santos RA, Brosnihan KB, Jacobsen DW, DiCorleto PE, Ferrario CM. Production of angiotensin-(1–7) by human vascular endothelium. Hypertension 19: II56 – 61, 1992. Santos RA, Castro CH, Gava E, Pinheiro SV, Almeida AP, Paula RD, Cruz JS, Ramos AS, Rosa KT, Irigoyen MC, Bader M, Alenina N, Kitten GT, Ferreira AJ. Impairment of in vitro and in vivo heart function in angiotensin-(1–7) receptor MAS knockout mice. Hypertension 47: 996 –1002, 2006. Santos RA, Ferreira AJ, Nadu AP, Braga AN, de Almeida AP, Campagnole-Santos MJ, Baltatu O, Iliescu R, Reudelhuber TL, Bader M. Expression of an angiotensin-(1–7)-producing fusion protein produces cardioprotective effects in rats. Physiol Genomics 17: 292–299, 2004. Santos RA, Ferreira AJ, Simoes e Silva AC. Recent advances in the angiotensin-converting enzyme 2-angiotensin(1–7)-Mas axis. Exp Physiol 93: 519 –527, 2008. Santos RA, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de Buhr I, Heringer-Walther S, Pinheiro SV, Lopes MT, Bader M, Mendes EP, Lemos VS, Campagnole-Santos MJ, Schultheiss HP, Speth R, Walther T. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci USA 100: 8258 – 8263, 2003. Santos SH, Fernandes LR, Mario EG, Ferreira AV, Porto LC, Alvarez-Leite JI, Botion LM, Bader M, Alenina N, Santos RA. Mas deficiency in Fvb/N mice produces marked changes in lipid and glycemic metabolism. Diabetes 57: 340 –347, 2008. Satofuka S, Ichihara A, Nagai N, Yamashiro K, Koto T, Shinoda H, Noda K, Ozawa Y, Inoue M, Tsubota K, Suzuki F, Oike Y, Ishida S. Suppression of ocular inflammation in endotoxininduced uveitis by inhibiting nonproteolytic activation of prorenin. Invest Ophthalmol Vis Sci 47: 2686 –2692, 2006. Sauter A, Machura K, Neubauer B, Kurtz A, Wagner C. Development of renin expression in the mouse kidney. Kidney Int 73: 43–51, 2008. Schiavone MT, Santos RA, Brosnihan KB, Khosla MC, Ferrario CM. Release of vasopressin from the rat hypothalamo-neurohypophysial system by angiotensin-(1–7) heptapeptide. Proc Natl Acad Sci USA 85: 4095– 4098, 1988. Schlatter E, Salomonsson M, Persson AE, Greger R. Macula densa cells sense luminal NaCl concentration via furosemide sensitive Na⫹2Cl⫺K⫹ cotransport. Pflügers Arch 414: 286 –290, 1989. Schliebe N, Strotmann R, Busse K, Mitschke D, Biebermann H, Schomburg L, Kohrle J, Bar J, Rompler H, Wess J, Schoneberg T, Sangkuhl K. V2 vasopressin receptor deficiency causes changes in expression and function of renal and hypothalamic components involved in electrolyte and water homeostasis. Am J Physiol Renal Physiol 295: F1177–F1190, 2008. Schmid C, Castrop H, Reitbauer J, Della Bruna R, Kurtz A. Dietary salt intake modulates angiotensin II type 1 receptor gene expression. Hypertension 29: 923–929, 1997. Scholz H, Jurk S, Kurtz A. Hypotonic swelling or stretch does not change cytosolic calcium in mouse renal juxtaglomerular cells. Acta Physiol Scand 157: 283–287, 1996. Scholz H, Kaissling B, Inagami T, Kurtz A. Differential response of renin secretion to vasoconstrictors in the isolated perfused rat kidney. J Physiol 441: 453– 468, 1991. 90 • APRIL 2010 • www.prv.org 668 CASTROP ET AL. 763. Scholz H, Kramer BK, Hamann M, Gotz KH, Kurtz A. Effects of endothelins on renin secretion from rat kidneys. Acta Physiol Scand 155: 173–182, 1995. 764. Scholz H, Kurtz A. Differential regulation of cytosolic calcium between afferent arteriol ar smooth muscle cells from mouse kidney. Pflügers Arch 431: 46 –51, 1995. 765. Scholz H, Kurtz A. Involvement of endothelium-derived relaxing factor in the pressure control of renin secretion from isolated perfused kidney. J Clin Invest 91: 1088 –1094, 1993. 766. Scholz H, Vogel U, Kurtz A. Interrelation between baroreceptor and macula densa mechanisms in the control of renin secretion. J Physiol 469: 511–524, 1993. 767. Schricker K, Hamann M, Kaissling B, Kurtz A. Role of the macula densa in the control of renal renin gene expression in two-kidney/one-clip rats. Pflügers Arch 427: 42– 46, 1994. 768. Schricker K, Hamann M, Kurtz A. Nitric oxide and prostaglandins are involved in the macula densa control of the renin system. Am J Physiol Renal Fluid Electrolyte Physiol 269: F825–F830, 1995. 769. Schricker K, Hamann M, Kurtz A. Prostaglandins are involved in the stimulation of renin gene expression in 2 kidney-1 clip rats. Pflügers Arch 430: 188 –194, 1995. 770. Schricker K, Hegyi I, Hamann M, Kaissling B, Kurtz A. Tonic stimulation of renin gene expression by nitric oxide is counteracted by tonic inhibition through angiotensin II. Proc Natl Acad Sci USA 92: 8006 – 8010, 1995. 771. Schricker K, Kurtz A. Liberators of NO exert a dual effect on renin secretion from isolated mouse renal juxtaglomerular cells. Am J Physiol Renal Fluid Electrolyte Physiol 265: F180 –F186, 1993. 772. Schricker K, Potzl B, Hamann M, Kurtz A. Coordinate changes of renin and brain-type nitric-oxide-synthase (b-NOS) mRNA levels in rat kidneys. Pflügers Arch 432: 394 – 400, 1996. 773. Schricker K, Ritthaler T, Kramer BK, Kurtz A. Effect of endothelium-derived relaxing factor on renin secretion from isolated mouse renal juxtaglomerular cells. Acta Physiol Scand 149: 347– 354, 1993. 774. Schunkert H, Danser AH, Hense HW, Derkx FH, Kurzinger S, Riegger GA. Effects of estrogen replacement therapy on the reninangiotensin system in postmenopausal women. Circulation 95: 39 – 45, 1997. 775. Schunkert H, Ingelfinger JR, Jacob H, Jackson B, Bouyounes B, Dzau VJ. Reciprocal feedback regulation of kidney angiotensinogen and renin mRNA expressions by angiotensin II. Am J Physiol Endocrinol Metab 263: E863–E869, 1992. 776. Schwartz J, Reid IA. Role of the vasoconstrictor and antidiuretic activities of vasopressin in inhibition of renin secretion in conscious dogs. Am J Physiol Renal Fluid Electrolyte Physiol 250: F92–F96, 1986. 777. Schweda F, Kammerl M, Wagner C, Kramer BK, Kurtz A. Upregulation of macula densa cyclooxygenase-2 expression is not dependent on glomerular filtration. Am J Physiol Renal Physiol 287: F95–F101, 2004. 778. Schweda F, Klar J, Narumiya S, Nusing RM, Kurtz A. Stimulation of renin release by prostaglandin E2 is mediated by EP2 and EP4 receptors in mouse kidneys. Am J Physiol Renal Physiol 287: F427–F433, 2004. 779. Schweda F, Kurtz A. Cellular mechanism of renin release. Acta Physiol Scand 181: 383–390, 2004. 780. Schweda F, Kurtz L, de Wit C, Janssen-Bienhold U, Kurtz A, Wagner C. Substitution of connexin40 with connexin45 prevents hyperreninemia and attenuates hypertension. Kidney Int 75: 482– 489, 2009. 781. Schweda F, Riegger GA, Kurtz A, Kramer BK. Store-operated calcium influx inhibits renin secretion. Am J Physiol Renal Physiol 279: F170 –F176, 2000. 782. Schweda F, Schweda A, Pfeifer M, Blumberg FC, Kammerl MC, Holmer SR, Riegger GA, Kramer BK. Role of endothelins for the regulation of renal renin gene expression. J Cardiovasc Pharmacol 36: S187–190, 2000. 783. Schweda F, Segerer F, Castrop H, Schnermann J, Kurtz A. Blood pressure-dependent inhibition of renin secretion requires A1 adenosine receptors. Hypertension 46: 780 –786, 2005. Physiol Rev • VOL 784. Schweda F, Wagner C, Kramer BK, Schnermann J, Kurtz A. Preserved macula densa-dependent renin secretion in A1 adenosine receptor knockout mice. Am J Physiol Renal Physiol 284: F770 –F777, 2003. 785. Schwertschlag U, Hackenthal E. Histamine stimulates renin release from the isolated perfused rat kidney. Naunyn-Schmiedebergs Arch Pharmacol 319: 239 –242, 1982. 786. Schwiebert EM, Kishore BK. Extracellular nucleotide signaling along the renal epithelium. Am J Physiol Renal Physiol 280: F945– F963, 2001. 787. Sealey JE, Itskovitz-Eldor J, Rubattu S, James GD, August P, Thaler I, Levron J, Laragh JH. Estradiol- and progesteronerelated increases in the renin-aldosterone system: studies during ovarian stimulation and early pregnancy. J Clin Endocrinol Metab 79: 258 –264, 1994. 788. Sequeira Lopez ML, Pentz ES, Nomasa T, Smithies O, Gomez RA. Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev Cell 6: 719 –728, 2004. 789. Sequeira Lopez ML, Pentz ES, Robert B, Abrahamson DR, Gomez RA. Embryonic origin and lineage of juxtaglomerular cells. Am J Physiol Renal Physiol 281: F345–F356, 2001. 790. Shaltout HA, Westwood BM, Averill DB, Ferrario CM, Figueroa JP, Diz DI, Rose JC, Chappell MC. Angiotensin metabolism in renal proximal tubules, urine, and serum of sheep: evidence for ACE2-dependent processing of angiotensin II. Am J Physiol Renal Physiol 292: F82–F91, 2007. 791. Shanmugam S, Corvol P, Gasc JM. Angiotensin II type 2 receptor mRNA expression in the developing cardiopulmonary system of the rat. Hypertension 28: 91–97, 1996. 792. Shanmugam S, Sandberg K. Ontogeny of angiotensin II receptors. Cell Biol Int 20: 169 –176, 1996. 793. Shek EW, Brands MW, Hall JE. Chronic leptin infusion increases arterial pressure. Hypertension 31: 409 – 414, 1998. 794. Shi Q, Black TA, Gross KW, Sigmund CD. Species-specific differences in positive and negative regulatory elements in the renin gene enhancer. Circ Res 85: 479 – 488, 1999. 795. Shi Q, Gross KW, Sigmund CD. NF-Y antagonizes renin enhancer function by blocking stimulatory transcription factors. Hypertension 38: 332–336, 2001. 796. Shi Q, Gross KW, Sigmund CD. Retinoic acid-mediated activation of the mouse renin enhancer. J Biol Chem 276: 3597–3603, 2001. 797. Shi SJ, Nguyen HT, Sharma GD, Navar LG, Pandey KN. Genetic disruption of atrial natriuretic peptide receptor-A alters renin and angiotensin II levels. Am J Physiol Renal Physiol 281: F665– F673, 2001. 798. Shin LH, Dovgan PS, Nypaver TJ, Carretero OA, Beierwaltes WH. Role of neuropeptide Y in the development of two-kidney, one-clip renovascular hypertension in the rat. J Vasc Surg 32: 1015–1021, 2000. 799. Shivakumar BR, Wang Z, Hammond TG, Harris RC. EP24.15 interacts with the angiotensin II type I receptor and bradykinin B2 receptor. Cell Biochem Funct 23: 195–204, 2005. 800. Shoaf SE, Bramer SL, Bricmont P, Zimmer CA. Pharmacokinetic and pharmacodynamic interaction between tolvaptan, a nonpeptide AVP antagonist, furosemide or hydrochlorothiazide. J Cardiovasc Pharmacol 50: 213–222, 2007. 801. Shoaf SE, Wang Z, Bricmont P, Mallikaarjun S. Pharmacokinetics, pharmacodynamics, and safety of tolvaptan, a nonpeptide AVP antagonist, during ascending single-dose studies in healthy subjects. J Clin Pharmacol 47: 1498 –1507, 2007. 802. Shricker K, Holmer S, Kramer BK, Riegger GA, Kurtz A. The role of angiotensin II in the feedback control of renin gene expression. Pflügers Arch 434: 166 –172, 1997. 802a.Sigmund CD, Okuyama K, Ingelfinger J, Jones CA, Mullins JJ, Kane C, Kim U, Wu CZ, Kenny L, Rustum Y et al. Isolation and characterization of renin-expressing cell lines from transgenic mice containing a renin-promoter viral oncogene fusion construct. J Biol Chem 265: 19916 –19922, 1990. 803. Sigmund CD, Jones CA, Mullins JJ, Kim U, Gross KW. Expression of murine renin genes in subcutaneous connective tissue. Proc Natl Acad Sci USA 87: 7993–7997, 1990. 90 • APRIL 2010 • www.prv.org PHYSIOLOGY OF KIDNEY RENIN 804. Silva-Antonialli MM, Tostes RC, Fernandes L, Fior-Chadi DR, Akamine EH, Carvalho MH, Fortes ZB, Nigro D. A lower ratio of AT1/AT2 receptors of angiotensin II is found in female than in male spontaneously hypertensive rats. Cardiovasc Res 62: 587–593, 2004. 805. Silva AQ, Santos RA, Fontes MA. Blockade of endogenous angiotensin-(1–7) in the hypothalamic paraventricular nucleus reduces renal sympathetic tone. Hypertension 46: 341–348, 2005. 806. Silva DM, Vianna HR, Cortes SF, Campagnole-Santos MJ, Santos RA, Lemos VS. Evidence for a new angiotensin-(1–7) receptor subtype in the aorta of Sprague-Dawley rats. Peptides 28: 702–707, 2007. 807. Simon AM, McWhorter AR. Decreased intercellular dye-transfer and downregulation of non-ablated connexins in aortic endothelium deficient in connexin37 or connexin40. J Cell Sci 116: 2223– 2236, 2003. 808. Singh I, Grams M, Wang WH, Yang T, Killen P, Smart A, Schnermann J, Briggs JP. Coordinate regulation of renal expression of nitric oxide synthase, renin, and angiotensinogen mRNA by dietary salt. Am J Physiol Renal Fluid Electrolyte Physiol 270: F1027–F1037, 1996. 809. Sinn PL, Sigmund CD. Human renin mRNA stability is increased in response to cAMP in Calu-6 cells. Hypertension 33: 900 –905, 1999. 810. Sjoquist M, Huang W, Jacobsson E, Skott O, Stricker EM, Sved AF. Sodium excretion and renin secretion after continuous versus pulsatile infusion of oxytocin in rats. Endocrinology 140: 2814 –2818, 1999. 811. Skalweit A, Doller A, Huth A, Kahne T, Persson PB, Thiele BJ. Posttranscriptional control of renin synthesis: identification of proteins interacting with renin mRNA 3⬘-untranslated region. Circ Res 92: 419 – 427, 2003. 812. Skott O. Do osmotic forces play a role in renin secretion? Am J Physiol Renal Fluid Electrolyte Physiol 255: F1–F10, 1988. 813. Skott O. Episodic release of renin from single isolated superfused rat afferent arterioles. Pflügers Arch 407: 41– 45, 1986. 814. Skott O, Baumbach L. Effects of adenosine on renin release from isolated rat glomeruli and kidney slices. Pflügers Arch 404: 232– 237, 1985. 815. Skott O, Briggs JP. Direct demonstration of macula densa-mediated renin secretion. Science 237: 1618 –1620, 1987. 816. Smyth DD, Umemura S, Yang E, Pettinger WA. Inhibition of renin release by alpha-adrenoceptor stimulation in the isolated perfused rat kidney. Eur J Pharmacol 140: 33–38, 1987. 817. Smyth JT, Dehaven WI, Jones BF, Mercer JC, Trebak M, Vazquez G, Putney JW Jr. Emerging perspectives in store-operated Ca2⫹ entry: roles of Orai, Stim and TRP. Biochim Biophys Acta 1763: 1147–1160, 2006. 818. Sohl G, Willecke K. Gap junctions and the connexin protein family. Cardiovasc Res 62: 228 –232, 2004. 819. Soler MJ, Wysocki J, Ye M, Lloveras J, Kanwar Y, Batlle D. ACE2 inhibition worsens glomerular injury in association with increased ACE expression in streptozotocin-induced diabetic mice. Kidney Int 72: 614 – 623, 2007. 820. Song Q, Wang DZ, Harley RA, Chao L, Chao J. Cellular localization of low-molecular-weight kininogen and bradykinin B2 receptor mRNAs in human kidney. Am J Physiol Renal Fluid Electrolyte Physiol 270: F919 –F926, 1996. 821. Spat A, Hunyady L. Control of aldosterone secretion: a model for convergence in cellular signaling pathways. Physiol Rev 84: 489 – 539, 2004. 822. Speidel D, Varoqueaux F, Enk C, Nojiri M, Grishanin RN, Martin TF, Hofmann K, Brose N, Reim K. A family of Ca2⫹dependent activator proteins for secretion: comparative analysis of structure, expression, localization, and function. J Biol Chem 278: 52802–52809, 2003. 823. Stamler R, Stamler J, Riedlinger WF, Algera G, Roberts RH. Weight and blood pressure. Findings in hypertension screening of 1 million Americans. JAMA 240: 1607–1610, 1978. 824. Stankovic AR, Fisher ND, Hollenberg NK. Prorenin and angiotensin-dependent renal vasoconstriction in type 1 and type 2 diabetes. J Am Soc Nephrol 17: 3293–3299, 2006. Physiol Rev • VOL 669 825. Steckelings UM, Kaschina E, Unger T. The AT2 receptor–a matter of love and hate. Peptides 26: 1401–1409, 2005. 826. Steege A, Fahling M, Paliege A, Bondke A, Kirschner KM, Martinka P, Kaps C, Patzak A, Persson PB, Thiele BJ, Scholz H, Mrowka R. Wilms’ tumor protein (-KTS) modulates renin gene transcription. Kidney Int 74: 458 – 466, 2008. 827. Stichtenoth DO, Marhauer V, Tsikas D, Gutzki FM, Frolich JC. Effects of specific COX-2-inhibition on renin release and renal and systemic prostanoid synthesis in healthy volunteers. Kidney Int 68: 2197–2207, 2005. 828. Stoeckel ME, Freund-Mercier MJ, Palacios JM, Richard P, Porte A. Autoradiographic localization of binding sites for oxytocin and vasopressin in the rat kidney. J Endocrinol 113: 179 –182, 1987. 829. Stumpf WE, Sar M, Narbaitz R, Reid FA, DeLuca HF, Tanaka Y. Cellular and subcellular localization of 1,25-(OH)2-vitamin D3 in rat kidney: comparison with localization of parathyroid hormone and estradiol. Proc Natl Acad Sci USA 77: 1149 –1153, 1980. 830. Su Z, Zimpelmann J, Burns KD. Angiotensin-(1–7) inhibits angiotensin II-stimulated phosphorylation of MAP kinases in proximal tubular cells. Kidney Int 69: 2212–2218, 2006. 831. Summers RJ, Stephenson JA, Kuhar MJ. Localization of beta adrenoceptor subtypes in rat kidney by light microscopic autoradiography. J Pharmacol Exp Ther 232: 561–569, 1985. 832. Sun D, Samuelson LC, Yang T, Huang Y, Paliege A, Saunders T, Briggs J, Schnermann J. Mediation of tubuloglomerular feedback by adenosine: evidence from mice lacking adenosine 1 receptors. Proc Natl Acad Sci USA 98: 9983–9988, 2001. 833. Sun J, Oddoux C, Gilbert MT, Yan Y, Lazarus A, Campbell WG Jr, Catanzaro DF. Pituitary-specific transcription factor (Pit-1) binding site in the human renin gene 5⬘-flanking DNA stimulates promoter activity in placental cell primary cultures and pituitary lactosomatotropic cell lines. Circ Res 75: 624 – 629, 1994. 834. Sun J, Oddoux C, Lazarus A, Gilbert MT, Catanzaro DF. Promoter activity of human renin 5⬘-flanking DNA sequences is activated by the pituitary-specific transcription factor Pit-1. J Biol Chem 268: 1505–1508, 1993. 835. Susic D, Lippton H, Knight M, Frohlich ED. Cardiovascular effects of nonproteolytic activation of prorenin. Hypertension 48: e113–114, 2006. 836. Suter PM, Locher R, Hasler E, Vetter W. Is there a role for the ob gene product leptin in essential hypertension? Am J Hypertens 11: 1305–1311, 1998. 837. Suttner SW, Boldt J. Natriuretic peptide system: physiology and clinical utility. Curr Opin Crit Care 10: 336 –341, 2004. 838. Suzuki H, Handa M, Kondo K, Saruta T. Role of renin-angiotensin system in glucocorticoid hypertension in rats. Am J Physiol Endocrinol Metab 243: E48 –E51, 1982. 839. Suzuki M, Satoh S. Suppression of bradykinin-induced renin release by indomethacin in anesthetized rats. Clin Exp Hypertens 6: 1227–1235, 1984. 840. Suzuki S, Franco-Saenz R, Tan SY, Mulrow PJ. Direct action of kallikrein and other proteases on the renin-angiotensin system. Hypertension 3: I13–17, 1981. 841. Suzuki S, Franco-Saenz R, Tan SY, Mulrow PJ. Direct action of rat urinary kallikrein on rat kidney to release renin. J Clin Invest 66: 757–762, 1980. 842. Tahmasebi M, Puddefoot JR, Inwang ER, Vinson GP. The tissue renin-angiotensin system in human pancreas. J Endocrinol 161: 317–322, 1999. 843. Takagi M, Matsuoka H, Atarashi K, Yagi S. Endothelin: a new inhibitor of renin release. Biochem Biophys Res Commun 157: 1164 –1168, 1988. 844. Takahashi N, Lopez ML, Cowhig JE Jr, Taylor MA, Hatada T, Riggs E, Lee G, Gomez RA, Kim HS, Smithies O. Ren1c homozygous null mice are hypotensive and polyuric, but heterozygotes are indistinguishable from wild-type. J Am Soc Nephrol 16: 125–132, 2005. 845. Takayanagi R, Ohnaka K, Sakai Y, Nakao R, Yanase T, Haji M, Inagami T, Furuta H, Gou DF, Nakamuta M. Molecular cloning, sequence analysis and expression of a cDNA encoding human type-1 angiotensin II receptor. Biochem Biophys Res Commun 183: 910 –916, 1992. 90 • APRIL 2010 • www.prv.org 670 CASTROP ET AL. 846. Tallant EA, Clark MA. Molecular mechanisms of inhibition of vascular growth by angiotensin-(1–7). Hypertension 42: 574 –579, 2003. 847. Tallant EA, Ferrario CM, Gallagher PE. Angiotensin-(1–7) inhibits growth of cardiac myocytes through activation of the mas receptor. Am J Physiol Heart Circ Physiol 289: H1560 –H1566, 2005. 848. Tallant EA, Lu X, Weiss RB, Chappell MC, Ferrario CM. Bovine aortic endothelial cells contain an angiotensin-(1–7) receptor. Hypertension 29: 388 –393, 1997. 849. Tamaki T, Kiyomoto K, He H, Tomohiro A, Nishiyama A, Aki Y, Kimura S, Abe Y. Vasodilation induced by vasopressin V2 receptor stimulation in afferent arterioles. Kidney Int 49: 722–729, 1996. 850. Tamura K, Chen YE, Horiuchi M, Chen Q, Daviet L, Yang Z, Lopez-Ilasaca M, Mu H, Pratt RE, Dzau VJ. LXRalpha functions as a cAMP-responsive transcriptional regulator of gene expression. Proc Natl Acad Sci USA 97: 8513– 8518, 2000. 851. Tamura K, Chen YE, Tanaka Y, Sakai M, Tsurumi Y, Koide Y, Kihara M, Pratt RE, Horiuchi M, Umemura S, Dzau VJ. Nuclear receptor LXRalpha is involved in cAMP-mediated human renin gene expression. Mol Cell Endocrinol 224: 11–20, 2004. 852. Tamura K, Umemura S, Nyui N, Yamaguchi S, Ishigami T, Hibi K, Yabana M, Kihara M, Fukamizu A, Murakami K, Ishii M. A novel proximal element mediates the regulation of mouse Ren-1C promoter by retinoblastoma protein in cultured cells. J Biol Chem 272: 16845–16851, 1997. 853. Tamura K, Umemura S, Yamaguchi S, Iwamoto T, Kobayashi S, Fukamizu A, Murakami K, Ishii M. Mechanism of cAMP regulation of renin gene transcription by proximal promoter. J Clin Invest 94: 1959 –1967, 1994. 854. Tan DY, Meng S, Manning RD Jr. Role of neuronal nitric oxide synthase in Dahl salt-sensitive hypertension. Hypertension 33: 456 – 461, 1999. 855. Tanaka Y, Tamura K, Koide Y, Sakai M, Tsurumi Y, Noda Y, Umemura M, Ishigami T, Uchino K, Kimura K, Horiuchi M, Umemura S. The novel angiotensin II type 1 receptor (AT1R)associated protein ATRAP downregulates AT1R and ameliorates cardiomyocyte hypertrophy. FEBS Lett 579: 1579 –1586, 2005. 856. Tanida M, Shen J, Horii Y, Matsuda M, Kihara S, Funahashi T, Shimomura I, Sawai H, Fukuda Y, Matsuzawa Y, Nagai K. Effects of adiponectin on the renal sympathetic nerve activity and blood pressure in rats. Exp Biol Med 232: 390 –397, 2007. 857. Tanimoto K, Sugiura A, Kanafusa S, Saito T, Masui N, Yanai K, Fukamizu A. A single nucleotide mutation in the mouse renin promoter disrupts blood pressure regulation. J Clin Invest 118: 1006 –1016, 2008. 858. Taugner C, Poulsen K, Hackenthal E, Taugner R. Immunocytochemical localization of renin in mouse kidney. Histochemistry 62: 19 –27, 1979. 859. Taugner R, Buhrle CP, Nobiling R. Ultrastructural changes associated with renin secretion from the juxtaglomerular apparatus of mice. Cell Tissue Res 237: 459 – 472, 1984. 860. Taugner R, Hackenthal E, Inagami T, Nobiling R, Poulsen K. Vascular and tubular renin in the kidneys of mice. Histochemistry 75: 473– 484, 1982. 861. Taugner R, Kirchheim H, Forssmann WG. Myoendothelial contacts in glomerular arterioles and in renal interlobular arteries of rat, mouse and Tupaia belangeri. Cell Tissue Res 235: 319 –325, 1984. 862. Taugner R, Metz R. Development and fate of the secretory granules of juxtaglomerular epithelioid cells. Cell Tissue Res 246: 595– 606, 1986. 863. Taugner R, Nobiling R, Metz R, Taugner F, Buhrle C, Hackenthal E. Hypothetical interpretation of the calcium paradox in renin secretion. Cell Tissue Res 252: 687– 690, 1988. 864. Taugner R, Schiller A, Kaissling B, Kriz W. Gap junctional coupling between the JGA and the glomerular tuft. Cell Tissue Res 186: 279 –285, 1978. 865. Taugner R, Whalley A, Angermuller S, Buhrle CP, Hackenthal E. Are the renin-containing granules of juxtaglomerular epithelioid cells modified lysosomes? Cell Tissue Res 239: 575–587, 1985. Physiol Rev • VOL 866. Theilig F, Campean V, Paliege A, Breyer M, Briggs JP, Schnermann J, Bachmann S. Epithelial COX-2 expression is not regulated by nitric oxide in rodent renal cortex. Hypertension 39: 848 – 853, 2002. 867. Therland KL, Stubbe J, Thiesson HC, Ottosen PD, Walter S, Sorensen GL, Skott O, Jensen BL. Cycloxygenase-2 is expressed in vasculature of normal and ischemic adult human kidney and is colocalized with vascular prostaglandin E2 EP4 receptors. J Am Soc Nephrol 15: 1189 –1198, 2004. 868. Thrasher TN. Baroreceptor regulation of vasopressin and renin secretion: low-pressure versus high-pressure receptors. Front Neuroendocrinol 15: 157–196, 1994. 869. Thrasher TN. Baroreceptors, baroreceptor unloading, and the long-term control of blood pressure. Am J Physiol Regul Integr Comp Physiol 288: R819 –R827, 2005. 870. Thrasher TN. Effects of chronic baroreceptor unloading on blood pressure in the dog. Am J Physiol Regul Integr Comp Physiol 288: R863–R871, 2005. 871. Thrasher TN. Unloading arterial baroreceptors causes neurogenic hypertension. Am J Physiol Regul Integr Comp Physiol 282: R1044 –R1053, 2002. 872. Thrasher TN, Chen HG, Keil LC. Arterial baroreceptors control plasma vasopressin responses to graded hypotension in conscious dogs. Am J Physiol Regul Integr Comp Physiol 278: R469 –R475, 2000. 873. Thrasher TN, Keil LC. Arterial baroreceptors control blood pressure and vasopressin responses to hemorrhage in conscious dogs. Am J Physiol Regul Integr Comp Physiol 275: R1843–R1857, 1998. 874. Thurau K, Schnermann J. The sodium concentration in the macula densa cells as a regulating factor for glomerular filtration (micropuncture experiments). Klin Wochenschr 43: 410 – 413, 1965. 875. Tikellis C, Cooper ME, Bialkowski K, Johnston CI, Burns WC, Lew RA, Smith AI, Thomas MC. Developmental expression of ACE2 in the SHR kidney: a role in hypertension? Kidney Int 70: 34 – 41, 2006. 876. Tikellis C, Johnston CI, Forbes JM, Burns WC, Burrell LM, Risvanis J, Cooper ME. Characterization of renal angiotensinconverting enzyme 2 in diabetic nephropathy. Hypertension 41: 392–397, 2003. 877. Timmermans PB, Wong PC, Chiu AT, Herblin WF, Benfield P, Carini DJ, Lee RJ, Wexler RR, Saye JA, Smith RD. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev 45: 205–251, 1993. 878. Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem 275: 33238 –33243, 2000. 879. Tkacs NC, Kim M, Denzon M, Hargrave B, Ganong WF. Pharmacological evidence for involvement of the sympathetic nervous system in the increase in renin secretion produced by a low sodium diet in rats. Life Sci 47: 2317–2322, 1990. 879a.Todd-Turla KM, Schnermann J, Fejes-Toth G, Naray-FejesToth A, Smart A, Killen PD, Briggs JP. Distribution of mineralocorticoid and glucocorticoid receptor mRNA along the nephron. Am J Physiol Renal Physiol 264: F781–F791, 1993. 880. Todorov V, Muller M, Schweda F, Kurtz A. Tumor necrosis factor-alpha inhibits renin gene expression. Am J Physiol Regul Integr Comp Physiol 283: R1046 –R1051, 2002. 881. Todorov VT, Desch M, Schmitt-Nilson N, Todorova A, Kurtz A. Peroxisome proliferator-activated receptor-gamma is involved in the control of renin gene expression. Hypertension 50: 939 –944, 2007. 882. Todorov VT, Desch M, Schubert T, Kurtz A. The Pal3 promoter sequence is critical for the regulation of human renin gene transcription by PPAR␥. Endocrinology 149: 4647– 4657, 2008. 883. Todorov VT, Volkl S, Friedrich J, Kunz-Schughart LA, Hehlgans T, Vermeulen L, Haegeman G, Schmitz ML, Kurtz A. Role of CREB1 and NFB-p65 in the down-regulation of renin gene expression by tumor necrosis factor ␣. J Biol Chem 280: 24356 – 24362, 2005. 884. Todorov VT, Volkl S, Muller M, Bohla A, Klar J, KunzSchughart LA, Hehlgans T, Kurtz A. Tumor necrosis factor- 90 • APRIL 2010 • www.prv.org PHYSIOLOGY OF KIDNEY RENIN alpha activates NFkappaB to inhibit renin transcription by targeting cAMP-responsive element. J Biol Chem 279: 1458 –1467, 2004. 885. Toffelmire EB, Slater K, Corvol P, Menard J, Schambelan M. Response of plasma prorenin and active renin to chronic and acute alterations of renin secretion in normal humans. Studies using a direct immunoradiometric assay. J Clin Invest 83: 679 – 687, 1989. 886. Tojo A, Madsen KM, Wilcox CS. Expression of immunoreactive nitric oxide synthase isoforms in rat kidney. Effects of dietary salt and losartan. Jpn Heart J 36: 389 –398, 1995. 887. Toma I, Kang JJ, Sipos A, Vargas S, Bansal E, Hanner F, Meer E, Peti-Peterdi J. Succinate receptor GPR91 provides a direct link between high glucose levels and renin release in murine and rabbit kidney. J Clin Invest 118: 2526 –2534, 2008. 888. Toubeau G, Nonclercq D, Zanen J, Laurent G, Schaudies PR, Heuson-Stiennon JA. Renal tissue expression of EGF and EGF receptor after ischaemic tubular injury: an immunohistochemical study. Exp Nephrol 2: 229 –239, 1994. 889. Trabold F, Pons S, Hagege AA, Bloch-Faure M, Alhenc-Gelas F, Giudicelli JF, Richer-Giudicelli C, Meneton P. Cardiovascular phenotypes of kinin B2 receptor- and tissue kallikrein-deficient mice. Hypertension 40: 90 –95, 2002. 890. Traynor TR, Smart A, Briggs JP, Schnermann J. Inhibition of macula densa-stimulated renin secretion by pharmacological blockade of cyclooxygenase-2. Am J Physiol Renal Physiol 277: F706 –F710, 1999. 891. Treschan TA, Peters J. The vasopressin system: physiology and clinical strategies. Anesthesiology 105: 599 – 612, 2006. 892. Troughton RW, Lewis LK, Yandle TG, Richards AM, Nicholls MG. Hemodynamic, hormone, and urinary effects of adrenomedullin infusion in essential hypertension. Hypertension 36: 588 –593, 2000. 893. Tsurumi Y, Tamura K, Tanaka Y, Koide Y, Sakai M, Yabana M, Noda Y, Hashimoto T, Kihara M, Hirawa N, Toya Y, Kiuchi Y, Iwai M, Horiuchi M, Umemura S. Interacting molecule of AT1 receptor, ATRAP, is colocalized with AT1 receptor in the mouse renal tubules. Kidney Int 69: 488 – 494, 2006. 894. Uckaya G, Ozata M, Sonmez A, Kinalp C, Eyileten T, Bingol N, Koc B, Kocabalkan F, Ozdemir IC. Plasma leptin levels strongly correlate with plasma renin activity in patients with essential hypertension. Horm Metab Res 31: 435– 438, 1999. 895. Ueda S, Masumori-Maemoto S, Ashino K, Nagahara T, Gotoh E, Umemura S, Ishii M. Angiotensin-(1–7) attenuates vasoconstriction evoked by angiotensin II but not by noradrenaline in man. Hypertension 35: 998 –1001, 2000. 895a.Uhrenholt TR, Schjerning J, Hansen PB, Norregaard R, Jensen BL, Sorensen GL, Skott O. Rapid inhibition of vasoconstriction in renal afferent arterioles by aldosterone. Circ Res 93: 1258 –1266, 2003. 896. Ujiie K, Yuen J, Hogarth L, Danziger R, Star RA. Localization and regulation of endothelial NO synthase mRNA expression in rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 267: F296 – F302, 1994. 897. Uriz J, Gines P, Cardenas A, Sort P, Jimenez W, Salmeron JM, Bataller R, Mas A, Navasa M, Arroyo V, Rodes J. Terlipressin plus albumin infusion: an effective and safe therapy of hepatorenal syndrome. J Hepatol 33: 43– 48, 2000. 898. Ushio-Fukai M, Alexander RW, Akers M, Lyons PR, Lassegue B, Griendling KK. Angiotensin II receptor coupling to phospholipase D is mediated by the betagamma subunits of heterotrimeric G proteins in vascular smooth muscle cells. Mol Pharmacol 55: 142–149, 1999. 899. Vallon V, Heyne N, Richter K, Khosla MC, Fechter K. [7-D-Ala]angiotensin 1–7 blocks renal actions of angiotensin 1–7 in the anesthetized rat. J Cardiovasc Pharmacol 32: 164 –167, 1998. 900. Van Kesteren CA, Danser AH, Derkx FH, Dekkers DH, Lamers JM, Saxena PR, Schalekamp MA. Mannose 6-phosphate receptor-mediated internalization and activation of prorenin by cardiac cells. Hypertension 30: 1389 –1396, 1997. 901. Vander AJ. Direct effects of prostaglandin on renal function and renin release in anesthetized dog. Am J Physiol 214: 218 –221, 1968. 902. Vander AJ. Inhibition of renin release in the dog by vasopressin and vasotocin. Circ Res 23: 605– 609, 1968. Physiol Rev • VOL 671 903. Vandongen R, Peart WS, Boyd GW. Andrenergic stimulation of renin secretion in the isolated perfused rat kidney. Circ Res 32: 290 –296, 1973. 904. Vandongen R, Peart WS, Boyd GW. Effect of angiotensin II and its nonpressor derivatives on renin secretion. Am J Physiol 226: 277–282, 1974. 905. Vandongen R, Strang KD, Poesse MH, Birkenhager WH. Suppression of renin secretion in the rat kidney by a nonvascular alpha-adrenergic mechanism. Circ Res 45: 435– 439, 1979. 906. Vane JR. Nobel lecture, 8th December 1982. Adventures and excursions in bioassay: the stepping stones to prostacyclin. Br J Pharmacol 79: 821– 838, 1983. 907. Varagic J, Trask AJ, Jessup JA, Chappell MC, Ferrario CM. New angiotensins. J Mol Med 86: 663– 671, 2008. 908. Vargas SL, Toma I, Kang JJ, Meer EJ, Peti-Peterdi J. Activation of the succinate receptor GPR91 in macula densa cells causes renin release. J Am Soc Nephrol 20: 1002–1011, 2009. 909. Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev 52: 269 –324, 2000. 910. Vaz M, Jennings G, Turner A, Cox H, Lambert G, Esler M. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation 96: 3423–3429, 1997. 911. Veeraveedu PT, Watanabe K, Ma M, Palaniyandi SS, Yamaguchi K, Kodama M, Aizawa Y. Effects of V2-receptor antagonist tolvaptan and the loop diuretic furosemide in rats with heart failure. Biochem Pharmacol 75: 1322–1330, 2008. 912. Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F, Acton S, Patane M, Nichols A, Tummino P. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem 277: 14838 –14843, 2002. 913. Villarreal D, Freeman RH, Davis JO, Verburg KM, Vari RC. Renal mechanisms for suppression of renin secretion by atrial natriuretic factor. Hypertension 8: II28 –35, 1986. 914. Villarreal D, Reams G, Freeman RH, Taraben A. Renal effects of leptin in normotensive, hypertensive, and obese rats. Am J Physiol Regul Integr Comp Physiol 275: R2056 –R2060, 1998. 915. Vio CP, An SJ, Cespedes C, McGiff JC, Ferreri NR. Induction of cyclooxygenase-2 in thick ascending limb cells by adrenalectomy. J Am Soc Nephrol 12: 649 – 658, 2001. 916. Vio CP, Cespedes C, Gallardo P, Masferrer JL. Renal identification of cyclooxygenase-2 in a subset of thick ascending limb cells. Hypertension 30: 687– 692, 1997. 917. Vitzthum H, Abt I, Einhellig S, Kurtz A. Gene expression of prostanoid forming enzymes along the rat nephron. Kidney Int 62: 1570 –1581, 2002. 918. Voigtlander T, Ganten D, Bader M. Transcriptional regulation of the rat renin gene by regulatory elements in intron I. Hypertension 33: 303–311, 1999. 919. Voigtlander T, Ripperger A, Ganten D, Bader M. Transcriptional silencer in intron I of the rat renin gene. Adv Exp Med Biol 377: 285–292, 1995. 920. Waeber B, Evequoz D, Aubert JF, Fluckiger JP, Juillerat L, Nussberger J, Brunner HR. Prevention of renal hypertension in the rat by neuropeptide Y. J Hypertens 8: 21–25, 1990. 921. Wagner C, de Wit C, Gerl M, Kurtz A, Hocherl K. Increased expression of cyclooxygenase 2 contributes to aberrant renin production in connexin 40-deficient kidneys. Am J Physiol Regul Integr Comp Physiol 293: R1781–R1786, 2007. 922. Wagner C, de Wit C, Kurtz L, Grunberger C, Kurtz A, Schweda F. Connexin40 is essential for the pressure control of renin synthesis and secretion. Circ Res 100: 556 –563, 2007. 923. Wagner C, Godecke A, Ford M, Schnermann J, Schrader J, Kurtz A. Regulation of renin gene expression in kidneys of eNOSand nNOS-deficient mice. Pflügers Arch 439: 567–572, 2000. 924. Wagner C, Kees F, Kramer BK, Kurtz A. Role of sympathetic nerves for the stimulation of the renin system by angiotensin II receptor blockade. J Hypertens 15: 1463–1469, 1997. 925. Wagner C, Kurtz A. Regulation of renal renin release. Curr Opin Nephrol Hypertens 7: 437– 441, 1998. 90 • APRIL 2010 • www.prv.org 672 CASTROP ET AL. 926. Wagner C, Kurtz L, Schweda F, Simon AM, Kurtz A. Connexin 37 is dispensable for the control of the renin system and for positioning of renin-producing cells in the kidney. Pflügers Arch 459: 151–158, 2009. 927. Wagner C, Pfeifer A, Ruth P, Hofmann F, Kurtz A. Role of cGMP-kinase II in the control of renin secretion and renin expression. J Clin Invest 102: 1576 –1582, 1998. 928. Wagner D, Metzger R, Paul M, Ludwig G, Suzuki F, Takahashi S, Murakami K, Ganten D. Androgen dependence and tissue specificity of renin messenger RNA expression in mice. J Hypertens 8: 45–52, 1990. 929. Wagner J, Jan Danser AH, Derkx FH, de Jong TV, Paul M, Mullins JJ, Schalekamp MA, Ganten D. Demonstration of renin mRNA, angiotensinogen mRNA, and angiotensin converting enzyme mRNA expression in the human eye: evidence for an intraocular renin-angiotensin system. Br J Ophthalmol 80: 159 –163, 1996. 930. Wakahara S, Konoshita T, Mizuno S, Motomura M, Aoyama C, Makino Y, Kato N, Koni I, Miyamori I. Synergistic expression of angiotensin-converting enzyme (ACE) and ACE2 in human renal tissue and confounding effects of hypertension on the ACE to ACE2 ratio. Endocrinology 148: 2453–2457, 2007. 931. Walters PE, Gaspari TA, Widdop RE. Angiotensin-(1–7) acts as a vasodepressor agent via angiotensin II type 2 receptors in conscious rats. Hypertension 45: 960 –966, 2005. 932. Wang BC, Flora-Ginter G, Leadley RJ Jr, Goetz KL. Ventricular receptors stimulate vasopressin release during hemorrhage. Am J Physiol Regul Integr Comp Physiol 254: R204 –R211, 1988. 933. Wang D, Yoshida H, Song Q, Chao L, Chao J. Enhanced renal function in bradykinin B(2) receptor transgenic mice. Am J Physiol Renal Physiol 278: F484 –F491, 2000. 934. Wang JL, Cheng HF, Harris RC. Cyclooxygenase-2 inhibition decreases renin content and lowers blood pressure in a model of renovascular hypertension. Hypertension 34: 96 –101, 1999. 935. Wang T, Brown MJ. Differential expression of adenylyl cyclase subtypes in human cardiovascular system. Mol Cell Endocrinol 223: 55– 62, 2004. 936. Wang Y, Marsden PA. Nitric oxide synthases: biochemical and molecular regulation. Curr Opin Nephrol Hypertens 4: 12–22, 1995. 937. Wang Y, Marsden PA. Nitric oxide synthases: gene structure and regulation. Adv Pharmacol 34: 71–90, 1995. 938. Wang Y, Roman R, Lidofsky SD, Fitz JG. Autocrine signaling through ATP release represents a novel mechanism for cell volume regulation. Proc Natl Acad Sci USA 93: 12020 –12025, 1996. 939. Warner FJ, Lew RA, Smith AI, Lambert DW, Hooper NM, Turner AJ. Angiotensin-converting enzyme 2 (ACE2), but not ACE, is preferentially localized to the apical surface of polarized kidney cells. J Biol Chem 280: 39353–39362, 2005. 940. Watkins BE, Davis JO, Lohmeier TE, Freeman RH. Intrarenal site of action of calcium on renin secretion in dogs. Circ Res 39: 847– 853, 1976. 941. Weaver DR, Reppert SM. Adenosine receptor gene expression in rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 263: F991–F995, 1992. 942. Weekley LB. Renal renin secretion rate and norepinephrine secretion rate in response to centrally administered angiotensin-II: role of the medial basal forebrain. Clin Exp Hypertens A 14: 923–945, 1992. 943. Weihprecht H, Lorenz JN, Schnermann J, Skott O, Briggs JP. Effect of adenosine1-receptor blockade on renin release from rabbit isolated perfused juxtaglomerular apparatus. J Clin Invest 85: 1622–1628, 1990. 944. Weinberger MH, Aoi W, Henry DP. Direct effect of beta-adrenergic stimulation on renin release by the rat kidney slice in vitro. Circ Res 37: 318 –324, 1975. 945. Weir MR. Effects of renin-angiotensin system inhibition on endorgan protection: can we do better? Clin Ther 29: 1803–1824, 2007. 946. Weir MR, Dzau VJ. The renin-angiotensin-aldosterone system: a specific target for hypertension management. Am J Hypertens 12: 205S–213S, 1999. 947. Wells JA, de Vos AM. Hematopoietic receptor complexes. Annu Rev Biochem 65: 609 – 634, 1996. Physiol Rev • VOL 948. Wendel M, Knels L, Kummer W, Koch T. Distribution of endothelin receptor subtypes ETA and ETB in the rat kidney. J Histochem Cytochem 54: 1193–1203, 2006. 949. Wharton J, Gordon L, Byrne J, Herzog H, Selbie LA, Moore K, Sullivan MH, Elder MG, Moscoso G, Taylor KM. Expression of the human neuropeptide tyrosine Y1 receptor. Proc Natl Acad Sci USA 90: 687– 691, 1993. 950. Whitworth JA, Gordon D, Andrews J, Scoggins BA. The hypertensive effect of synthetic glucocorticoids in man: role of sodium and volume. J Hypertens 7: 537–549, 1989. 951. Widdop RE, Jones ES, Hannan RE, Gaspari TA. Angiotensin AT2 receptors: cardiovascular hope or hype? Br J Pharmacol 140: 809 – 824, 2003. 952. Wilcox CS, Welch WJ, Murad F, Gross SS, Taylor G, Levi R, Schmidt HH. Nitric oxide synthase in macula densa regulates glomerular capillary pressure. Proc Natl Acad Sci USA 89: 11993– 11997, 1992. 953. Winaver J, Abassi Z. Role of neuropeptide Y in the regulation of kidney function. EXS 123–132, 2006. 954. Wolf G. Novel aspects of the renin-angiotensin-aldosterone-system. Front Biosci 13: 4993–5005, 2008. 955. Wolf K, Castrop H, Hartner A, Goppelt-Strube M, Hilgers KF, Kurtz A. Inhibition of the renin-angiotensin system upregulates cyclooxygenase-2 expression in the macula densa. Hypertension 34: 503–507, 1999. 956. Wolfle SE, Schmidt VJ, Hoepfl B, Gebert A, Alcolea S, Gros D, de Wit C. Connexin45 cannot replace the function of connexin40 in conducting endothelium-dependent dilations along arterioles. Circ Res 101: 1292–1299, 2007. 957. Wong DW, Oudit GY, Reich H, Kassiri Z, Zhou J, Liu QC, Backx PH, Penninger JM, Herzenberg AM, Scholey JW. Loss of angiotensin-converting enzyme-2 (Ace2) accelerates diabetic kidney injury. Am J Pathol 171: 438 – 451, 2007. 958. Wright FS, Schnermann J. Interference with feedback control of glomerular filtration rate by furosemide, triflocin, and cyanide. J Clin Invest 53: 1695–1708, 1974. 959. Wyse B, Waters M, Sernia C. Stimulation of the renin-angiotensin system by growth hormone in Lewis dwarf rats. Am J Physiol Endocrinol Metab 265: E332–E339, 1993. 960. Wysocki J, Ye M, Soler MJ, Gurley SB, Xiao HD, Bernstein KE, Coffman TM, Chen S, Batlle D. ACE and ACE2 activity in diabetic mice. Diabetes 55: 2132–2139, 2006. 961. Xu P, Costa-Goncalves AC, Todiras M, Rabelo LA, Sampaio WO, Moura MM, Santos SS, Luft FC, Bader M, Gross V, Alenina N, Santos RA. Endothelial dysfunction and elevated blood pressure in MAS gene-deleted mice. Hypertension 51: 574 –580, 2008. 962. Yamada K, Iyer SN, Chappell MC, Ganten D, Ferrario CM. Converting enzyme determines plasma clearance of angiotensin(1–7). Hypertension 32: 496 –502, 1998. 963. Yamaguchi I, Yao L, Sanada H, Ozono R, Mouradian MM, Jose PA, Carey RM, Felder RA. Dopamine D1A receptors and renin release in rat juxtaglomerular cells. Hypertension 29: 962–968, 1997. 964. Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7: 941–946, 2001. 965. Yamazato M, Yamazato Y, Sun C, Diez-Freire C, Raizada MK. Overexpression of angiotensin-converting enzyme 2 in the rostral ventrolateral medulla causes long-term decrease in blood pressure in the spontaneously hypertensive rats. Hypertension 49: 926 –931, 2007. 966. Yan Y, Jones CA, Sigmund CD, Gross KW, Catanzaro DF. Conserved enhancer elements in human and mouse renin genes have different transcriptional effects in As4.1 cells. Circ Res 81: 558 –566, 1997. 967. Yanai K, Saito T, Kakinuma Y, Kon Y, Hirota K, TaniguchiYanai K, Nishijo N, Shigematsu Y, Horiguchi H, Kasuya Y, Sugiyama F, Yagami K, Murakami K, Fukamizu A. Renin-de- 90 • APRIL 2010 • www.prv.org PHYSIOLOGY OF KIDNEY RENIN 968. 969. 970. 971. 972. 973. 974. 975. 976. 977. 978. 979. pendent cardiovascular functions and renin-independent bloodbrain barrier functions revealed by renin-deficient mice. J Biol Chem 275: 5– 8, 2000. Yang EK, Lee WJ, Park YY, Ahn DK, Park JS, Kim HJ. Effects of chronic central administration of losartan on the cardiovascular and hormonal responses to hemorrhage in conscious rats. Regul Pept 67: 107–113, 1996. Yang T, Endo Y, Huang YG, Smart A, Briggs JP, Schnermann J. Renin expression in COX-2-knockout mice on normal or low-salt diets. Am J Physiol Renal Physiol 279: F819 –F825, 2000. Yang T, Huang YG, Singh I, Schnermann J, Briggs JP. Localization of bumetanide- and thiazide-sensitive Na-K-Cl cotransporters along the rat nephron. Am J Physiol Renal Fluid Electrolyte Physiol 271: F931–F939, 1996. Yang T, Huang YG, Ye W, Hansen P, Schnermann JB, Briggs JP. Influence of genetic background and gender on hypertension and renal failure in COX-2-deficient mice. Am J Physiol Renal Physiol 288: F1125–F1132, 2005. Yang T, Singh I, Pham H, Sun D, Smart A, Schnermann JB, Briggs JP. Regulation of cyclooxygenase expression in the kidney by dietary salt intake. Am J Physiol Renal Physiol 274: F481–F489, 1998. Yao J, Suwa M, Li B, Kawamura K, Morioka T, Oite T. ATPdependent mechanism for coordination of intercellular Ca2⫹ signaling and renin secretion in rat juxtaglomerular cells. Circ Res 93: 338 –345, 2003. Ye M, Wysocki J, William J, Soler MJ, Cokic I, Batlle D. Glomerular localization and expression of angiotensin-converting enzyme 2 and angiotensin-converting enzyme: implications for albuminuria in diabetes. J Am Soc Nephrol 17: 3067–3075, 2006. Ying L, Morris BJ, Sigmund CD. Transactivation of the human renin promoter by the cAMP/protein kinase A pathway is mediated by both cAMP-responsive element binding protein-1 (CREB)-dependent and CREB-independent mechanisms in Calu-6 cells. J Biol Chem 272: 2412–2420, 1997. Yosipiv IV, Dipp S, El-Dahr SS. Targeted disruption of the bradykinin B(2) receptor gene in mice alters the ontogeny of the renin-angiotensin system. Am J Physiol Renal Physiol 281: F795– F801, 2001. Yosipiv IV, el-Dahr SS. Developmental regulation of ACE gene expression by endogenous kinins and angiotensin II. Am J Physiol Renal Fluid Electrolyte Physiol 269: F172–F179, 1995. Young D, O’Neill K, Jessell T, Wigler M. Characterization of the rat mas oncogene and its high-level expression in the hippocampus and cerebral cortex of rat brain. Proc Natl Acad Sci USA 85: 5339 –5342, 1988. Young D, Waitches G, Birchmeier C, Fasano O, Wigler M. Isolation and characterization of a new cellular oncogene encoding Physiol Rev • VOL 980. 981. 982. 983. 984. 985. 986. 987. 988. 989. 990. 991. 992. 673 a protein with multiple potential transmembrane domains. Cell 45: 711–719, 1986. Yuan W, Pan W, Kong J, Zheng W, Szeto FL, Wong KE, Cohen R, Klopot A, Zhang Z, Li YC. 1,25-Dihydroxyvitamin D3 suppresses renin gene transcription by blocking the activity of the cAMP response element in the renin gene promoter. J Biol Chem 282: 29821–29830, 2007. Yun J, Schoneberg T, Liu J, Schulz A, Ecelbarger CA, Promeneur D, Nielsen S, Sheng H, Grinberg A, Deng C, Wess J. Generation and phenotype of mice harboring a nonsense mutation in the V2 vasopressin receptor gene. J Clin Invest 106: 1361–1371, 2000. Zelis R, Nussberger J, Clemson B, Waeber B, Grouzmann E, Brunner HR. Neuropeptide Y infusion decreases plasma renin activity in postmyocardial infarction rats. J Cardiovasc Pharmacol 24: 896 – 899, 1994. Zhang J, Hill CE. Differential connexin expression in preglomerular and postglomerular vasculature: accentuation during diabetes. Kidney Int 68: 1171–1185, 2005. Zhang MZ, Wang SW, Cheng H, Zhang Y, McKanna JA, Harris RC. Regulation of renal cortical cyclooxygenase-2 in young rats. Am J Physiol Renal Physiol 285: F881–F888, 2003. Zhang MZ, Yao B, Fang X, Wang S, Smith JP, Harris RC. Intrarenal dopaminergic system regulates renin expression. Hypertension 53: 564 –570, 2009. Zhang Y, Morgan T. Role of the macula densa in renin synthesis and secretion. Am J Hypertens 7: 448 – 452, 1994. Zhang Y, Morgan TO. Sodium depletion, renal denervation, beta adrenergic blockage and renin secretion and synthesis. Blood Press 3: 67–71, 1994. Zhong JC, Huang DY, Yang YM, Li YF, Liu GF, Song XH, Du K. Upregulation of angiotensin-converting enzyme 2 by all-trans rats. Hypertension 44: 907–912, 2004. Zhou X, Davis DR, Sigmund CD. The human renin kidney enhancer is required to maintain base-line renin expression but is dispensable for tissue-specific, cell-specific, and regulated expression. J Biol Chem 281: 35296 –35304, 2006. Zhou X, Sigmund CD. Chorionic enhancer is dispensable for regulated expression of the human renin gene. Am J Physiol Regul Integr Comp Physiol 294: R279 –R287, 2008. Zhuo J, Dean R, MacGregor D, Alcorn D, Mendelsohn FA. Presence of angiotensin II AT2 receptor binding sites in the adventitia of human kidney vasculature. Clin Exp Pharmacol Physiol Suppl 3: S147–S154, 1996. Zhuo J, Ohishi M, Mendelsohn FA. Roles of AT1 and AT2 receptors in the hypertensive Ren-2 gene transgenic rat kidney. Hypertension 33: 347–353, 1999. 90 • APRIL 2010 • www.prv.org