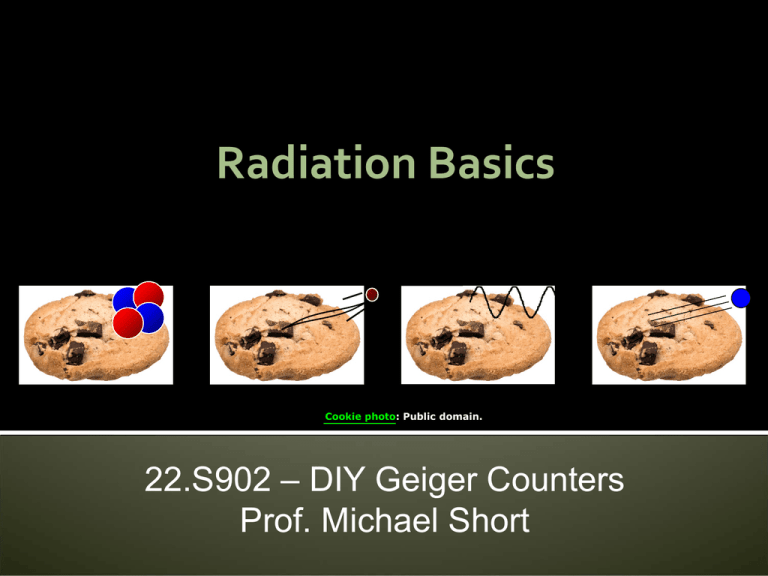
Radiation Basics
Cookie photo: Public domain.
22.
02 DI
eiger Counters
Prof. Michael hort
Old 22.01 Final Exam Question
ou have four highl radioactive coo ies:
α
β
ou must:
γ
Put one in our poc et
at one
What do ou do
n
old one in our hand
ive one to a friend
Cookie photo: Public domain.
2
Motivation
Introduce ioni ing radiation basics
stablish common notation and terminolog
Understand t pes of radiation
Intuitivel understand range of radiation
Derive and use nuclear reaction e uations,
half lives of common isotopes
3
Types of Ionizing Radiation
Alpha
–
elium nucleus
2
eav , charged
eta
A free electron or positron
1
– ight, charged
amma
A nuclear photon light
eutron n
A free neutron
–
–
o mass, no charge
eav , no charge
4
Ranges of Ionizing Radiation
Paper
Plastic
Steel
Lead
alpha
beta
beta
gamma
Image by MIT OpenCourseWare.
5
Relative Energy Deposition
Which do ou thin deposits the
most energ
Where
2
1
Over what range
Which t pe s is/are safer outside
the bod
Which t pe s is/are safer inside
the bod
6
Old 22.01 Final Exam Question
ou have four highl radioactive coo ies:
α
β
ou must:
γ
Put one in our poc et
at one
n
old one in our hand
ive one to a friend
What do ou do
Cookie photo: Public domain.
7
Ions, Isotopes
Atoms are determined b the number of protons
–
ample:
elium A WA
has two protons
Two special t pes:
– Ion
• Same # protons neutrons
• Different electrons charge
– Isotope
• Same # protons
• Different neutrons mass
Notation
4He
4He+
4He
3He
Ion An atom with a charge different of protons electrons
Isotope An atom with a different number of neutrons mass
8
Isotopic Notation
3He
Image
Notation
Notation
𝑨𝑨
𝒁𝒁
𝑵𝑵𝑵𝑵𝑵𝑵𝑵𝑵
±𝒄𝒄
Template
Atomic Number (Z) The number of protons in an atom
Mass Number (A) The total number of nucleons in an atom
9
Notation Examples
𝟗𝟗𝟐𝟐𝟐𝟐 𝟎𝟎
𝟗𝟗𝟗𝟗𝑼𝑼
𝟗𝟗𝟐𝟐𝟐𝟐 +𝟒𝟒
𝟗𝟗𝟗𝟗𝑼𝑼
=
=
𝟗𝟗𝟐𝟐𝟐𝟐
𝑼𝑼
𝟗𝟗𝟐𝟐𝟐𝟐 +𝟒𝟒
𝑼𝑼
𝟔𝟔𝟎𝟎
𝟎𝟎
𝟔𝟔𝟎𝟎
𝑪𝑪𝑪𝑪
𝟗𝟗𝟐𝟐𝑪𝑪𝑪𝑪 =
𝟏𝟏𝟐𝟐𝟐𝟐
+𝟏𝟏
𝟏𝟏𝟐𝟐𝟐𝟐
+𝟏𝟏
=
𝑪𝑪𝑪𝑪
𝟐𝟐𝟐𝟐𝑪𝑪𝑪𝑪
𝟒𝟒
+𝟗𝟗
𝟒𝟒
+𝟗𝟗
= 𝑯𝑯𝑵𝑵 =
𝟗𝟗𝑯𝑯𝑵𝑵
𝛂𝛂
10
Stable Isotopes
http://atom.kaeri.re.kr/
table isotopes do not undergo spontaneous
radioactive deca
Which are stable Consult the Table of
Notation
Stable Isotope An isotope which does not undergo
spontaneous, natural radioactive deca
uclides
11
Stable Isotopes, Atomic Weight
http://atom.kaeri.re.kr/
oo closer at one section
Same neutron
number (N)
Which isotopes of chlorine are stable
Same
atomic
number
(Z)
12
Stable Isotopes, Atomic Weight
http://atom.kaeri.re.kr/
oo closer at one section
Which isotopes of chlorine are stable In which percentages
13
Stable Isotopes, Atomic Weight
http://atom.kaeri.re.kr/
oo closer at one section
Which isotopes of chlorine are stable In which percentages
𝑀𝑀𝑀𝑀𝑀𝑀𝑀𝑀𝐶𝐶𝐶𝐶 = 0.7577 ∗ 34.98 + 0.2423 ∗ 36.97 = 35.46 𝑀𝑀𝑎𝑎𝑎𝑎
Notation
iterature value: 35.453 ± 0.002 𝑀𝑀𝑎𝑎𝑎𝑎
Atomic Weight – The average stable element isotopic weight
Atomic Mass Unit (amu) – Exactly 1⁄12 the weight of 12𝐶𝐶
14
More Features of the Nuclide Table
http://atom.kaeri.re.kr/
cess mass
tabilit
ields half life and
specific activit
information
Parent nuclides
Notation
ields energetics and
nuclear reaction
information
ields nuclear reaction
mechanism information
Excess Mass (Δ) – The mass of a nucleus not accounted for b
the weight of its protons and neutrons alone
15
More Features of the Nuclide Table
http://atom.kaeri.re.kr/
cess mass
tabilit
ields half life and
specific activit
information
Mode of deca
Notation
ields energetics and
nuclear reaction
information
ields nuclear reaction
mechanism information
Half Life – The time it ta es 0 of an isotope to deca
Decay Energy – The total energ involved in this radioactive deca
16
Next Three Topics
Writing nuclear reactions
uantif ing energetics of reactions
Predicting radiation t pe and energ
17
Nuclear Reactions
adioactive Deca
atural process
Fission
plitting atoms
Fusion
Combining atoms
Today’s
focus
– Thermal energ is collected from inetic energ
energ of fission products
recoil
– Thermal energ is collected from multiple sources
Notation
Decay The natural process of unstable isotope change
Fission The process of splitting an isotope into fission fragments
Fusion – The process of combining two isotopes into a new one
18
Nuclear Reaction Principles
CO
almost
Mass number of nucleons
Charge
nerg
OT necessaril protons and neutrons
ample: Tritium deca
T I
+2
𝛼𝛼
𝟐𝟐
𝟐𝟐
±
𝑯𝑯 → 𝑯𝑯𝑵𝑵 + ? ? ? 𝛽𝛽
𝛾𝛾
19
Nuclear Reaction Principles (β)
CO
almost
Mass number of nucleons
Charge
nerg
OT necessaril protons and neutrons
T I
ample: Tritium deca
𝟐𝟐
𝟐𝟐
+
−
𝑯𝑯 → 𝑯𝑯𝑵𝑵 + 𝛽𝛽 + 𝑬𝑬𝒓𝒓𝒓𝒓𝒓𝒓
What is
r n
What else is missing
20
Nuclear Reaction Energetics (β)
𝟐𝟐
𝟐𝟐
+
−
𝑯𝑯 → 𝑯𝑯𝑵𝑵 + 𝛽𝛽 + 𝑬𝑬𝒓𝒓𝒓𝒓𝒓𝒓
Total Erxn: oo at differences in excess mass
𝑄𝑄 𝑀𝑀𝑀𝑀𝑀𝑀 = ∆𝑃𝑃 − ∆𝐷𝐷
The Q value gives this amount
Parents P are all species on the left side
Daughters D are all on the right side
21
Nuclear Reaction Energetics (β)
http://atom.kaeri.re.kr/
𝟐𝟐
𝟐𝟐
+
−
~0
𝑯𝑯 → 𝑯𝑯𝑵𝑵 + 𝛽𝛽 + 𝑬𝑬𝒓𝒓𝒓𝒓𝒓𝒓
𝑸𝑸 𝑴𝑴𝑵𝑵𝑴𝑴 = ∆𝑷𝑷 − ∆𝑫𝑫 = 𝟏𝟏𝟒𝟒. 𝟗𝟗𝟐𝟐𝟎𝟎 − 𝟏𝟏𝟒𝟒. 𝟗𝟗𝟐𝟐𝟏𝟏 = 𝟎𝟎. 𝟎𝟎𝟏𝟏𝟗𝟗 𝑴𝑴𝑵𝑵𝑴𝑴22
Nuclear Reaction Energetics (β)
𝟐𝟐
𝟐𝟐
+
−
𝑯𝑯 → 𝑯𝑯𝑵𝑵 + 𝛽𝛽 + 𝑬𝑬𝒓𝒓𝒓𝒓𝒓𝒓
Total
r n:
oo at differences in excess mass
𝑸𝑸 𝑴𝑴𝑵𝑵𝑴𝑴 = ∆𝑷𝑷 − ∆𝑫𝑫 = 𝟏𝟏𝟒𝟒. 𝟗𝟗𝟐𝟐𝟎𝟎 − 𝟏𝟏𝟒𝟒. 𝟗𝟗𝟐𝟐𝟏𝟏 = 𝟎𝟎. 𝟎𝟎𝟏𝟏𝟗𝟗 𝑴𝑴𝑵𝑵𝑴𝑴
ot all energ goes to the beta particle
Average beta energ : . e
ome, not all, goes to the daughter nuclide s recoil
Where does the rest go
23
Nuclear Reaction Energetics (β)
3
3
+
−
𝑯𝑯 → 𝑯𝑯𝑵𝑵 + 𝜷𝜷 +
0
𝝂𝝂
0�
+ 𝑬𝑬𝒓𝒓𝒓𝒓𝒓𝒓
An antineutrino carries awa the e cess energ
Chargeless, essentiall massless particles
Extremely hard to detect!
How did we know of their existence? Missing
energy in the reaction balance!
24
Other Types of Radiation
http://www-sk.icrr.u-tokyo.ac.jp/sk/gallery/wme/sk_01h-wm.jpg
http://www.ps.uci.edu/~tomba/sk/tscan/pictures.html
eutrino ν
Chargeless, nearl massless particle
Anti neutrino ν�
Antiparticle e uivalent of the neutrino
Super Kamiokande neutrino
detector, Japan
Courtesy of Kamioka Observatory, ICRR (Institute for Cosmic Ray
Research), The University of Tokyo. Used with permission.
Cherenkov radiation
rings produced by
© Tomasz Barszczak. All rights reserved. This content is
excluded from our Creative Commons license. For more
information, see http://ocw.mit.edu/help/faq-fair-use/.
25
Nuclear Reaction Principles (γ)
ometimes beta deca leaves the nucleus in an
excited state
This energ can be lost through gamma
emission, bringing the nucleus to the ground
state
ample: Cobalt 0
𝟔𝟔𝟎𝟎
𝟔𝟔𝟎𝟎∗
𝑪𝑪𝑪𝑪 →
∗
𝟔𝟔𝟎𝟎
+
𝑵𝑵𝑵𝑵 →
𝟔𝟔𝟎𝟎
Denotes excited
state
+
−
𝑵𝑵𝑵𝑵 + 𝛽𝛽 +
+
𝑵𝑵𝑵𝑵 + γ
0
�
0𝝂𝝂
26
Nuclear Reaction Principles (γ)
http://atom.kaeri.re.kr/
ow to determine gamma energ levels
Use energy level diagrams
27
Nuclear Reaction Principles (γ)
http://atom.kaeri.re.kr/
How can this be?
Internal conversion – competing
process with gamma emission
28
Nuclear Reaction Principles (γ)
Internal conversion IC
𝟔𝟔𝟎𝟎
cited state ic s out an inner shell electron
ample: Cobalt 0 most li el mechanism
𝑪𝑪𝑪𝑪 →
𝟔𝟔𝟎𝟎𝟗𝟗.𝟐𝟐𝟏𝟏 𝑴𝑴𝑵𝑵𝑴𝑴
𝟔𝟔𝟎𝟎𝟗𝟗.𝟏𝟏𝟔𝟔 𝑴𝑴𝑵𝑵𝑴𝑴
𝟔𝟔𝟎𝟎𝟏𝟏.𝟐𝟐𝟐𝟐 𝑴𝑴𝑵𝑵𝑴𝑴
𝟔𝟔𝟎𝟎∗
+
𝑵𝑵𝑵𝑵+ + 𝛽𝛽 − + 00𝝂𝝂�
𝑵𝑵𝑵𝑵 →
𝑵𝑵𝑵𝑵
+𝟗𝟗
𝟔𝟔𝟎𝟎𝟗𝟗.𝟏𝟏𝟔𝟔 𝑴𝑴𝑵𝑵𝑴𝑴
→
𝑵𝑵𝑵𝑵+𝟗𝟗 →
𝑵𝑵𝑵𝑵+𝟗𝟗 + 𝑀𝑀 −
𝟔𝟔𝟎𝟎𝟏𝟏.𝟐𝟐𝟐𝟐 𝑴𝑴𝑵𝑵𝑴𝑴
𝟔𝟔𝟎𝟎
𝑵𝑵𝑵𝑵+𝟗𝟗 + γ
𝑵𝑵𝑵𝑵+𝟗𝟗 + γ
β
IC
γ
γ
29
Nuclear Reaction Principles (γ)
Resulting radiation spectrum can be complex
Example: Cesium-137
Courtesy of Korea Atomic Energy Research Institute. Used with permission.
Graph © Wiley-VCH, from J. Turner, Atoms, Radiation, and Radiation
Protection (2007). All rights reserved. This content is excluded from
our Creative Commons license. For more information, see
http://ocw.mit.edu/help/faq-fair-use/.
͛͘
Nuclear Reaction Principles (γ)
http://atom.kaeri.re.kr/
31
Nuclear Reaction Principles (α)
elativel heav isotopes can emit a helium
nucleus alpha particle
ample: Americium 2 1
𝟗𝟗𝟒𝟒𝟏𝟏
𝑨𝑨𝑵𝑵 →
𝟗𝟗𝟐𝟐𝟐𝟐∗
−𝟗𝟗
𝑵𝑵𝑵𝑵
𝟗𝟗𝟐𝟐𝟐𝟐∗
→
−𝟗𝟗
𝑵𝑵𝑵𝑵
𝟗𝟗𝟐𝟐𝟐𝟐
𝑵𝑵𝑵𝑵
−𝟗𝟗
+ α
+γ
+2
32
Nuclear Reaction Principles (α)
http://en.wikipedia.org/wiki/Americium
Uses of Americium:
33
Nuclear Reaction Principles (α)
http://atom.kaeri.re.kr/
ow s this deca chain
34
Half Life
Time it ta es for half of an isotope to deca
The decay constant relates this to an
e ponential form of the same rule
1.0
λ=
A
A0
0.693
T
λt
A
= eA0
0.5
0.25
0.125
0
T
2T
3T
t
Image by MIT OpenCourseWare.
35
Half Life
Define an activity in terms of deca s per second
𝑑𝑑𝑑𝑑
= 𝜆𝜆𝑑𝑑
𝐴𝐴 = −
𝑑𝑑𝑑𝑑
olve the ordinar differential e uation:
𝑑𝑑𝑑𝑑
= −𝜆𝜆 ∗ 𝑑𝑑𝑑𝑑
𝑑𝑑
ln 𝑑𝑑 = −𝜆𝜆𝑑𝑑 + ln 𝑑𝑑0
𝐴𝐴 = 𝐴𝐴0 @ 𝑑𝑑 = 0
ln 𝑑𝑑 = −𝜆𝜆𝑑𝑑 + 𝑐𝑐
𝑑𝑑 = 0
𝑑𝑑
𝑙𝑙𝑙𝑙
= −𝜆𝜆𝑑𝑑
𝑑𝑑0
ln 𝑑𝑑0 = 𝑐𝑐
𝑑𝑑 = 𝑑𝑑0 𝑀𝑀 −𝜆𝜆𝜆𝜆
λ describes how uic l the number of atoms N
changes b a factor of e
3
Half Life
𝑑𝑑𝑑𝑑
= 𝜆𝜆𝑑𝑑
𝐴𝐴 = −
𝑑𝑑𝑑𝑑
𝑑𝑑 = 𝑑𝑑0 𝑀𝑀 −𝜆𝜆𝜆𝜆
Find the half life T :
𝑑𝑑
1
= = 𝑀𝑀 −𝜆𝜆𝑇𝑇
𝑑𝑑0 2
Notation
𝐴𝐴 = 𝐴𝐴0 @ 𝑑𝑑 = 0
𝐴𝐴 𝐴𝐴0 −𝜆𝜆𝜆𝜆
=
𝑀𝑀
𝜆𝜆
𝜆𝜆
ln 0.5 = −𝜆𝜆𝜆𝜆
0.693
𝜆𝜆 =
𝜆𝜆
Activity A measure of the number of radioactive deca s per second
Decay Constant The constant for an activit to decrease b a factor of e
Half Life – The time it ta es a sample s activit to decrease b a factor of 2
37
Measuring Activity
Activit is measured in ec uerels
1 𝐵𝐵𝐵𝐵 = 1 𝐷𝐷𝐷𝐷𝑀𝑀𝐷𝐷𝑙𝑙𝑑𝑑𝑀𝑀𝐷𝐷𝐷𝐷𝑀𝑀𝑑𝑑𝐷𝐷𝐷𝐷𝑙𝑙 𝑝𝑝𝑀𝑀𝐷𝐷 𝑀𝑀𝑀𝑀𝑐𝑐𝐷𝐷𝑙𝑙𝑑𝑑
A more convenient unit is the Curie Ci
1 𝐶𝐶𝐷𝐷 = 3.7 × 1010 𝐵𝐵𝐵𝐵
ou will often see reduced units mCi, Ci on
real devices and sources
Notation
One Curie is a lot of radiation
Becquerel (Bq) The fundamental unit of radioactivit , e ual to one
disintegration per second
Curie (Ci) A more convenient activit unit, 1 Ci 3.
1010
38
Activity on Sources and Devices
en.wikipedia.org/wiki/Americium
www.prc68.com/I/Rad_Det.shtml
39
Thinking Ahead for the Lab
ow do ou measure the activit of a source
ow do ou account for older sources
What else will ma e measuring the activit of a
source difficult
In other words, what are possible sources of error
and/or confusion
40
41
MIT OpenCourseWare
http://ocw.mit.edu
22.S902 Do-It-Yourself (DIY) Geiger Counters
January IAP 2015
For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms.