Document 13117817
advertisement

AN ABSTRACT OF THE THESIS OF Kathleen O'Hara for the degree of Master of Science in Veterinary Science presented on
December 27, 2000. Title: Effect of Dietary (n-3) and (n-6) Fatty Acids on the Immune
Response of Normal Horses.
Redacted for Privacy
Abstract approved: _ _ _
_
Jean A. Hall
Objective - To determine whether dietary (n-6) and (n-3) polyunsaturated fatty acids (PUFA) can
modulate the immune response of normal horses, and if dietary (n-3) fatty acids are more
suppressive than (n-6) fatty acids.
Animals - Ten healthy female horses.
Procedure - Two groups of normal horses were fed a diet supplemented with corn oil, an
(n-6) PUFA, or fish oil, an (n-3) PUFA, for a period of 14 weeks. Physical examinations and
body condition scores were recorded at the beginning and end of the feeding trial. Food
consumption was recorded daily and body weights were recorded periodically throughout
the trial. Plasma fatty acid profiles were evaluated at 0,6,8, 12 and 18 weeks (4 weeks after
discontinuing the PUFA supplements). The humoral immune response was evaluated by
measuring antibodies to keyhole limpet hemocyanin (KLH). Cell-mediated immunity was
assessed using a delayed-type hypersensitivity skin test (DTH) response to KLH.
Leukotriene production (LTB4 and LTBs) and cytokine production (TNF-a) by stimulated
immune cells were measured. Phagocytic activity of pulmonary alveolar macrophages was
also evaluated.
Biochemical measurements included plasma lipids (cholesterol and
triglycerides), plasma a-tocopherol, complete blood counts, white cell differential counts and
serum biochemistries.
Results - Food consumption was
~
95%, except for one horse which showed decreased food
consumption (25-50%) during the last 4 weeks of the feeding trial. Plasma fatty acid profiles of the
horses revealed significant changes as early as 6 weeks after initiating dietary supplementation. The
horses supplemented with (n-3) fatty acids showed an increase in plasma eicosapentaenoic acid
(EPA), docosahexaenoic acid (DHA), and arachidonic acid (AA), whereas the horses fed the (n-6)
fatty acids showed an increase predominantly in plasma linoleic acid. There was no difference
between the two groups of horses in their response to a DTH skin test. However, the immediate
response (30 minutes and 24 hours) was greater in the horses fed the (n-3) fatty supplement
compared to the horses fed the (n-6) fatty acid supplement. The KLH antibody titers showed no
significant difference between the groups of horses. At 0weeks, there was no difference in LTB4 and
LTB5 production between horses. After 12 weeks, leukotriene B4 and B5 production was significantly
increased in the horses consuming the (n-3) fatty acid supplement compared to the horses
consuming the (n-6) fatty acid supplement (pSO.02). The ratio of LTB5 to LTB4 was also significantly
higher in the horses consuming the (n-3) fatty acid supplement compared to the horses fed the (n 6)
e
fatty acid supplement (p=0.002). TNF-a production by pulmonary alveolar macrophages increased
significantly (p=0.05) within the two groups of horses at 6 weeks compared to the predietary period
but there was no difference between groups. Horses fed the (n-3) fatty acid supplement continued to
produce Significantly higher levels of TNF-a at 8weeks (p=0.04) and 12 weeks (p=0.0001) compared
to their predietary levels. Phagocytic activity of pulmonary alveolar macrophages was increased at 12
weeks in the horses consuming the (n-6) fatty acid supplement (p=0.03) compared to 0 weeks.
Cholesterol levels were significantly higher at 6 weeks in both groups of horses (P=0.03) and at 8
weeks (p=O.01) in the horses fed the (n-3) fatty acid supplement compared to the pretrial period.
Plasma u-tocopherollevels were also significantly increased in the horses fed the (n-3) fatty acid
supplement compared to 0 weeks (p=O.02).
Conclusion - The horses readily ate the com oil and fish oil supplements and no significant side
effects were noted. The plasma fatty acid profiles of the horses were significantly altered by dietary
PUFA supplementation by 6 weeks and changes disappeared within 4 weeks of discontinuing the
PUFA supplements.
Dietary supplementation with (n-6) and (n-3) fatty acids modulated the
inflammatory response of normal horses in several ways. Both fatty acid supplements increased the
production of the proinflammatory cytokine TNF-u, whereas only the (n-3) PUFA supplement
increased the production of the proinflammatory eicosanoid LTB4.
Production of the less
inflammatory eicosanoid LTB5 was also increased by dietary (n-3) PUFA supplementation. The ratio
of plasma EPA to AA concentrations corresponded to the ratio of LTB5 to LTB4 concentrations
produced by equine neutrophils.
Phagocytic activity was increased by dietary (n-6) PUFA
supplementation but not by dietary (n-3) PUFA supplementation. The immediate inflammatory
response to a DTH skin test was suppressed by dietary (n-3) PUFA supplementation but not by
dietary (n-6) PUFA supplementation and neither PUFA had an effect on the antibody response to
KLH.
@Copyright by Kathleen O'Hara December 27, 2000 All Rights Reserved Effect of (n-3) and (n-6) Fatty Acids on the Immune Response of Normal Horses
By
Kathleen O'Hara
ATHESIS submitted to Oregon State University in partial fulfillment of the requirements for the degree of Master of Science Presented December 27, 2000 Commencement June 2001 Master of Science thesis of Kathleen O'Hara presented on December 27,2000
APPROVED:
Redacted for Privacy
Major Professor, representing Veterinary Science
Redacted for Privacy
Dean ofOeQOtvete= ary M~dicine ;
Redacted for Privacy
I understand that my thesis will become part of the permanent collection of Oregon State
University libraries. My signature below authorizes release of my thesis to any reader upon
request.
Redacted for Privacy
Klthieen O'Hara, Author
ACKNOWLEDGEMENT
The author thanks the following people for their help and/or guidance, which made
this thesis possible. Lisa Boeder, Dr. Karyn Bird, Shi-Hua Du, Dr. Joe Gradin, Dr. Jean Hall,
Dr. Loren Koller, Ye-Sun Lee, Dr. Erwin Pearson, Bernadette Stang, Dr. Susan Tornquist, Dr.
Robert Van Saun, Dr. Anthony Vella, and Dr. Rosemary Wander.
TABLE OF CONTENTS INTRODUCTION ................................................................................................................... 1 REVIEW OF THE LITERATURE .......................................................................................... 6 Dietary Considerations.......................................................................................... 6 Equine Dietary Practices............................................................................ 6 Dietary Study Designs................................................................................ 8 Immune Responses ............................................................................................... 9 Inflammation ............................................................................................... 9 Hypersensitivity Reactions ....................................................................... 10 Effect of Dietary Fatty Acids on Immune Responses ..................................... 13 Membrane fluidity .. ................................................................................... 14 Cell-mediated immunity............................................................................ 17 Antibody production.................................................................................. 18 Cytokine production.................................................................................. 20 Eicosanoid synthesis................................................................................ 23 Leukotriene synthesis............................................................................... 27 MATERIALS AND METHODS .. .......................................................................................... 32 Animals .................................................................................................................. 32 Diets ....................................................................................................................... 32 Study Design.........................................................................................................39 Plasma Fatty Acid Profiles.................................................................................. 40 Immunological Measurements ........................................................................... 41 De/ayed-type hypersensitivity skin test ................................................... 41 Keyhole limpet hemocyanin antibody titer............................................... 43 Leukotriene 84 and 85 quantification ....................................................... 44 Tumor Necrosis Factor-a production ...................................................... 48 Phagocytosis '" ......................................................................................... 51 TABLE OF CONTENTS continued
Biochemical Measurements ............................................................................... 52 Plasma lipids (cholesterol and triglyceride) ............................................. 52 Plasma a-tocopherol ............................................................................... 53 Complete blood count (CBC) and white cell differential count............... 53 Serum biochemistries............................................................................... 53 Statistical Analysis .............................................................................................. 54 RESULTS ............................................................................................................................. 55 Animals .................................................................................................................. 55 Diets .......................................................................................................................55 Plasma Fatty Acid Profiles.................................................................................. 55 Immunological Measurements ........................................................................... 64 Delayed-type hypersensitivity skin test ................................................... 64 Keyhole limpet hemocyanin antibody titer............................................... 65 Leukotriene 84 and B5 quantification ....................................................... 65 Tumor Necrosis Factor-a production ...................................................... 70 Phagocytosis ............................................................................................ 71 Biochemical Measurements ............................................................................... 72 Plasma lipids (cholesterol and triglyceride) ............................................. 72 Plasma a-tocopherol ............................................................................... 74 Complete blood count (CBC) and white cell differential count............... 75 Serum biochemistries............................................................................... 76 DISCUSSION ....................................................................................................................... 78 REFERENCES..................................................................................................................... 93 APPENDIX .......................................................................................................................... 100 LIST OF FIGURES 1a. Diagrammatic representation of a membrane's transition between a gel state
and a liquid-crystalline state..............................................................................................16
1b. Diagrammatic represenation of cholesterol's effect on a membrane's transition
between a gel state and a liquid-crystalline state............................................................16
2. Metabolism of (n-6) polyunsaturated fatty acids and (n-3) polyunsaturated fatly
acids through the cyclooxygenase and lipoxygenase pathways.....................................25
3. Metabolism of arachidonic acid, eicosapentaenoic acid and docosahexaenoic acid
through the 5-lipoxygenase pathway................................................................................29
4. Effect of feeding horses diets that differed in the (n-6) and (n-3) fatly acid content
on the plasma concentrations of saturated fatty acids, mononsaturated fatty acids
and polyunsaturated fatty acids at 0,6,8, 12, and 18 weeks .........................................57
5. Effect of feeding horses diets that differed in the (n-6) and (n-3) fatty acid content
on the sum of the (n-6) and the sum of the (n-3) fatty acids in plasma at 0,6,8,12
and 18 weeks. The ratio of the sum of (n-6) to the sum of (n-3) fatty acids is also
compared at 0,6,8,12 and 18 weeks.............................................................................60
6. Effect of feeding horses diets that differed in the (n-6) and (n-3) fatty acid content
on the concentration offatty acids in plasma. Total plasma PUFA is subdivided
into plasma linoleic acid (LA) and the sum of the remaining PUFA (rest). These
are shown for each group of horses at 0,6, 12 and 18 weeks.......................................63
7. Effect of feeding horses diets that differed in the (n-6) and (n-3) fatty acid content
on the production of LTB4 and LTB5 by stimulated peripheral blood neutrophils at 12
weeks compared to baseline (0 weeks). The ratio of LTB5 to LT84 is compared
at 0 and 12 weeks .............................................................................................................67
8. Effect of feeding horses diets that differed in the (n-6) and (n-3) fatty acid content
on the concentration of AA, EPA, and DHA in plasma is compared to LTB4 and
LTB5 production by stimulated neutrophils at 0 weeks in panel A and 12 weeks in
panel B. The ratios of EPA to AA and LTB5 to LTB4 concentrations are included........69
LIST OF FIGURES (Continued)
9. Effect of feeding horses diets that differed in the (n-6) and (n-3) fatty acid content on the production of TNF-a production by pulmonary alveolar macrophages at 0, 6, 8, and 12 weeks ................................................................................................................71 10. Effect of feeding horses diets that differed in the (n-6) and (n-3) fatty acid content on phagocytic activity of alveolar macrophages at 0, 6, 8 and 12 weeks.......................72 11. Effect of feeding horses diets that differed in the (n-6) and (n-3) fatty acid content on plasma lipid concentrations (cholesterol and triglyceride) at 0,6,8 and 12 weeks .........................................................................................................73 12. Effect of feeding horses diets that differed in the (n-6) and (n-3) fatty acid content on plasma a-tocopherol concentrations in horses at 0, 6, 8 and 12 weeks ......75 LIST OF TABLES 1. Composition of selected fatty acids of the oils used in the study diets ............................. 34 2. Nutrient composition of the diets on a dry matter basis ..................................................... 35 3. Vitamin and mineral analysis of the vitamin/mineral supplement...................................... 36 4. Nutrient analysis of the grass hay....................................................................................... 37 5. Nutrient analysis of the legume hay.................................................................................... 38 LIST OF APPENDIX TABLES 1. Effect of feeding diets that differed in the (n-6) and (n-3) fatty acid content on
plasma fatty acid profiles of horses after 6,8 and 12 weeks compared to
baseline (0 weeks}.............................................................................................................. 101
2. Effect of feeding diets that differed in the (n-6) and (n-3) fatty acid content on
plasma fatty acid profiles of horses after 12 weeks compared to baseline (0 weeks)
and 4 weeks after discontinuing the fatty acid supplement (18 weeks) ........................... 103
3. Effect of feeding diets that differed in the (n-6) and (n-3) fatty acid content on
the delayed type hypersensitivity skin test in horses at 30 minutes, 24, 48, 72
and 96 hours....................................................................................................................... 105
4. Effect of feeding diets that differed in the (n-6) and (n-3) fatty acid content on
the keyhole limpet hemocyanin (KLH) antibody log titers in horses after vaccination
with KLH .............................................................................................................................. 106
5. Effect of feeding diets that differed in the (n-6) and (n-3) fatty acid content on
leukotriene 84 and leukotriene 85 production by horse peripheral blood neutrophils
stimulated with calcium ionophore A23187; and the ratio of LTBs to LT84 at
12 weeks compared to baseline (0 weeks)....................................................................... 107
6. Effect of feeding diets that differed in (n-6) and (n-3) fatty acid content on
TNF-a production by pulmonary alveolar macrophages (PAM) and peripheral
blood mononuclear cells (P8MC) from horses after 6, 8, and 12 weeks compared
to baseline (0 weeks) .......................................................................................................... 108
7. Effect of feeding diets that differed in the (n-6) and (n-3) fatty acid content on
fluorescent bead engulfment by pulmonary alveolar macrophages (PAM) from
horses after 6, 8 and 12 weeks compared to baseline (0 weeks) .................................... 109
8. Effect of feeding diets that differed in the (n-6) and (n-3) fatty acid content on plasma
lipid concentrations (cholesterol and triglyceride) of horses after 6,8, and 12 weeks
compared to baseline (0 weeks)........................................................................................ 110
9. Effect of feeding diets that differed in the (n-6) and (n-3) fatty acid content on
plasma a-tocopherol concentrations of horses after 6, 8, and 12 weeks compared
to baseline (0 weeks} .......................................................................................................... 111
LIST OF APPENDIX TABLES (Continued)
10. Effect of feeding diets that differed in the (n-6) and (n-3) fatty acid content on complete blood counts (CSC) of horses after 6, 8 and 12 weeks compared to baseline (0 weeks).............................................................................................................. 112 11. Effect of feeding diets that differed in the (n-6) and (n-3) fatty acid content on serum biochemistries of horses after 8 and 12 weeks compared to baseline (0 weeks) ........... 113 LIST OF ABBREVIATIONS AA =arachidonic acid
BALF =bronchoalveolar lavage fluid
DHA =docosahexaenoic acid
DMEM =Dulbecco's modified essential medium
DPBS =Dulbecco's phosphate buffered saline
DTH =delayed-type hypersensitivity reaction
EPA =eicosapentaenoic acid
HBSS =Hank's balanced salt solution
IL =interleukin
=keyhole limpet hemocyanin
LT =leukotriene
MUFA =monounsaturated fatty acids
KLH
PAM =pulmonary alveolar macrophages
PBMC =peripheral blood mononuclear cells
PG =prostaglandin
PUFA =polyunsaturated fatty acids
SFA =saturated fatty acids
TX =thromboxane
DEDICATION
This work is dedicated to the memory of my father, William, and to my mother, Carleen.
Effect of Dietary (n-3) and (n-6) Fatty Acids on the Immune Response of Normal Horses
INTRODUCTION
Equine chronic obstructive pulmonary disease (COPD) is an inflammatory condition
of the lower airways of older horses (usually> 6 years). It is often associated with exposure
to dusty or moldy hay, straw or bedding. The two most frequently implicated molds are
Aspergillus fumigatus and Micropo/yspora 'aeni. Clinical signs of the disease include
dyspnea, cough and nasal discharge, which can vary with the severity of the disease. The
condition is characterized by intermittent airway obstruction resulting from bronchospasm,
mucus plugs and pathologic changes in the bronchiolar walls. There is also an increase in
pulmonary neutrophils. These pathologic changes are associated with an increase in
pulmonary airway resistance and subsequent hypoxemia.
The horse works harder to
breathe with a greater effort made on the expiratory phase rather than the inspiratory phase.
In an attempt to expel air, the horse's forced expirations result in hypertrophy of the external
abdominal oblique muscles and a "heave line".1,2
The conventional treatment for this chronic inflammatory condition is corticosteroids.
Although corticosteroids decrease inflammation, they also have a number of harmful effects
that include laminitis, infections and a Cushing-like syndrome with signs of muscle wasting,
dry hair coat, polydipsia, and polyuria. 1 Because of these negative effects associated with
long term steroid use, alternative treatments are needed in the management of immune­
mediated conditions such as COPD. One alternative treatment may be the use of dietary
2
supplements, e.g., polyunsaturated fatty acids, which have been shown to modulate the
immune system in several ways.3-5
All mammals can synthesize fatty acids with the exception of linoleic acid and a­
linolenic acid, which must be provided in the diet. These are termed essential fatty acids.
Linoleic acid, an (n-6) PUFA, is found in corn oil and soy oil, while a-linolenic acid, an (n-3)
PUFA, is found in fish oil and linseed oil. Further metabolism of linoleic acid, e.g., elongation
and desaturation, results in the production of arachidonic acid (AA), which is subsequently
incorporated into cellular membranes.
Further metabolism of a-linolenic acid, e.g.,
elongation and desaturation, results in the production of eicosapentaenoic acid (EPA) and
docosahexaenoic acid (DHA), which are also incorporated into cellular membranes. It is
thought that by increasing the amount of dietary (n-3) PUFA relative to (n-6) PUFA, that (n-3)
fatty acids are preferentially incorporated into cell membranes compared to (n-6) fatty acids. 6
When cells are activated by a chemical or physical insult, fatty acids are metabolized
into eicosanoids. Eicosanoids are mediators of inflammation. The type of eicosanoid that a
cell produces can be modulated through dietary supplementation of the essential fatty acids.
Arachidonic acid metabolism by cyclooxygenase produces the proinflammatory eicosanoids
of the 2-series (e.g., thromboxane (TX) A2 and prostaglandin (PG) E2), whereas metabolism
of AA by lipoxygenase yields the 4-series eicosanoids (e.g., leukotriene (LT) 84).
Alternatively, metabolism of EPA produces the less inflammatory eicosanoids of the 3- and
5-series {e.g., T){Aa, PGE3, and LT8s).3
In addition to affecting eicosanoid production, dietary modulation with PUFA can also
affect production of some cytokines. Cytokines affect the immune response by altering
3
lymphocyte proliferation, differentiation, and activation. Production of the proinflammatory
cytokines interleukin (IL)-1, IL-6 and TNF-a has been shown to be decreased when a diet is
supplemented with (n-3) PUFA 7-9. Dietary consumption of PUFA has been shown to affect
other immune functions such as phagocytosis, cell-mediated immune responses and
antibody production. 10-14
It is well documented that PUFA have the ability to either suppress or enhance the
immune response. Epidemiological studies have shown that populations of people who
consume large amounts of fish, a source of high polyunsaturated (n-3) fatty acids, have a
lower incidence of inflammatory conditions and autoimmune diseases. 15,16 Kremer et a/. 17
demonstrated that consumption of (n-3) fatty acids resulted in decreased symptoms related
to rheumatoid arthritis. In this study, patients with rheumatoid arthritis were randomized into
two groups and placed on diets for 12 weeks. One group consumed a diet with a
polyunsaturated/saturated (PIS) fatty acid ratio of 1.4:1, while the other group consumed a
control diet with a PIS fatty acid ratio of 1:4. Onset of morning stiffness was delayed and joint
tenderness was improved in the group consuming the highly PUFA diet.
Consumption of polyunsaturated fatty acids has been shown to exert effects on
some inflammatory lung disorders. For example, increased fish consumption in people
seems to have some protective qualities against conditions such as childhood asthma and
adult bronchitis, although not against adult asthma. 18 Data from the Second National Health
and Nutrition Examination Survey (NHANESII) showed that diets supplemented with fish oil
protected against wheezing in smokers with bronchitis. 19
4
Animal models have been used to demonstrate the anti-inflammatory effects of (n-3)
fatty acids on lung tissue. Koch et al. 20 perfused isolated rabbit lungs with a 10% fish oil
emulsion, a 10% soy oil emulsion, or 2 ml of saline solution for 3 hours. The lungs were
stimulated with small doses of calcium ionophore A23187 (10-SM) during the 3 hour period,
followed by a challenge with a higher dose of calcium ionophore (1Q-7M) after the lipids were
washed out by exchange of perfusion fluid. Edema (indicated by increased lung weight) and
pulmonary artery pressure were reduced by 50010 in the fish oil group compared to the soy oil
and control groups. In addition, the lungs perfused with fish oil shifted the production of
leukotrienes from LTC4, which constricts respiratory tract smooth muscle, to LTCs, which is
less vasoconstrictive.
Murray et a/. 21 compared the effects of linoleic acid, an (n-6) PUFA, with EPA and
gamma-linolenic acid, both of which are (n-3) fatty acids, on acute lung injury in pigs. Three
groups of pigs were fed a diet supplemented with linoleic acid, EPA or EPA plus gamma­
linolenic acid for 8 days. Acute lung injury was induced by a continuous 4-hour infusion with
E. coli endotoxin. Pigs that received the diets supplemented with EPA, or EPA and gamma­
linolenic acid produced less thromboxane (TX) 82 than those fed linoleic acid. Those groups
fed higher amounts of (n-3) PUFA also showed an improvement in oxygen delivery as
indicated by measurements in arterial P02levels.
The goal of this investigation was to evaluate the effect of feeding a dietary
supplement of fish oil versus corn oil on the immune response of healthy horses. Parameters
to be evaluated included immune cell function of the equine lung (phagocytic ability of
pulmonary alveolar macrophages), systemic antibody production to a foreign antigen, and a
5
delayed type hypersensitivity (OTH) skin test. In addition, and of particular interest in this
study, was the effect of different sources of PUFA on eicosanoid and cytokine production. If
it could be demonstrated that dietary supplementation with (n-3) PUFA leads to a reduction in
proinflammatory cytokines and eicosanoids in normal horses, then there is justification for
studying the effects of (n-3) PUFA supplementation in horses with chronic obstructive
pulmonary disease.
6
REVIEW OF THE LITERATURE Dietary Considerations
Equine Dietary Practices
Equine feeding practices since the early 1900's have been quite varied. The main
staples fed to horses over the years have been hay, oats, straw, maize, beans or dried
grains. However, some dietary supplements included meat rolled in bran, and Norwegian
horses were accustomed to eating boiled fish soup mixed with other food. Although this
practice is not recommended today, some Icelandic ponies may still be fed herring on
occasion. Over the last century, oats have been the preferred feed, although the use of corn
has increased mostly because of a decline in oat production in some areas. 22
Recently there has been an interest in supplementing horse diets with fat. This
notion began in the 1970's, when a study reported higher blood glucose levels in horses after
an endurance ride when they were fed a high fat diet.22 While the major emphasis on fat
supplementation in horses has been for performance horses (Le., endurance horses and
thoroughbreds), there are some practical reasons for feeding this energy source to horses in
general. For example, in addition to improving coat condition, there is some indication that
diets containing fat rather than soluble carbohydrate may decrease the risk of founder. 23
Founder, or laminitis, occurs when there is an overabundance of carbohydrate or
starch in the horse's diet. An excess of this type of nutrient overwhelms the digestive
capacity of the small intestine and starch is passed to the large intestine where it is
7
fermented. This results in increased lactic acid production and a decrease in pH, which can
lead to the release of endotoxins (because of bacterial death) and subsequently to colitis or
laminitis.
Substituting fat for carbohydrates can reduce the risk of these metabolic
disturbances. Neelley and Herthel24 have shown that laminitis in horses can be prevented
by dietary fatty acid supplementation.
Because of the interest in feeding fat to horses in order to improve performance and
health, several investigators have examined the horse's ability to digest fats. 25-28 Each of
these studies revealed that fats are readily digested in the equine intestinal tract regardless
of their source (animal vs. vegetable origin) or degree of saturation. One long-term study
conducted by Harris et al. 29 compared the effects of feeding a saturated versus an
unsaturated fatty acid dietary supplement to Thoroughbred horses. These investigators
found no apparent adverse affects of feeding either a saturated (coconut oil) or unsaturated
(soy oil) fatty acid supplement to horses over a 6 month feeding period.
Although the degree of saturation of a dietary fat has no affect on digestibility in the
horse, the degree of saturation or unsaturation may affect cell function because of changes in
membrane fluidity. For example, polyunsaturated fatty acids may increase cell membrane
fluidity, compared to saturated fatty acids, which in turn may alter cell function. Thus, there is
a need to determine whether one fatty acid is superior to another when supplementing
equine diets with fat. This is true for healthy horses in general, as well as for horses with
specific underlying disease conditions.
8
Dietary Study Designs
Various study designs have been used to investigate the role of dietary fatty acid
supplementation on immune responses of horses.
For example, some studies have
compared the effect of a PUFA supplement, usually (n-3) fatty acids, with that of a "control"
diet (diet not supplemented with fatty aCids).30,31 Another study design used by Morrris et
al. 32 compared animals before and 8 weeks after they were given dietary PUFA
supplementation. In a third study, Harris et al. 29 compared two groups of horses, one
receiving a saturated fatty acid source versus the other receiving an unsaturated fatty acid
source in their diet. Based on these studies, it appears that the use of a traditional control
group is not always necessary. In these cases, a pre/post study design and/or a study
design with two treatment groups have yielded valuable information.
Treatment groups for this investigation were selected based upon the following
criteria. The primary question of interest was whether immune responses of normal horses
are affected when their diet is supplemented with either (n-6) fatty acids or (n-3) fatty acids.
The effects that these polyunsaturated fatty acids have on immune function are well
documented in other species. In general, their effects are considered proinflammatory, (n-6)
fatty acids, or anti-inflammatory, (n-3) fatty acids. It was an important consideration in this
study that the diets were balanced in terms of the same energy source (Le., the two diets
were balanced in fat and carbohydrate content). This would allow any differences between
groups to be attributed to differences in the type of polyunsaturated fatty acid fed rather than
the overall fat or carbohydrate content of the diet. The study was also designed such that
9
each animal could serve as its own control, (Le., prestudy evaluations were used as baseline
values for each animal).
Immune Responses
Inflammation
Inflammation can be classified as either acute, which usually occurs within an hour of
injury or infection, or chronic, which occurs hours to days later. The characteristics of
inflammation include swelling, redness, heat and pain.
Vasoactive mediators such as
histamine and cytokines cause blood vessel dilation and increased vascular permeability,
which leads to swelling, redness and heat. Pain is caused by migratory neutrophils and
macrophages releasing inflammatory mediators such as eicosanoids. 33,34
Acute inflammation is mediated by histamine, kinins and AA metabolites such as
LTB4 and PGE2 produced by mast cells; by proinflammatory cytokines such as TNF-a, IL-1
and IL-6 produced by phagocytes; and by respiratory burst and digestive enzymes contained
in granules released by neutrophils. Histamine causes vasodilation of arterioles and venules,
vasoconstriction of larger arteries; and kinins cause smooth-muscle contraction (e.g.,
bronchi, G.!. tract and bladder).
Leukotriene B4 stimulates neutrophil and eosinophil
chemotaxis, and PGE2 causes vasodilation and platelet aggregation.
TNF-a activates
vascular endothelium to increase the expression of cell adhesion molecules and increases
vascular permeability.
This favors the extravasation of leukocytes and lymphocytes.
Interleukin-1 activates vascular endothelium, and along with IL-6 activates lymphocytes (Le.,
10
B cells and T helper-2 cells) and macrophages.
Respiratory burst enzymes, such as
NADPH-oxidase, produce superoxide anion (0-2), which is then metabolized to hydrogen
peroxide (H202) by the enzyme superoxide dismutase. Digestive enzymes, such as acid
hydrolases, lipase and proteases, along with metabolites from the respiratory burst enzymes
cause cellular damage that contributes to the inflammatory process. 33,34
The acute inflammatory response can lead to chronic inflammation.
Chronic
inflammation is mediated by enzymes (collagenase, lipase, elastase, hyaluronidase and
proteases) released by macrophages during formation of phagolysomes. These enzymes
damage cellular membranes and mast cells. Damage to the latter results in the release of
histamine. Macrophages also produce proinflammatory cytokines (TNF-a, IL-1, and IL-6)
and arachidonic acid metabolites, such as leukotrienes and prostaglandins. Sustained
inflammation is also characterized by the presence of eosinophils and T helper-2
lymphocytes, which are recruited to the site by mediators released from mast cells (e.g., LTB4
and histamine).33
Hypersensitivity Reactions
Immediate hypersensitivity, mediated by IgE antibody, is an allergic response to a
soluble allergen. Allergens that elicit a Type 1 hypersensitivity response pass through
mucous membranes, such as the conjunctiva, respiratory tract and gastrointestinal tract.
Allergens that produce an IgE antibody response are usually delivered in small doses (Le., <1
J-lg). Exposure to antigen activates T-helper 2 cells to produce IL-4, a cytokine that induces
isotype switching from IgM to IgE. Mast cells are activated when IgE antibodies that are
11
bound to their surface by specific Fc receptors are cross-linked by antigen. Upon activation,
the mast cells release their granules containing histamine and kinins, and the cell membrane
produces leukotrienes and prostaglandins.
The type of IgE-mediated hypersensitivity reaction that occurs depends on the dose
of allergen and the location of the mast cell that is stimulated. Allergen (usually a high dose)
that enters the circulatory system affects masts cells located in connective tissue. There is a
generalized release of histamine and systemic anaphylaxis can follow.
low doses of
allergen that gain access through the skin (subcutaneous route) result in localized histamine
release and local inflammation.
Allergens that invade the body through inhalation or
ingestion affect the mast cells that are located in mucosal tissue. Ingestion of allergens can
produce a localized contraction of smooth muscle, which leads to vomiting. It can also
produce a systemic response, e.g., hives or anaphylaxis. low doses of antigen within the
respiratory tract result in mast cell release of histamine and kinins. These cause smooth
muscle contraction of the smaller airways.33,34
Type 2 hypersensitivity reactions are the result of drug interactions in susceptible
individuals. Drugs that bind to cell surfaces (e.g., red blood cells) can elicit the production of
IgG antibodies.
These anti-drug antibodies target the cells for destruction by
macrophages. 33 ,34
Type 3 hypersensitivity reactions, or immune-complex disease, occur in response to
a soluble antigen.
When antigen and antibody (lgG mediated) react, small immune­
complexes form, which are then deposited in tissues instead of being cleared from the
circulation through the action of complement.
The immune complexes cause local
12
inflammation and increased vascular permeability by activating mast cells, leukocytes and
complement (proinflammatory CSa). Leukocytes, especially neutrophils, enter the site from
local vessels. This local inflammation, which is followed by neutrophil infiltration to the area,
is known as an Arthus Reaction. It is characterized by a necrotic lesion resulting from
antigen-antibody complexes accumulating in the tissue. Some allergens can elicit type 3
hypersensitivity reactions when they are present in large amounts. For example, inhalation
of high doses of allergen may lead to the production of IgG rather than IgE antibody. This
occurs in farmers who have continual exposure to hay-dust or molds. The inhaled allergens
react with antibodies to form immune complexes in the alveolar walls. Accumulation of fluid
and cells in the alveolar walls compromise lung function. 33 ,34
Delayed-type hypersensitivity reactions (type 4 hypersensitivity) are mediated by T
helper-1 cells and cytotoxic T cells. This type of hypersensitivity occurs after there has been
a previous exposure to the same antigen. Soluble protein antigens (e.g., insect venom) elicit
this type of reaction and cause local swelling. Erythema (reddening of the area), induration
(hardening) and dermatitis are characteristic of this response. When antigen enters under
the skin (subcutaneously), a T cell mediated response evolves over the next 24 to 72 hours.
T helper-1 cells enter the site and recognize the antigen through interactions with antigen
presenting cells.
After T helper-1 cells are activated, they release growth factors,
chemokines, and cytokines. The release of growth factors such as IL-3 and granulocyte
macrophage-colony stimulating factor (GM-CSF) act to stimulate the production of early
monocyte/macrophage precursors in the bone marrow. The release of chemokines such as
Macrophage Chemotactic Factor direct the migration of macrophages into the area.
13
T helper-1 cells produce interferon-8 (IFN-8), which activates macrophages causing them to
release inflammatory mediators such as IL-1 and TNF-a. Release of TNF-a and TNF-j3 by T
helper-1 cells causes tissue destruction and increased expression of adhesion molecules on
local blood vessels. Adhesion molecules aid in the recruitment of other cells to the area
(e.g., phagocytes and Tcells).33,34
Although the exact cause of chronic obstructive pulmonary disease in horses is
unknown, it is thought to be a Type 1 and/or Type 3 hypersensitivity reaction. 1,34,35 Its
characteristics are consistent with Type 1 hypersensitivity (allergic asthma) in that the clinical
signs improve when the allergen is removed from the horse's environment. 36 Additionally, it
has been shown that horses with COPO have higher levels of IgE antibody in the lung. 37
The manifestation of chronic bronchiolitis is consistent with a Type 3 hypersensitivity
reaction. 34
Effect of Dietary Fatty Acids on Immune Responses
To achieve an understanding of the immune modulating effects of fatty acids, it is
helpful to know how they affect cellular function. Some of the ways in which fatty acids can
affect cellular functions include changing cell membrane fluidity, cell-mediated immunity,
antibody production, production of cytokines, or altering eicosanoid metabolism.
14
Membrane fluidity
The Singer-Nicholson fluid mosaic model perhaps best illustrates the structure of
biomembranes. 38 In this model, biomembranes consist of a phospholipid bilayer with
globular proteins and glycoproteins embedded in the bilayer in varying degrees. Each
phospholipid is composed of a polar hydrophilic head and two non-polar, hydrophobic fatty
acyl chains. The phosphate-containing head group has a third hydrophilic group such as
choline, serine, inositol or ethanolamine. One of the acyl chains is usually an unsaturated
fatty acid, while the other is a saturated fatty acid. The degree of unsaturation of these acyl
groups affects their physical properties. Saturated fatty acids have no double bonds between
the carbons in the chain, whereas unsaturated fatty acids have one or more double bonds.
Each double bond introduces a bend or "kink" in the molecule, which causes more disorder
and lowers the melting point. Therefore, cell membranes containing higher amounts of
unsaturated fatty acid are more fluid at lower temperatures.
An interesting example of how membrane fluidity is affected by temperature can be
seen with cholesterol, a constituent of plasma membranes. Membranes can exist in a gel­
crystalline state or in a liquid-crystalline state depending upon the temperature (see Figure
1a). Cholesterol affects membrane fluidity by broadening the temperature range over which
the membrane transitions between these two states. Cholesterol, a precursor to some
hormones, is made up of cyclohexane rings with a hydroxyl group at one end. Its bulky
conformation causes more disorder within the membrane in the gel-phase, which means that
it begins to transition from a gel to a liquid at a lower temperature. But because of its rigid
15
structure, it stiffens the membrane above the transition state, which means it takes longer to
liquefy (see Figure 1b).38
These points help illustrate the fact that membrane fluidity can be altered by the
cholesterol to phospholipid ratio, the degree of fatty acid unsaturation, and temperature.
Increasing the amount of PUFA in the diet increases the ratio of PUFA to SFA (PIS ratio) in
cell membranes and thus increases membrane fluidity.6 It makes sense that cell function is
related to the degree of fluidity of its membranes. For example, an important cell function
that is likely related to membrane fluidity is phagocytosis of foreign antigen by cells involved
in the innate immune response. Calder39 summarizes the findings of several animal studies
that indicate that fish oil, an (n-3) PUFA, has either no effect or a suppressive effect on
phagocytic activity of resident macrophages in different organs.
A review by Peck40
indicates that while consumption of saturated fatty acids suppresses phagocytosis, the type
of polyunsaturated fatty acid, i.e., (n-3) or (n-6), has no effect on phagocytic activity. A study
conducted by Virella et al. 41 tested the ability of human polymorphonuclear leukocytes to
engulf fluorescent latex beads after a volunteer consumed a fish oil supplement for 6 weeks.
Phagocytic activity of polymorphonuclear leukocytes was slightly decreased at 3 weeks and
markedly suppressed at 6 weeks compared to baseline activity levels. In another study in
which cells were cultured with fatty acids, Calder et al. 10 demonstrated a positive correlation
between phagocytic activity of mouse macrophages and an increasing degree of
unsaturation of fatty acids.
16
Heat
Cool
Gel state
More ordered
Liquid state
Disordered, irregular
Figure 1a. Diagrammatic representation of a membrane's transition between a gel state
and a liquid-crystalline state.
Tm = transition (meltlng)
temperature
Pure phospholipid
bilayer
---.
u0
-,
c:
0
:;:::;
a.
....
0
(/)
.0
co
m
Q)
-
.r:.
0
.£B
co
0:::
0
10
20
30
40
50
60 Figure1b. Diagrammatic representation of cholesterol's effect on a membrane's
transition between a get state and a liquid-crystalline state. (Adapted from Mathews
and Van Holde, 1995)
17
Cell-mediated immunity
Cell-mediated immunity is mediated by T lymphocytes. Two subsets of T cells that
are important in this response are the cytotoxic CD8+ T cells and the CD4+ T helper-1 cells.
The CD8+ T lymphocyte is activated when it recognizes antigen presented by an antigen­
presenting cell. It undergoes proliferation in response to the T cell growth factor interleukin
(IL)-2, and this is followed by differentiation into an armed effector T cell. The activated
cytotoxic T cell kills antigen-invaded cells through direct interactions. The CD4+ T cells, upon
activation, differentiate into either aT helper-1 cell or a T helper-2 cell. The T helper-2 cell is
involved in humoral immunity. The T helper-1 cell mediates the cell-mediated immune
response through interactions with antigen presenting cells such as the macrophage. 33
Fish oil, an (n-3) PUFA, has been shown to exert suppressive effects on T
lymphocyte proliferation. Meydani et al. 8 gave young and old women a fish oil supplement
for 3 months and measured T lymphocyte proliferation at 0,4,8 and 12 weeks. Proliferation
was suppressed in the older women at all timepoints compared to the younger women,
emphasizing that age also has an effect on T lymphocyte proliferation. Nonetheless, even
within the same age group, an increase in proliferation was seen at 4 weeks in the older
women compared to 0 weeks, but it was followed by a decrease at 8 and 12 weeks. In a
study conducted by Wander et a1.42 , geriatric beagle dogs were fed three experimental diets
that had a ratio of (n-6) to (n-3) PUFA of 31:1, 5.4:1 or 1.4:1 for 12 weeks. Dogs that were on
the high (n-3) fatty acid diet had a suppressed cell mediated immune response based on the
results of a delayed-type hypersensitivity skin response (DTH). Fritsche and Johnston12 fed
18
weanling mice a diet of 10% corn oil, 10% linseed oil, or 8% fish oil plus 2% corn oil for 8 to
12 weeks. Cytotoxic T cell activity of spleen cells was measured 7 days after the mice were
given a viral challenge. The cytotoxic T cell activity of splenocytes from fish oil-fed mice was
significantly lower than that of the linseed oil-fed mice. Although the fish oil-fed mice had
lower cytotoxic Tcell activity than the corn oil-fed mice, it was not statistically Significant.
Antibody production
The humoral immune response is mediated by B cells that proliferate into antibody­
secreting plasma cells. B cell activation and differentiation into plasma cells is triggered by
antigen and is mediated by T helper-2 cells. This type of acquired immunity differs from cell
mediated immunity in that it is a response to extracellular antigen. How (n-3) fatty acids
affect the humoral immune response has been evaluated in several studies that reveal
contradictory results.
Lim et al. 43 studied the effect of cells incubated in vitro with (n-3) PUFA on antibody
production by spleen lymphocytes from Sprague-Dawley rats. The specific PUFA studied
included DHA, EPA, and a-linolenic acid. Cells were cultured with (n-3) PUFA alone, or with
(n-3) PUFA and concavalin A or pokeweed mitogen for 6 hours. Data from this study showed
that cells incubated with (n-3) PUFA alone had suppressed IgA, IgM and IgG antibody
production. Marked inhibition of IgM antibody production and mild inhibition of IgG and IgA
antibody production occurred when cells were incubated with Concavalin A alone, while no
inhibition occurred when cells were incubated with pokeweed mitogen alone.
When
lymphocytes were cultured with PUFA in the presence of concavalin A, PUFA attenuated the
19
inhibitory effect of ConA on IgG and IgM antibody production but strengthened the inhibition
on IgA antibody production. Cells cultured with PUFA and pokeweed mitogen showed
inhibition of IgA and IgG, but not IgM antibody production.
In another study conducted by Yamada et a1. 14, rat lymphocytes had decreased
production of IgM, IgG and IgA antibodies and increased production of IgE antibodies when
cultured with unsaturated fatty acids such as AA and linoleic acid. The authors showed that
enhanced production of IgE was dependent on the type of PUFA as follows: linoleic acid <a­
linolenic acid=EPA<AA<DHA. Additionally, the authors showed that the enhancing effect of
PUFA on lymphocyte production of IgE could be suppressed if a-tocopherol (vitamin E) was
present in the culture.
In a human study conducted by Virella et al.,41 peripheral blood mononuclear cells
were cultured with pokeweed mitogen alone or with pokeweed mitogen plus EPA, an (n-3)
PUFA. There was a more pronounced depression of B cell responses when lymphocytes
were cultured with pokeweed mitogen and EPA than when they were cultured with pokeweed
mitogen alone.
Wander et al. 42 studied the effect of dietary supplementation with (n-6) and (n-3)
PUFA on antibody production in geriatric beagle dogs. Dogs were fed three experimental
diets that had a ratio of (n-6) to (n-3) PUFA of 31:1, 5.4:1 or 1.4:1 for 12 weeks. The dogs
were then sensitized to a keyhole limpet hemocyanin (KLH) protein, and antibodies to KLH
were measured 4 weeks post vaccination. No significant differences were seen in antibody
titers between dogs from the three dietary groups. In a study by Fritsche et a1.44 , the effect
of dietary fat source on antibody production in chickens was evaluated. Chickens were fed a
20
diet containing lard, corn oil, canola oil, linseed oil, or fish oil for 3 weeks. Antibody titer to an
injection of sheep red blood cells was measured by hemagglutination. Antibody titers in the
fish oil-fed chickens were significantly higher compared with titers in chickens fed the other
fat sources.
Cytokine production
Cytokines are proteins produced by cells that can affect their own function and/or the
function of other cells, locally and at a distance. Lymphokines are cytokines that are
produced by lymphocytes. They are given the name interleukin (IL) followed by a number.
Chemokines, a subset of cytokines, play a role in inflammatory processes through their
involvement in the migration and activation of cells (especially phagocytes and lymphocytes).
Cytokines alter cell function by binding to receptors on the cell, which activates secondary
messengers. There are subsequent changes in gene expression as well as changes in cell
proliferation and differentiation.
Tumor necrosis factor-a (TNF-a), IL-1 and IL-6 are proinflammatory cytokines.
TNF-a activates neutrophils and monocytes to initiate killing (or destruction) of antigen
(phagocytosis). It stimulates T and 8 cell function and the production of IL-1 and IL-6.
Together these cytokines affect responses in the liver (acute phase protein synthesis), bone
marrow (neutrophil mobilization), hypothalamus (increased body temperature), dendritic cells
(migration to lymph nodes) and muscle and fat (protein and energy metabolism).
Other cytokines that are important in the discussion of this thesis include those that
are involved in cell-mediated immunity and antibody responses. Different cytokines are
21
involved in each of these immune responses and it is the balance of cytokines that dictates
which type of immune response will predominate. Delayed-type hypersensitivity responses
and cell mediated responses are mediated by the cytokines IL-12 and interferon-o (IFN-o),
which are produced by antigen presenting cells and T helper-1 cells, respectively.
Interleukin-2, a T cell growth factor, is important in cell-mediated responses since it induces T
cell proliferation and differentiation. The cytokines IL-4 and IL-6 promote differentiation of
CD4 T cells into T helper-2 cells, which help mediate antibody responses. These cytokines
have inhibitory effects as well. Interferon-o inhibits T helper-2 cell proliferation; IL-4 and
IL-10 inhibit T helper-1 cells; and IL-4 inhibits IFN-o.
Polyunsaturated fatty acids can have direct effects on cytokine production. Several
studies have demonstrated the effects of dietary (n-3) and (n-6) PUFA on the production of
IL-2, IL-1, IL-6, and TNF-a. Less data is available that describe the effects of PUFA on IL-4,
IL-10 or IFN-o production.
Jolly et al.45 measured IL-2 production in mice after they were fed safflower oil alone
(control) or oil supplements containing safflower oil plus DHA, EPA, or AA. Mice fed the
safflower plus EPA diet had the lowest production of IL-2, whereas mice fed the safflower
plus AA supplemented diet had the highest production of IL-2. This study also revealed that
consumption of EPA and DHA caused a suppressive effect on T lymphocyte proliferation
compared to AA plus safflower or safflower alone. Wallace et al. 46 fed mice diets containing
coconut oil, olive oil, safflower oil, or fish oil for 6 weeks. Mice that were fed the safflower
and fish oil diets (polyunsaturated fatty acids) produced less IFN-o than mice that were fed
olive oil (monounsaturated fatty acids) or coconut oil (saturated fatty acids). Mice fed the
22
polyunsaturated fatty acids diet produced less IL-2 than mice fed the coconut oil (saturated
fatty acids). Interleukin-4 production was not significantly different in any of the groups,
however, the fish oil-fed mice produced less than the other groups. Based on cytokine
production, these results indicate that mice consuming PUFA can have a decreased T
helper-1 cell response compared to those consuming saturated fatty acids, but that neither
PUFA nor SFA has significant effects on T helper-2 responses.
Meydani et al. 8 conducted a study in which young and old women took dietary (n-3)
PUFA supplements for three months. Production of IL-1, IL-2, IL-6 and TNF-a by peripheral
blood mononuclear cells was measured before supplementation and at 1, 2, and 3 months
after supplementation. Production of all cytokines was reduced in both young and old
women (old>young) after supplementation with (n-3) PUFA. Proliferation of T cells was also
significantly decreased in the older women. The pronounced suppression noted in older
women was correlated to an increase in EPA and DHA and a decrease in AA in their plasma
fatty acid profiles after (n-3) supplementation.
In another human study, Endres et al. 47 studied the effect of dietary consumption of
(n-3) PUFA on IL-1 and TNF-a production by peripheral blood mononuclear cells.
Volunteers supplemented their diets with 18 grams of fish oil, an (n-3) PUFA, for six weeks.
After six weeks of supplementation, IL-1a, IL-113 and TNF-a production by
lipopolysaccharide (LPS)-stimulated peripheral blood mononuclear cells was measured.
Production of all cytokines was decreased at 6 weeks, but only IL-113 was significantly
decreased from baseline. Interestingly, 10 weeks after (n-3) supplementation was ceased
23
and peripheral blood mononuclear cells were again stimulated with LPS, production of all
three cytokines was significantly decreased compared to week zero.
In a study by Morris et al. 48 it was demonstrated that LPS-induced TNF-a
production by equine peritoneal macrophages was decreased compared to baseline levels
after horses were fed a diet containing 8% linseed oil as a source of a-linolenic acid, an (n-3)
PUFA, for 8 weeks. Calcium ionophore was also used to stimulate macrophages to produce
TNF-a, but endotoxin-induced TNF-a production was greater than that induced by calcium
ionophore. The a-linolenic acid supplement had no effect on calcium ionophore-induced
TNF-a production. These results led the authors to suggest that the mechanism by which
dietary supplementation with (n-3) fatty acids decrease TNF-a production may be unrelated
to AA metabolism.
Eicosanoid synthesis
Eicosanoids are products of cell membrane phospholipid metabolism that can
modulate immune function. Typically, arachidonic acid is found as a main constituent of
membrane phospholipids. There are three pathways of arachodonic acid metabolism. The
prostanoids - PGE2 and TXA2 - are produced through the cyclooxygenase pathway.
Leukotrienes and hydroxyeicosatetraenoic acids are derived from the lipoxygenase pathway.
A third pathway involving cytochrome P450 yields epoxides that are converted to
hydroxy-fatty acids. The focus of this thesis is on end-products of the first two major
pathways so the cytochrome P450 pathway will not be discussed. Arachodonic acid (also
24
known as eicosatetraenoic acid) is a constituent of membrane phospholipids such as
phosphatidylcholine and phosphatidylinositol. When these phospholipids are acted upon by
phospholipase A2 and phospholipase C, respectively, AA is released. Further metabolism of
arachidonic acid by cyclooxygenase and lipoxygenase yield the proinflammatory eicosanoids
PGE2, TXA2 and LTB449. An increase in PGE2 levels results in fever, erythema, edema,
vasodilation, and pain. Increased TXA2 causes platelet aggregation and vasoconstriction,
while LTB4 causes chemotaxis of eosinophils, monocytes and neutrophils to sites of
inflammation. 34 ,50
In addition, PGE2 and LTB4 can modulate cellular activity through their effects on
secondary messengers such as cAMP and cGMP. The cAMP to cGMP ratio defines the
cell's response. For example, degranulation of mast cells and contraction of bronchial
smooth muscle is dependent upon the ratio of cAMP to cGMP. High cAMP levels inhibit
release of granules, whereas high cGMP levels promote granule release. Levels of cAMP
are increased by PGE2, and cGMP levels are increased by LTB46. Another effect of
increased cAMP levels is that TNF-a synthesis is suppressed. Conversely, when cGMP is
increased, synthesis of TNF-a is increased. The production of cytokines such as IL-1, IL-2,
and IL-6 is also inhibited by PGE2, whereas LTB4 increases production of these cytokines. 39
Dietary supplementation with (n-3) fatty acids results in incorporation of EPA and
DHA into cellular membrane phospholipids (see Figure 2). Dietary a-linolenic acid, an (n-3)
fatty acid, competes with linoleic acid, an (n-6) fatty acid, for the same enzymes - desaturase
and elongase - that lead to the production of either EPA or AA, respectively. By increasing
the dietary ratio of (n-3) to (n-6) PUFA, (n-3) PUFA outcompete the (n-6) PUFA, which
25
ultimately results in greater concentrations of EPA and DHA in lipid membranes compared to
AA. In turn, a higher EPA to AA ratio leads to a shift in the type of metabolites produced.
linoleic acid
18:2(n-6)
~ A6
18:3(n-6)
1
elongase
1 series PG and TX ""­
/20"3(n-6)
3 series LT
AS
2 series PG and TX
...... arachidonic acid
/
4 series LT
1
20:4(n-6)
el009ase
22"4(n-6)
r
elongase
T::
r~o~datioo
24"S(n-6)
22:S(n-6)
A1S
~
a-linolenic acid
18:3(n-3)
~
A6
1
1
1-
18:4(n-3)
eloogase
20:4(n-3)
AS
eicosapentaenoic acid
.--J..senes PG ami TX
,,~
20:S(n-3)
-S series LT
9ase
22:f(n-3)
-+
elongase
24r:s
24J"(n-3)
13 oxidation
docosahexaenoic acid
Figure 2. Metabolism of (n-6) polyunsaturated fatty acids and (n-3) polyunsaturated fatty
acids through the cyclooxygenase and lipoxygenase pathways. CO = cyclooxygenase; LO =
lipoxygenase; LT =leukotriene; PG =prostaglandin; TX =thromboxnane; ~5, ~6 and ~ 12
indicate desaturase enzymes.
26
While AA metabolism leads to production of PGE2 and LTB4 (pro-inflammatory
eicosanoids), PGE3 and LTBs (less inflammatory eicosanoids) are produced from EPA.
Additionally, (n-3) PUFA inhibit cyclooxygenase, further reducing the AA by-product, PGE2. 6
In the studies conducted by Meydani et al. 8 and Endres et a1. 47 , dietary (n-3) PUFA
supplementation decreased PGE2 production by peripheral blood mononuclear cells. A study
conducted by Wu et al. 51 evaluated the production of PGE2 by peripheral blood mononuclear
cells of Cynomolgus monkeys after 28 weeks of dietary supplementation with a-linolenic
acid, or EPA plus DHA. Both diets caused a significant decrease in PGE2 production in the
monkeys (a-linolenic acid< EPA plus DHA) compared to baseline. Whelan et al. 52 fed
Syrian hamsters diets enriched in oleic acid, linoleic acid, AA, or EPA for 3 weeks. The
hamsters receiving the EPA enriched diet showed the lowest production of PGE2 and TXB2
by peritoneal macrophages compared to hamsters fed the other diets (EPA<oleic
acid<linoleic acid<AA).
Studies have been done that suggest that dietary a-linolenic acid can affect
eicosanoid production in horses. Linseed oil, a rich source of (n-3) fatty acids, contains
44g/100g of a-linolenic acid, but also has a considerable amount of (n-6) fatty acids - linoleic
acid (22g/100g) and monounsaturated fatty acids - oleic acid (22g/100g). One study showed
a decrease in TXB2 and LTB4 production by equine monocytes (P=0.08) after horses' diets
were supplemented with linseed oil, for 8 weeks. 31 In another study, where horses were fed
a diet containing 8% linseed oil for 8 weeks, peritoneal macrophages showed decreased
production of TXB2 when stimulated with LPS.32
27
Leukotriene synthesis
Leukotrienes are potent mediators of inflammatory reactions and have been termed
the 'slow reacting substance of anaphylaxis' because of their role in mediating respiratory
smooth muscle contraction. Leukotrienes C4, 04, and E4 are potent stimulators of airway
smooth muscle cells and are associated with bronchoconstriction, whereas leukotriene B4
has been identified as the agent involved in inflammatory processes. 53 Leukotriene 84 is
chemotactic to leukocytes and induces neutrophil adherence to endothelium and migration
into tissues. 49 Because leukotrienes are produced by the enzyme 5~lipoxygenase, this
pathway and its regulation have received growing attention from researchers.54
The effect that PUFA have on
end~products
of the
5~lipoxygenase
pathway can be
seen in Figure 3. This diagram illustrates that AA metabolism leads to the production of
LTA4, which is further metabolized to LTB4 or conjugated with reduced glutathione to form
LTC4.
Increased production of LTC4 and its metabolites, LT04 and LTE4, have been
associated with such disease processes as asthma and allergic rhinitis, whereas increased
production of LTB4 is associated with rheumatoid arthritis, gout and inflammatory bowel
disease.55 Metabolism of EPA and OHA lead to the production of 5~series leukotrienes such
as LTAs, which is then metabolized to LTCs (which is less vasoconstrictive than LTC4}, and
LTBs, which is less active «10% bioactivity) than LTB4 in terms of its chemotactic ability and
ability to cause release of lysosomal enzymes. 56.20
Studies have been conducted in animals and humans, which investigate the role of
(n-3) and (n-6) PUFA on the production and effect of LTB4 and LTBs. In one study, two
28
groups of rats were maintained on a standard rat diet for 4 weeks. One group of rats also
received a supplement of EPA via a gastric tube, while the second group of rats received
vehicle only (water). Leukocytes were isolated from peritoneal exudates and then stimulated
with calcium ionophore. Leukotriene B4 production decreased in the EPA-supplemented rats
compared to controls, whereas LTB5 production was increased. The investigators showed a
direct correlation between the ratio of EPA to AA in the leukocyte phospholipids and the ratio
of LTB5 to LTB4 produced after stimulation with calcium ionophore. 57
Vaughn et al. 58 evaluated the effect of varying the dietary ratio of (n-6) to (n-3)
PUFA on the production of leukotriene B4 in Beagle dogs. Five groups of dogs were fed
different ratios of (n-6) to (n-3) fatty acids (i.e., 5 to 1, 10 to 1, 23 to 1, 50 to 1 and 100 to 1)
for 12 weeks and LTB4 and LTB5 production in skin and in neutrophils were measured at 6
and 12 weeks. At 12 weeks, the dogs consuming the diets containing high amounts of (n-3)
PUFA showed significantly increased levels of LTB5 synthesis in skin compared to time zero.
Peripheral blood neutrophils from the dogs receiving the high (n-3) PUFA diets (ratios were 5
to 1 and 10 to 1) produced significantly less LTB4 and significantly more LTB5 at 6 and 12
weeks compared to dogs from the other diet groups.
Kragballe et al. 59 demonstrated that LTB5 was a less potent stimulator of DNA
synthesis in human keratinocytes and inhibited LTB4 stimulation of DNA synthesis. The
researchers also showed that LTB5 was a less potent chemoattractant of human neutrophils
and that it had an inhibitory effect on LTB4-induced chemotaxis of neutrophils. The results of
this study prompted the authors to suggest that the biological effects of LTB4 may be reduced
29
with a LTBs to LTB4 ratio equal to or greater than 10, but that at these higher ratios LTB4 still
has significant stimulatory activity.
Arachidonic Acid
20:4(n-6) PUFA
Eicosapentaenoic Acid
20:5(n-3) PUFA
Oocosahexaenoic Acid
22:6(n-3) PUFA
5-Lipoxygenase
~
LTB4
LTB5
(attenuated
activity)
1
LTCS ... LTD5-.LTES
LTC4 +LTD4 +LTE4
Figure 3. Metabolism of arachidonic acid, eicosapentaenoic acid and docosahexanoic acid
through the 5-lipoxygenase pathway. LT = leukotriene. (Adapted from Sperling, 1998)
Blue arrows indicate production pathways of 5-series LTs from DHA and EPA. Red arrows
indicate prodcuction pathways of 4-series LTs from AA.
In another investigation, Cleland et al. 60 compared the effect of dietary oils (linseed,
olive, sunflower and fish) on the production of LTB4 and LTBs by rat peritoneal exudate cells.
They compared the phospholipid composition of the rat peritoneal exudate cells to the
amounts of LTB4 and LTBs produced. There was a positive (but weak) correlation between
cellular content of AA and LTB4 synthesis as well as a correlation between cellular content of
30
cellular content of AA and LTB4 synthesis as well as a correlation between cellular content of
EPA and LTBs production. An inverse relationship was shown to exist between EPA content
and LTB4 production. The authors also demonstrated a linear relationship between the ratio
of EPA to AA in the peritoneal exudate cells and the ratio of LTBs to LTB4 produced by these
cells. The ratios for EPA to AA in the peritoneal exudate cells of rats ranged from 0.23 to
1.22 and the ratios of LTBs to LTB4 ranged from 0.21 to 1.01.
In a human study involving asthmatic subjects, Broughton et al. 53 evaluated the
effects of feeding diets with different (n-3) to (n-6) fatty acid ratios on respiratory measures
and leukotriene metabolites. Urine was selected for analysis because it provides an accurate
estimate of leukotriene synthesis. One group of volunteers who took supplements with a
lower (n-3) to (n-6) PUFA ratio showed increased excretion of the 4-series of leukotrienes in
their urine. A second group of volunteers who took supplements with a higher (n-3) to (n-6)
PUFA ratio had significantly lower levels of 4-series leukotrienes excreted in their urine
compared with volunteers who took supplements with a lower (n-3) to (n-6) PUFA ratio. The
volunteers in this second group also had a marked increase in LTEs (a 5-series leukotriene)
excretion compared to baseline measurements. There was also a positive and significant
correlation between the levels of 5-series leukotrienes excreted the in urine and the ability of
the subjects to respond to a methacholine induced-asthmatic challenge.
The authors
suggested that the respiratory benefits associated with (n-3) PUFA ingestion in these
volunteers may result from the inability of 5-series leukotrienes to elicit an asthmatic
response, or the 5-series leukotrienes may competitively inhibit the 4-series leukotrienes.
31
In summary, a diet high in (n-3) PUFA has been shown to ameliorate inflammatory
conditions.
The effect of dietary (n-3) PUFA supplementation on the production of
proinflammatory mediators such as cytokines and eicosanoids are well documented. In the
present study, the effects of feeding PUFA on the immune responses of normal horses will
be examined. Based on published data, it is expected that horses fed a dietary supplement
of (n-3) PUFA will show decreased production of pro-inflammatory mediators compared to
horses fed a dietary supplement of (n-6) PUFA.
32
MATERIALS AND METHODS Animals
Ten healthy mares donated to the College of Veterinary Medicine were selected for
this study. The mean weight of the horses was 503 kg (range, 430 to 555 kg). The average
age was 14.3 y (range, 5 to 20 y). Horses were determined to be healthy based upon
physical examinations, complete blood counts, and serum biochemical evaluations. All of the
horses had been vaccinated for encephalomyelitis, tetanus, influenza and rhinopneumonitis.
A commercially available oral paste dewormer (pyrantel pamoate, Strongid® Paste, Pfizer
Inc.) was used to eliminate internal parasites.
Prior to the start of the feeding trial, the mares were kept on pasture and fed Timothy
hay. Horses were randomly assigned to two groups of five and were acclimated to a beet
pulp supplement for two weeks prior to the start of the study. During the dietary intervention
period, the mares were allowed access to pasture and hay during the day and housed in
stalls overnight when the study diets were fed. The experimental protocol was reviewed and
approved by the Oregon State University Animal Care and Use Committee according to the
principles outlined by the National Institutes of Health.61
Diets
Feed-grade Menhaden fish oil (Omega Protein Inc., Reedville, VA) was used as the
source of oil for the (n-3) enriched diet. Mazola® corn oil (CPC International, Englewood
33
Cliffs, NJ) was used as the source of oil for the (n-6) enriched diet.
The fatty acid
composition of the oils is shown in Table 1. Oils were kept refrigerated and containers were
capped with N2.
The oils were evaluated for oxidation before and after the feeding period. Oxidation
of the oils was evaluated by measuring peroxide levels. Because peroxide can decompose
into aldehydes and ketones, these parameters were also measured using a P-anisidine
Value Assay. The fatty acid composition of the oils and analysis for oxidation was performed
by Dr. Rosemary C. Wander, Department of Nutrition and Food Serve Systems, University of
North Carolina, Greensboro, NC 27402-6170.
Components of the diet on an as fed basis included grass hay and alfalfa hay
(86.4%), beet pulp (10.?>1o), either com oil or fish oil (2.5%), and a commercially available
vitamin-mineral supplement (0.9%). See Table 2 for nutrient composition of the diets on a
dry matter basis and Table 3 for analysis of the vitamin-mineral supplement. Nutrient
analysis of the grass and legume hay was performed by DHI Forage Testing Laboratory,
Ithaca, NY (see Tables 4 and 5). Beet pulp, which contained 0.5% fat, 10.5% protein and
17.0% fiber, was obtained from Kropf Inc. (Harrisburg, OR).
The daily ration was prepared by soaking beet pulp for 6 to 8 hours prior to adding
0.27 kilograms of either com oil or fish oil. Oils were warmed to room temperature prior to
feeding. Fifty-six grams of the vitamin-mineral supplement and 15 grams of limestone were
also added to the beet pulp. In order to increase the palatability of the beet pulp and oils, 0.23
kilograms of grain was added to each diet. Dr. Robert Van Saun, the nutritionist who
34
balanced the nutrient content of the diets, did not deem the addition of this small amount of
grain to be a concern as all animals were treated identically.
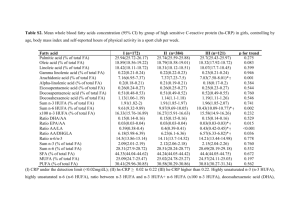
Table 1. Composition of selected fatty acids of the oils used in the study diets. 1
Fatty Acid
Fish oil
Corn oil
mg/g of oil
Area %
mg/g of oil
Area %
C14:0
ND2
ND
58.9
8.8
C16:0
101.2
10.7
150.9
22.5
C16:1(n-7)
ND
ND
85.1
12.7
C18:0
18.6
2.0
27.0
4.0
C18:1(n-9)
253.9
26.8
107.3
16.0
C18:2(n-6)
565.5
59.6
12.1
1.8
C20:0
ND
ND
2.3
0.3
C18:3(n-3)
8.3
0.9
10.5
1.6
C20:1(n-9)
ND
ND
27.7
4.1
18:4(n-3)
ND
ND
2.3
0.3
C20:2(n-6)
ND
ND
1.3
0.2
C20:3(n-6)
ND
ND
1.3
0.2
C20:4(n-6)
ND
ND
6.7
1.0
C20:5(n-3)
ND
ND
84.3
12.6
C22:1(n-9)
ND
ND
5.4
0.8
C22:0
ND
ND
2.2
0.3
C22:5(n-3)
ND
ND
13.3
2.0
C22:6(n-3)
ND
ND
72.0
10.7
947.6
99.9
670.5
100.0
Totals
lAnalysis performed by Dr. Rosemary C. Wander, Department of Nutrition and Food
Service Systems, University of North Carolina, Greensboro, NC 27402-6170.
2ND::: Not Detectable
35
Table 2. Nutrient composition of the diets on a dry matter basis 1
Com nent
acid diet
acid diet
Crude protein (%)
8.51
8.51
Crude fat (%)
4.9
4.9
Digestible energy (Mcal/kg)
2.19
2.19
Acid detergent fiber (%)
35.2
35.2
Calcium (%)
0.34
0.34
Phosphorus (%)
0.25
0.25
Copper (ppm)
31.8
31.8
Zinc (ppm)
46
46
Manganese (ppm)
85
85
Selenium (mg/kg)
0.14
0.14
Vitamin A (IU/kg)
4,952
4,952
Vitamin 0 (lU/kg)
396
396
53.46
53.46
1Nutrient analysis provided by Dr. Robert Van Saun, DVM, PhD, Diplomate American
College of Veterinary Nutrition, Pennsylvania State University, Sate College, PA.
Calculations were made using Spartan Equine Ration Evaluator developed by
Cooperative Extension Service, Animal Science Department, College of Veterinary
Medicine, Michigan State University.
36
Table 3. Vitamin and mineral analysis of the vitamin/mineral supplement: 1
Nutrient
Minimum nutrient per pound
Vitamin A
Vitamin D
Vitamin E
Vitamin B1 (thiamin)
Vitamin B2 (riboflavin)
Vitamin B6 (pyridoxine)
Vitamin B12
Vitamin C
Folic acid
Pantothenic acid
Choline chloride
Biotin
Calcium (max)
Calcium (min)
Phosphorus ­ P
Iron ­ Fe
Copper- Cu
Magnesium - Mg
Manganese - Mn
Zinc-Zn
Iodine -I
Selenium ­ Se
Cobalt- Co
Yeast culture
Methionine
400,0001U
32,0001U
4,0001U
100mg
176mg
120mg
600 mcg
14,600 mcg
1,020 mg
152 mg
1,000 mg
36,000 mcg
5,600 mg (1.2%)
5,000 mg (1.1%)
4,500 mg
3,200 mg
1,200 mg (2640 ppm)
1,200 mg
1,400 mg
1,600 mg (3520 ppm)
16 mg
24 mg (52.8 ppm)
24 mg (52.8 ppm)
5,675 mg
5,675 mg
1Horse Guard® - minerals provided in a combination of protein-bound
(proteinates/chelates) and inorganic forms. Nutrient analysis provided by Horse
Guard Inc., Redmond, OR.
37
Table 4. Nutrient analysis of the grass hayl
Component
%moisture
%dry matter
%crude protein
%adjusted crude protein
%acid detergent fiber
%neutral detergent fiber
%crude fat
%NSC
% TDN
Net energy-lactation
(Mcai/lb)
Net energy ­
maintenance (Mcai/lb)
Net energy ­ gain
(Mcai/lb)
%calcium
%phosphorus
%magnesium
% potassium
%sodium
PPM iron
PPM zinc
PPM copper
PPM manganese
% molybdenum
Horse DE (Mcai/lb)
Horse TDN (%)
1 Nutrient analysis
8.3
91.7
8.0
8.0
34.6
54.9
ND
20.4
55
0.57
Dry matter
ND2
ND
8.7
8.7
37.7
59.9
ND
22.2
60
0.62
0.55
0.60
0.31
0.34
0.22
0.25
0.18
2.07
0.035
122
28
17
67
0.9
0.81
40
0.24
0.27
0.20
2.26
0.038
1.33
30
18
73
1.0
0.88
44
As sampled
of hay was performed by DHI Forage Testing Laboratory, Ithaca, New York. 2ND = Not Detectable 38
Table 5. Nutrient analysis of the legume hayl
Component
%moisture
%dry matter
%crude protein
% adjusted crude protein
% acid detergent fiber
%neutral detergent fiber
%crude fat
%NSC
%TDN
Net energy-lactation
(Mcalllb)
Net energy ­
maintenance (Mcalllb)
Net energy - gain
(Mcalllb)
%calcium
% phosphorus
% magnesium
%potassium
%sodium
PPM iron
PPM zinc
PPM copper
PPM manganese
% molybdenum
Horse DE (Mcaillb)
Horse TDN (%)
1 Nutrient analysis
As sampled
Dry matter
8.6
91.4
13.6
13.6
31.5
41.7
ND
25.1
53
0.55
ND2
ND
14.9
14.9
35.5
45.6
ND
27.5
58
0.60
0.52
0.57
0.28
0.31
1.32
0.19
0.21
2.07
0.008
322
18
9
58
1.2
0.93
47
1.44
0.21
0.23
2.26
0.009
352
20
10
63
1.3
1.02
51
of hay was performed by DHI Forage Testing Laboratory, Ithaca, New York. 2ND =Not Detectable 39
Study Design
Horses were supplemented with a diet of fish oil or corn oil for a period of 14 weeks.
Body condition scores were evaluated at the beginning and the end of the feeding period.
The horses were fed in the afternoon, feed buckets were left in the stalls overnight, and food
consumption was assessed the following morning. Food consumption was recorded daily
throughout the feeding period. Body weights were recorded once prestudy, weekly during
the first three weeks of the feeding trial and then biweekly through week 12. Plasma fatty acid
profiles were determined at 0, 6, 8, 12, and 18 weeks. At 8 and 10 weeks, horses were
vaccinated with keyhole limpet hemocyanin (KLH).
Cell-mediated immunity was evaluated at 10 weeks (one day after the second KLH
vaccination) using a delayed-type hypersensitivity skin test. Humoral immunity was assessed
at 12 weeks by measurement of KLH antibody titers. Immunological assays were conducted
at 0, 6, 8, and 12 weeks of study. These tests included LTB4 and LTB5 production by
peripheral blood neutrophils, TNF-a production by pulmonary alveolar macrophages and
peripheral blood mononuclear cells, and phagocytosis of latex beads by pulmonary alveolar
macrophages.
Plasma lipids (cholesterol and triglycerides) and plasma a-tocopherol levels were
measured at 0,6,8 and 12 weeks. Serum biochemistries were evaluated prestudy and at 8
and 12 weeks. Complete blood counts and white cell differential counts were evaluated
prestudy and at 6, 8, and 12 weeks.
40
Plasma Fatty Acid Profiles
Plasma fatty acid profiles were determined by gas chromatography as previously
described 62 using heptadecanoic acid as the internal standard. In short, 15 JlI of internal
standard (17:0) was added to a 100 x 100 mm test tube with a teflon cap and blown dry using
N2. To this test tube, 0.2 ml of plasma was added and 0.8 ml saline to make a final volume of
1 ml. Then 3.75 ml of a 2:1 methanol/chloroform mixture was added to the test tube, which
was then placed on a shaker (Labquake, Fisher Scientific, Pittsburgh, PA) for 1 hour. After
shaking, the test tube was centrifuged at 2500 rpm for 10 min and the supernatant
transferred to a 120 x 10 mm test tube to be used later. The residue in the original test tube
was extracted using 1 ml distilled water and 3.75 ml of methanol/chloroform mixture, then
centrifuged again for 10 minutes.
The supematant from this spin was added to the
supematant from the first spin. To the supernatant test tube, 2.5 ml distilled water and 2.5 ml
chloroform were added and the test tube was centrifuged for 10 minutes. At this point, two
fractions were visible; the top fraction was removed using a water aspirator. The lower
fraction (chloroform layer) was withdrawn using a pasteur pipette ad placed into another 100
x 10 mm test tube and dried under nitrogen. After the solvent was completely evaporated,
0.2 ml benzene and 1 ml of boron trichloride was added to the tube, which was then filled
with a nitrogen cap. The tube was then placed in a 95°C heating block for 90 minutes. After
heating, the test tube was removed and allowed to cool to room temperature. Once cooled, 5
ml of distilled water and 5 ml hexane were added and the tube was vortexed for 2 minutes.
Following the mixing, the test tube was centrifuged at 1500 rpm for 10 minutes. Again, two
fractions were visible. The top layer (hexane) was transferred to a 125 x 16 mm test tube.
41
Three ml of hexane was added to the first tube, which was then mixed for 30 seconds, and
centrifuged at 1500 rpm for 10 minutes. The hexane layer (top) was combined with the first
hexane layer that had been previously removed. Sodium sulfate (0.3g) was added to these
combined extracts and the tube was vortexed for 45 seconds. After mixing, the extract was
transferred to a 100 x 10 mm test tube and the hexane evaporated under nitrogen. The
samples were reconstituted in 0.25 ml isooctane and 2 JlI were injected into the gas
chromatograph. The concentration of the fatty acids was expressed as g/100g fatty acids.
Immunological Measurements
De/ayed-type hypersensitivity skin test
The cell-mediated immune response was evaluated using a delayed type
hypersensitivity (DTH) skin test. This test is an in vivo indicator of T cell-mediated immune
responsiveness and is measured as swelling and induration following an intradermal
challenge. Horses were sensitized with keyhole limpet hemocyanin (KLH; Calbiochem­
Behring Diagnostics, La Jolla, CAl suspension administered intramuscularly (500 Jlg of KLH
emulsified in 1.0 mg of T1501 adjuvant for a total volume of 0.5 ml) at 8 and 10 weeks after
the initiation of the feeding trial. The KLH and adjuvant were combined in an oil-water
emulsion as described by Woodward (1989), however, the preparation was sonicated instead
of ground. In short, the vaccine was prepared using 50 mg of KLH, 2.5 ml of hexadecane,
1.75 ml of Tween 80, 0.75 ml of Span 80, and 100 mg of T1501 adjuvant, which were
42
emulsified and added to 4S ml normal saline. The vaccine was stored at -20°C until it was
administered to the horses.
For the DTH skin test, a heat-aggregated form of the KLH protein was used. The
KLH was heat aggregated according to the method of Exon et al. 63 Briefly, 120 mg of
soluble KLH was added to 6 ml of sterile saline solution and then heat aggregated in an BO°C
water bath for 1 hour. The resultant gel was centrifuged twice at 400 x g for 10 minutes,
removing the saline layer each time. The gel was then dispersed by passing it through a
23-gauge needle once, and through a 2S-gauge needle twice, carefully avoiding formation of
air bubbles.
The DTH skin test was performed the day following the second KLH intramuscular
vaccination. Intradermal injections were administered at five different sites. Each O.OS ml
injection of heat-aggregated KLH contained -3 mg of KLH.
Two O.OS ml intradermal
injections were administered 2.S cm apart on the external pinna of the left ear, which had
been previously shaved.
The right lateral neck was also shaved and three O.OS ml
intradermal injections were administered at least 2.S cm apart. An intradermal injection of
histamine base (0.1g/L; Histatrol Center Laboratories, Port Washington, NY) was
administered as a positive control, and saline (0.9%) was administered as a negative control.
These injections were also spaced 2.S cm apart on the right lateral neck. A 2S-gauge needle
was used to administer the intradermal reagents.
Ear thickness was measured prior to injecting the ear.
Reactions were then
recorded at 30 minutes and at 24, 4B and 96 hours after intradermal injections. Ear thickness
and wheal diameter were measured using calipers.
According to the manufacturer's
43
instructions, a reaction larger than the negative control was considered a positive reaction.
Histamine produced an induration typically 15 mm larger than the saline control at 30
minutes, after which the reaction subsided. These controls ruled out trauma or the volume of
substance injected as the cause of the DTH response. The same person administered the
test to all horses.
Keyhole limpet hemocyanin antibody titer
Humoral immune response was measured as the antibody response to KLH. Horses
were injected intramuscularly with 0.5 ml KLH vaccine, prepared as previously described for
the DTH skin test, at 8 and 10 weeks. Blood was collected via venipuncture at 12 weeks (14
days after the booster vaccination) and the serum was assayed for KLH antibody levels by an
indirect ELISA. Briefly, 96-well microtiter plates (Costar, Cambridge, MA) were coated with 5
Ilg/ml of KLH (100 Ill/well; Sigma Chemical Co., St. Louis, MO) in 0.1M sodium phosphate
buffer, sealed to prevent evaporation and incubated at at 4°C overnight. The next day the
coating solution was removed and 100 III of StabilCoat (SurModics Inc., Eden Praire, MN)
was added to each well to block nonspecific binding sites and the plates were incubated for
30 minutes at room temperature. After incubation, the StabilCoat was removed, the plates
were re-sealed and stored at 4°C until used.
Serum samples were serially diluted in 0.05M phosphate buffered saline with 0.05%
Tween-20 (T-PBS; pH 8.0), added to the plate in triplicate, and incubated for 30 minutes at
room temperature. Positive and negative control serums were also assayed on each plate.
After incubation, plates were washed 4 times with T-PBS and 100 III of horseradish
44
peroxidase conjugated to rec-Protein G (Zymed Laboratories Inc., San Francisco, CA) was
added to each well at a previously determined dilution (1 :20,000). Rec-Protein G binds to
IgG immunoglobulin through their Fc regions and was used instead of an anti-species
conjugate as it resulted in equivalent results with a stronger signal and less background.
After an additional 30 minute incubation at room temperature, plates were again washed and
100 JlI of 1.6JlM ABTS/H202 (2,2'-Azino-bis (3-ethylbenzthiazoline-6-sulfonic acid; Sigma
Chemical Co., St. Louis, MO) in 0.1 Mcitrate buffer (pH 4.0) was added to all wells. Plates
were incubated at room temperature in the dark and were read when a positive reference
sample reached an 0.0. of 1.5. The optical density of each well at 405 nm was determined
using a Bio-Tek EL312 microplate reader (Bio-Tek Instruments Inc., Winooski, VT). The
antibody titer was expressed as an endpoint titer for each sample, which was calculated from
a regression line of optical density against sample dilution, with a threshold of 0.200 optical
density (approximately 3 times the optical density generated by the backgound), using a
software program (KinetiCalc, Bio-Tek Instruments Inc., Winooski, VT). Final results were
expressed as the log of the antibody titer.
Leukotriene 84 and 85 quantification
Neutrophils (PMN) were isolated and purified as previously described. 64 Briefly,
PMN were separated from a1:1 dilution of whole blood and Dulbecco's phosphate buffered
saline (DPBS, Sigma Chemical, St. Louis, MO) by layering the mixture over a gradient of
Histopaque 1077, which was layered over Histopaque 1119, and then centrifuging for 30
minutes at 700 x g. After removing the PMN layer, these cells were washed and lysed with a
45
solution of 17mM Tris-HCI buffer with 0.83% NH4CI (Sigma) until contaminating red blood
cells were no longer visible. After this, the PMN were washed twice with Hank's Balanced
Salt Solution (HBSS) without CaCI2, and then centrifuged for 5 minutes at 300 x g. Next,
these cells were resuspended in 2 ml HBSS with 0.8mM CaCI2. An aliquot of the cell
suspension was used to count the cells with a Coulter ZBI Counter (Coulter Electronics Inc.,
Hialeah, FL).
Aliquots of 1 x 107 cells were transferred to 5 ml plastic tubes and HBSS containing
0.8mM CaCI2 was added to achieve a final volume of 495 /-11. To initiate leukotriene
synthesis, 5 /-11 of calcium ionophore A23187 (Sigma, 10 uM-final concentration) was added
to each tube of cells. Control tubes received 5 /-11 of 0.2% dimethyl sulfoxide (DMSO, Syntex,
West Des Moines, IA) instead of calcium ionophore. Following this, tubes were incubated for
5 minutes in a 37°C water bath. After the incubation, 2 ml of ice-cold methanol was added to
each tube and the tubes were incubated on ice for 20 minutes. Next, the tubes were
centrifuged for 5 minutes at 1000 x g. Finally, the supernatants were transferred to clean 5
ml plastic tubes and stored at -70°C until subsequent LTB4 and LTBs measurements were
made (stored for 15 months).
Leukotrienes were extracted and separated using a modified version of the methods
described by Terano et al. 57 and Vaughn et al. 58 Briefly, the stored supernatants from the
leukotriene stimulation procedure were centrifuged for 5 minutes at 400 x g.
The
supernatants (-2.5 ml) were transferred to 15 ml centrifuge tubes and 100 ng of
Prostaglandin B3, which served as an internal standard, was added to each sample prior to
extraction. In order to reduce the concentration of methanol in these leukotriene samples to
46
less than 15%, citrate buffer (pH 4.0) was added to achieve a final volume of 14.5 ml. Next,
a 12 ml syringe was attached to a C-18 Solid Phase Extraction cartridge (Sep-Pak Classic,
Waters Inc., Milford, MA) and 5-ml of HPLC-grade methanol was passed through the
cartridge. Next, 5 ml of distilled-deionized water (dd-H20) was passed through the cartridge.
After the cartridges were prewetted, samples were loaded onto the cartridges by gravity flow
and leukotrienes were absorbed onto the extraction column. Next, the cartridges were rinsed
with 5 ml of distilled-deionized (dd)-H20 followed by 5 ml of HPLC-grade hexane.
Leukotrienes were then eluted from the column into a 5 ml plastic test tube using gravity flow
with 5 ml of a 90:10 mixture of methanol and dd-H20. Following this, samples were placed in
a 30°C water bath and solvent was evaporated under a stream of N2. Once evaporated, the
residues were reconstituted in 125 J,tl of mobile phase (methanol:water:glacial acetic acid
(75:25:01), pH adjusted to 5.7 with NH40H), capped with N2 and stored at -70°
(~
1 month)
untilleukotrienes could be separated by HPLC.
Leukotrienes B4 and B5 were separated by HPLC using a C-18 reversed phase
column (Nova-pak, 3.9 mm x 300 mm, 60A pore size, 4 J,tm particle size, Waters Inc., Milford,
MA) fitted with a pre-column C-18 guard column (Nova-pak Sentry, 3.9 mm x 20 mm, Waters
Inc., Milford, MA). The mobile phase was methanol:water:glacial acetic acid (75:25:01), pH
adjusted to 5.7 with NH40H. The flow rate of the pump (Model 110B, Beckman, Fullerton,
CA) was set at 0.7 ml/minute and the variable wavelength UV detector (System Gold
Mode1166, Beckman) was fixed at 254 nm.
Authentic PGB3, LTB5 and LTB4 standards (Cayman Chemical Co., Ann Harbor, MI)
were prepared and injected onto the HPLC column. They eluted at 5.2,6.7, and 8.9 minutes,
47
respectively. Peaks were integrated using a PerkinElmer LCI-100 Laboratory Computing
Integrator (Boston, MA). Fractions were collected from 6.2-8.0 minutes for LTBs and from
8.5-10.3 minutes for LTB4. Fractions were also collected at the start (0-6.2 minutes) and at
the end (10.3-15 minutes) of each run and between the leukotriene fractions (8.0-8.5
minutes). The fractionated samples were capped with N2 and stored at -70°C for subsequent
analysis by enzyme immunoassay (S 1 month).
The concentration of LTB4 and LTBs in the samples was determined using enzyme
immunoassay kits (Cayman Chemical Co., Anna Harbor, MI). First, the stored samples
obtained from the HPLC separation of leukotrienes were placed in a 37°C water-bath and
evaporated to dryness under a stream of N2. Next, all samples were reconstituted in 150 J.. l1
of enzyme-immunoassay buffer and stored at 4°C until they were assayed by EIA (S 3 days).
The protocol included with the EIA kit was followed. Briefly, the buffered samples were
loaded onto a 96-well ELISA plate as neat (undiluted) or as a 1:10 dilution in EIA buffer. For
the 1:10 dilution, the neat solution (Le., part of the original 150 ul sample) was diluted 1:10
with EIA buffer by adding 15 J.. l1 from the 150 J.. l1 sample to 135 J.. l1 of EIA buffer. All samples
(neat and 1:10 dilutions) were analyzed in duplicate (50 J...ll/well).
Next, 50 J.. l1 of LTB4 acetylcholinesterase tracer were added to all wells except the
Total Activity (TA) and the blank wells. Then, 50 J.. l1 of LTB4 antiserum was added to each
well except the TA, the non-specific binding, and the blank wells. After this, the plates were
covered with plastic film and incubated for 18 hours at room temperature.
After the
incubation period, the plates were emptied and washed 5X with wash buffer provided in the
kit. Next, Ellman's Reagent (from the kit) was reconstituted in dd-H20 and 200 J.. l1 were
48
added to each well and 5 l.tI of tracer were added to the TA wells. The plates were then
covered with plastic film and incubated in the dark for 60 minutes on an orbital shaker. The
plates were read at 405 nm until an 0.0. of 0.500 was reached. The antiserum used had a
cross-reactivity of 100% for LTB4 and 50% for LTBs. An additional standard curve for LTBs
was prepared in the same manner as the LTB4 standard to accurately quantify LTBs.
Concentrations for LTB4 and LTB5 were calculated using the software program Kinetic Calc
version 2:12 (Bioteck Instruments Inc. Winooski, VT).
Tumor Necrosis Factor-a production
Peripheral blood mononuclear cells (PBMC) were isolated for stimulation of TNF-a
production according to the methods of Coligan et al. 65 and Krakowka et al. 66 In short, cells
were separated from a 1:1 dilution of blood and OPBS (OPBS, Sigma) by layering the blood­
OPBS mixture over Histopaque 1077 (Sigma) and centrifuging for 30 minutes at
~ x g at
4°C. Cells were washed once with OPBS and centrifuged for 10 minutes at 300 x g. Cells
were resuspended in 2 ml OPBS. An aliquot of the cell suspension was used to count the
cells using a Coulter ZBI Counter (Coulter Electronics Inc.).
Pulmonary alveolar macrophages (PAM) were isolated for stimulation of TNF-a
production and analysis of phagocytic activity. Bronchoalveolar lavage fluid (BALF) was
collected from all horses to obtain and isolate pulmonary alveolar macrophages. Horses
were first sedated with 0.5 ml of detomidine (Oormosedan, SmithKline Beecham Animal
Health, Exton, PA) given intravenously. A bronchoalveolar lavage tube was introduced
through the nostril, nasopharynx and trachea and advanced until it wedged in a bronchus.
49
While advancing the tube, the bronchial surface was anesthetized using 10 ml of lidocaine
administered through the tube. This was done to alleviate coughing. A 100 ml aliquot
volume of pre-warmed physiological saline was infused into the bronchus and immediately
aspirated with a 140 ml syringe. This was repeated twice more, each time using 100 ml
saline. As the BALF was collected, it was transferred to a sterile 250 ml Erlenmeyer flask
and kept on ice until subsequent steps were performed to isolate pulmonary alveolar
macrophages. Approximately 200 ± 30 ml of BALF was obtained from each horse.
Back in the lab, the BALF in the 250 ml Erlenmeyer flasks was transferred to 50 ml
centrifuge tubes and centrifuged for 20 minutes at 400 x g. Supernatants were discarded
and cell pellets were combined and resuspended in 2 ml of HBSS and then transferred to 15
ml centrifuge tubes. An aliquot of the cell suspension was used to determine cell counts
using a Coulter ZBI Counter (Coulter Electronics Inc.).
After counting, aliquots of 1 x 107 PBMC or PAM cells were transferred to 1.5 ml
microcentrifuge tubes and centrifuged for 10 minutes at 300 x g. Following this, supernatants
were discarded and the cell pellets were resuspended in 1 ml of RPMI-1640 containing 30
ug/ml of LPS (E. coli 055: B5; Sigma). Media was also supplemented with 100,000 U/L
penicillin, 100 mg/L streptomycin, 2 mmol/L L-glutamine and 5% fetal calf serum (Sigma).
Each 1 ml cell suspension was added to a tissue culture flask (25-ml, Corning, Corning, NY)
containing 4 ml of RPMI-1640 media. Cells were incubated for 40 hours at 37°C in 95% air
and 5% C02. After the incubation period, the cells in media were transferred to 15 ml
centrifuge tubes and centrifuged for 10 minutes at 900 x g. Supernatants were filtered
through a 0.45-micron filter (UNIFLO-Plus, Scheicher and Schuell, Keene, NH). Cell-free
50
supematants were stored at -70°C until TNF-a concentrations were determined. (Samples
were assayed for TNF-a by Dr. Susan Tornquist's laboratory, Department of Biomedical
Sciences, College of Veterinary Medicine, Oregon State University.)
TNF-a was quantified by a method adapted from Branch et al. 67 Briefly, on day
one, L929 cells were trypsinized to ensure they were in log phase by day two. On day two, a
96-well microtiter plate was prepared by adding 50 JlI of Dulbecco's modified essential media
(DMEM, Sigma) with 15% calf serum and 2 Jlg/ml of actinomycin 0 to each well. After this,
the trypsinized L929 cells were counted and 4 X 104cells were added to each well. Next, the
supematants from the stimulated PBMC or PAM were added to separate wells (50 Jll/well) in
quadruplicate.
The PBMC-supematants were added directly (undiluted) to the wells, whereas the
PAM-supernatants were first diluted 1:10 with DMEM. Reference wells were prepared in
order to measure minimum and maximum lysis of L929 cells. Deionized water (200 JlI) was
added to the appropriate wells for maximum lysis. For minimum lysis, 50 JlI of OMEM was
added to the appropriate wells. After all the samples were added to the wells, plates were
incubated at 37°C and 5% C02 for 20 hours. TNF-a present in supernatants from the
stimulated PBMC or PAM caused lysis of L929 cells. After the incubation, 50JlI of Neutral
Red solution was added to each well to stain remaining L929 cells and plates were incubated
for 2 hours at 37°C. Next, the media was removed from the wells and the plates were rinsed
once with phosphate buffered saline. Following this, 100 JlI of a NaH2P04-ethanol solution
was added to each well. After 20 minutes of gentle mixing, the plates were read at 570 nm.
Cell lysis was measured against a standard curve that was prepared with human
51
recombinant TNF-a. High cell lysis, and thus high TNF-a concentration, corresponded to
low absorbance.
Phagocytosis
Pulmonary alveolar macrophages were isolated as described above and
phagocytosis of latex beads was assessed. Aliquots of 1 x 106 PAM in HBSS were placed
into 1.5 ml microcentrifuge tubes. An appropriate volume (63 J.l1) of latex beads (1.75 micron,
Polysciences Inc., Warrington, PA) was added to the suspension, which resulted in a final
bead to macrophage ratio of 25:1. The final volume in the tubes was adjusted to 1 ml with
Eagle's minimum essential medium (Gibco) plus 10% fetal calf serum. After this, the cells
were incubated on a Labquake (Fisher Scientific, Pittsburgh, PA) for 2 hours at 37°C. After
incubation, tubes were centrifuged for 5 minutes at 500 x g. Next, supernatants were
discarded from the tubes and cells were resuspended in 100 J.l1 of phosphate buffered saline
with sodium azide and bovine serum albumin (PAB) and placed into the wells of a V-bottom
96-well microtiter plate. A mouse, anti-equine, pan-granulocyte/monocyte surface marker
(cell-line D59B, VMDR Inc., Pullman, WA) was used to identify the alveolar macrophages.
Additional wells containing cells only were prepared to provide controls for the flow cytometry
analysis. A purified mouse IgG1 isotype standard (Pharmingen Int'l, San Diego, CA) was
used as a negative control in these wells.
The above reagents were added to the appropriate wells (50 J.lllwell) followed by
addition of 50 J.l1 of PAB to each well. Next, the plate was placed on ice and incubated in the
dark for 5 minutes. After the incubation, 50 J.l1 of PAB was added to each well and the plate
52
was centrifuged at 500 x g for 3 minutes. Next, the supernatant in each well was aspirated,
the plate was gently vortexed and 125 JlI of PAS was added to each well followed by
centrifugation for 3 minutes at 500 x g. After this, the supernatant in each well was again
aspirated, the plate was gently vortexed and 50 JlI of PAS was added to each well.
The secondary antibody was a goat anti-mouse IgG, F (ab')2 conjugated to
phycoerythrin fluorescent stain (Jackson ImmunoResearch Inc., West Grove, PA). This
secondary antibody was added to each well (50 Jll/well) followed by addition of 50 JlI of PAS.
The plate was then placed on ice and incubated in the dark for 5 minutes. After this
incubation, 50 JlI of PAS was added to each well and the plate was centrifuged at 500 x g for
3 minutes. Next, the supernatant in each well was aspirated, the plate was gently vortexed
and 125 JlI of PAS was added to each well followed by centrifugation for 3 minutes at 500 x
g. Following this, supernatant was again aspirated, the plate was gently vortexed and 175 JlI
of PAS was added to each well. Fluorescent bead engulfment was assessed using an
EPICS IX flow cytometer (Coulter Electronics Inc.)
Biochemical Measurements
Plasma lipids (cholesterol and triglyceride)
These were measured separately from the serum biochemistry profile, using
techniques previously discussed. 68 The sum of cholesterol and triglycerides was taken as
an estimate oftotallipid. 69
53
Plasma a-tocopherol
This was determined by HPLC using a fluorometric detector as discussed previously Wander
et a1. 42 . In short, a-tocopherol was extracted into hexane, dried, resuspended in methanol,
then injected into a C18, reversed-phase column (250 X 4.6 mm, 5 /-lm CLS-ODS, Shim­
pack, Shimadzu). The column was fitted with a precolumn filter (0.5 /-lm frit, A-318; Upchurch
Scientific, Oak Harbor, WA), the temperature was maintained at 35°C and the flow rate for
100% methanol was 1.5mllmin. The excitation wavelength was 292 nm; the emission
wavelength was 330 nm. (i-Tocopherol was used as the internal standard.
Complete blood count (CaC) and white cell differential count
The CBC was determined using a hematology analyzer (Baker 9010, Serono-Baker
Instrument, Inc., Allentown, PA). The white cell differential was determined by a certified
medical technologist by microscopic examination of blood smears after Wright-Giemsa
staining.
Serum biochemistries
Serum was frozen and later analyzed using a Roche FARA II system (Roche Inc.,
Somerville, NJ).
54
Statistical Analysis
The statistical analysis of data was done using the Statgraphics statistical software
program.
A two-sample t-test was used to compare data.
Values were considered
significant at a P ~ 0.05. Mean values for horses in the two diet groups were compared at
each timepoint for each assay. Mean values for horses within a diet group were assessed at
each timepoint for significant changes from prestudy values. Data such as the KLH antibody
titer required a log transformation before comparing groups.
Outliers were excluded from
comparisons only if there was reasonable justification for doing so.
55
RESULTS Animals
All horses readily consumed the oil enriched diets and average weekly food
consumption was calculated to be greater than 95 percent for both diet groups. One horse in
the (n-3) fatty acid supplemented diet group had decreased food consumption (25-50%)
beginning the 10th week of the trial and lasting through the end of the feeding period at 14
weeks. Mean body weights within each group of horses did not show any significant
changes compared to the pretrial period. Differences from week zero in mean body weights
were compared between groups at each timepoint and showed no significant differences.
Body condition scores of the horses were the same at the beginning and end of the feeding
trial.
Diets
The peroxide levels and P-anisidine values used to evaluate oxidation of the oils
showed no significant oxidation of the oils at the beginning or end of the feeding trial.
Plasma Fatty Acid Profiles
The plasma fatty acid compositions of the horses at the beginning of the study were
similar. A summary of the changes that occurred in the plasma fatty acid profiles after dietary
supplementation with polyunsaturated fatty acids can be found in the Appendix. The plasma
56
fatty acid profiles showed significant changes six weeks after supplementation began with (n­
6) or (n-3) polyunsaturated fatty acids. In general, the changes seen at six weeks persisted
throughout the 14-week dietary trial and returned to predietary levels by four weeks after the
end of the supplementation period.
The changes in plasma concentrations of the sum of the saturated fatty acids (SFA),
the sum of the monounsaturated fatty acids (MUFA) and the sum of the PUFA are depicted in
separate panels of Figure 4. The top panel (panel A) shows that the plasma concentrations
of saturated fatty acids in horses from each diet group were relatively unaffected by the
dietary supplementation with PUFA.
The group supplemented with (n-6) PUFA had
significantly lower levels of plasma saturated fatty acids at 6 weeks compared to baseline
(P=O.05). This change resulted in horses from the (n-6) diet group having significantly lower
plasma saturated fatty acids at 6 weeks compared to horses from the (n-3) diet group
(P=O.02). No other significant differences were noted within the groups or between the
groups over time.
Panel B illustrates the changes in plasma concentrations of monounsaturated fatty
acids. Compared to the predietary period, plasma levels of monounsaturated fatty acids
decreased in horses from both diet groups and remained lower than baseline levels at 6, 8,
and 12 weeks. There was a highly significant decrease from baseline in the (n-6) diet group
at 6 and 8 weeks (psO.00001). The changes noted in this group at 12 weeks were still
significantly lower than baseline (P=O.007) although not as pronounced.
The plasma
concentrations of monounsaturated fatty acids in horses fed the (n-3) diet were lower than
the baseline levels at 6 (P=O.07) and 8 weeks (P=O.09). Four weeks after discontinuing the
57
Sum of Saturated Fatty Acids
A
U)
"t:I
~
~
u.
g
50
40
30
20
10
o
~==~~u=~-U~~~~~~~~
o
6
8
18
12
week
B
Sum of Monounsaturated Fatty Acids
25 ~--------------------------20
~
u.
8
~
+-~~
=-_
__~____~__________
15
10
5
o
C
6
12
8
18
week
Sum of Polyunsaturated Fatty Acids
+*
+*
60 ~----------------------~---
o
6
12
8
18
week
lZJ (n-6) fatty acid diet
(n-3) fatty acid diet
I
Figure 4. Effect of feeding horses diets that differed in the (n-6) and (n-3) fatty
acid content on the plasma concentrations of saturated fatty acids, monounsaturated
fatty acids, and polyunsaturated fatty acids at 0, 6, 8, 12, and 18 weeks. The fatty acids
are expressed in g/100g fatty acids (mean ± SEM). + Denotes a significant change
(p:$;0.05) from week 0 within a group of horses. * Denotes a significant difference
(p:$;0.05) between group means at that timepoint.
58
fatty acid supplement (18 weeks), plasma concentrations of monounsaturated fatty acids
were still significanijy decreased in horses fed the (n-6) fatty acid supplement, whereas there
was no significant difference form baseline in horses fed the (n-3) fatty acid supplement.
Comparisons between diet groups at each timepoint showed that horses in the group
supplemented with (n-6) PUFA had significantly lower concentrations of MUFA at 6 weeks
(P=O.0002) and 8 weeks (P=O.00001) compared to the (n-3) PUFA-supplemented group.
Panel C shows that the concentration of the PUFA in the plasma of all horses
appeared to increase because of the dietary supplements.
Specifically, plasma
concentrations of PUFA increased in both groups at 6 weeks compared to baseline values,
but the change was only significant in horses fed the (n-6) fatty acids (P=O.003). Plasma
concentrations of PUFA remained significantly higher in horses from the (n-6) fatty acid
supplemented group at 8 weeks (P=O.001) compared to the predietary period. The plasma
concentrations of PUFA in horses fed the (n-3) fatty acids did not increase significantly above
predietary levels at any time. Comparisons between the groups at each timepoint revealed
that horses fed the (n-6) fatty acid supplement had significantly higher plasma PUFA
concentrations than horses fed the (n-3) fatty acid supplement at 6 weeks (P=O.01) and 8
weeks (P::;O.0002). No differences were noted between the groups at 12 weeks. However,
at 18 weeks (four weeks after the fatty acid supplementation period had ended), horses fed
the (n-6) fatty acid supplement had higher concentrations of plasma PUFA than horses fed
the (n-3) fatty acid supplement (P::;O.02).
Although plasma levels of total PUFA appeared to increase in both groups of horses
as a result of supplementation with (n-6) or (n-3) PUFA, the concentrations of individual fatty
59
acids comprising the total plasma PUFA changed quite differently between groups. Changes
in plasma concentrations of the sum of (n-6) PUFA and the sum of the (n-3) PUFA are
depicted in separate panels in Figure 5.
Panel A illustrates that the horses that consumed the (n-6) fatty acid diet had an
increase in plasma (n-6) fatty acid levels at 6 weeks (P::;;O.003) and 8 weeks (P::;;O.002).
Although plasma (n-6) fatty acids were still increased at 12 weeks compared to baseline, this
increase was not significant.
Four weeks after the (n-6) fatty acid supplement was
discontinued (18 weeks), the plasma (n-6) fatty acids had decreased and were not
significantly higher than baseline values. Conversely, horses consuming the high (n-3) fatty
acid diet showed a decrease in plasma (n-6) fatty acids at 6 weeks (P::;;O.004) and the (n-6)
fatty acid levels remained Significantly lower than baseline values at 8 weeks (P::;;O.002) and
12 weeks (P::;;O.03). Plasma levels of (n-6) fatty acids were not significantly different from
baseline levels at 18 weeks, which was 4 weeks after discontinuing the (n-3) fatty acid
supplement. Comparisons between the two groups of horses showed that the horses fed the
(n-6) fatty acid supplement had significantly higher concentrations of (n-6) PUFA levels than
horses fed the (n-3) fatty acid supplement at 6, 8, and 12 weeks (P::;;O.01), and the (n-6)
PUFA levels remained higher 4 weeks after discontinuing the fatty acid supplements (18
weeks) in the horses fed the (n-6) fatty acid supplement.
Panel B of Figure 5 demonstrates the changes that occurred in plasma
concentrations of (n-3) fatty acids in horses fed the two fatty acid supplements. The group of
horses consuming the (n-3) fatty acids showed marked increases in plasma concentrations of
(n-3) PUFA at 6,8, and 12 weeks (P::;;O.0002). The horses consuming the (n-6) fatty acids
60
Sum of (n-6) polyunsaturated fatty
A
*
60
50
~
u..
8.....
en
*
*
,-----~~--~~-------------
+-----~~~~~--~~-------
4 0 +---"~~--l:
30
20
10
o
~~~~~~~~~-==-~~~~
o
B
6
12
8
week
18
Sum of (n-3) polyunsaturated fatty
20 ,-------~*=---~*~--~
*-------15 +--------1
10 -+---------1
5 -+-----,--------1
o
~~~~=-~~~~~~~~~
o
6
12
8
18 week
C Ratio of the Sum of (n-6) to the Sum of (n-3) fatty acids
70 ,------------------~---------
60 -+-------------------4r=~-----50 +-----------------~~-------
40 +-------~----~--4
30 -+------~~--~~---I
20 +-~~~~--~~--~
10
O ~LL.4..-'--,__L'==---,..u:L4::=>____;_-=<="-r--LLL"'--~
o
6
1'21 (n-6) fatty acid diet
8
12
18
week
(n-3) fatty acid diet
Figure 5. Effect of feeding horses diets that differed in the (n-6) and (n-3) fatty acid content
on the sum of the (n-6) and the sum of the (n-3) fatty acids in plasma at 0, 6, 8, 12, and 18
weeks. The ratio of the sum of (n-6) to the sum of (n-3) fatty acids is also compared at zero,
6, 8, 12, and 18 weeks. The fatty acids are expressed in g/100g fatty acids (mean ± SEM).
+ Denotes a significant change (p:::;0.05) from week 0 within a diet group. * Denotes a
significant difference (p:::;0.05) between group means within a timepoint.
61
showed a decrease in plasma concentrations of (n-3) PUFA, but this was significantly
different from baseline only at 12 weeks (P=O.04). Comparisons between the two groups of
horses showed that horses fed the (n-3) fatty acid supplement had significantly higher
concentrations of (n-3) PUFA levels than the horses fed the (n-6) fatty acid supplement at 6,
8, and 12 weeks
(P~O.00002).
By 4 weeks after the fatty acid supplements were
discontinued, there was no difference between the two groups of horses in the plasma (n-3)
fatty acids.
An interesting contrast between groups was seen when the ratio of the plasma (n­
6) to (n-3) fatty acids was compared. Within a group of horses, Panel C shows that the
plasma ratio of (n-6) to (n-3) fatty acids increased in horses fed the (n-6) fatty acid
supplement by 6 weeks. This ratio was only significantly different from baseline at 12
weeks (P=O.03). The ratio decreased significantly in horses fed the (n-3) fatty acid
supplement at 6 weeks (P=O.04) and remained lower than baseline values at 8 and 12
weeks (P=O.05). Horses consuming the (n-3) fatty acid supplement had a significantly
lower ratio of plasma (n-6) to (n-3) fatty acids than those consuming the (n-6) fatty acid
supplement at 6,8, and 12 weeks
(P~O.002).
Four weeks after discontinuing the fatty acid
supplements (18 weeks), the plasma ratio of (n-6) to (n-3) fatty acids in both groups of
horses had returned to predietary values and there was no significant difference between
the two groups.
Further comparison of the changes in the concentrations of the individual plasma
fatty acids that comprise the total PUFA are depicted in Figure 6. In this figure, total PUFA
are subdivided into the linoleic acid constituent; 18:2(n-6) and the sum of the remaining
62
PUFA, which includes a-linolenic acid, 18:3(n-3); EPA 20:5(n-3); DHA 22:6(n-3); and AA
20:4(n-6). As can be seen from the graph at time 0 weeks, linoleic acid makes up the largest
portion of the total plasma PUFA in horses fed either the (n-6) or the (n-3) fatty acid
supplement. At 6 and 12 weeks, in those horses fed the (n-6) fatty acid supplement, linoleic
acid continues to be the predominant plasma fatty acid. Linoleic acid is significantly higher
than baseline at 6 weeks (P=O.004). In those horses fed the (n-3) fatty acid supplement,
however, there is a significant decrease in plasma linoleic acid at 6 (P=O.001) and 12 weeks
(P=O.007) compared to baseline values. Four weeks after discontinuing the diets (18 weeks),
plasma linoleic acid concentrations increased in this group of horses, but values were still
significantly lower than pretrial levels (P=O.05). Horses fed the (n-3) fatty acid supplement
showed a concomitant increase in plasma concentrations of the remaining PUFA, which are
predominantly AA, EPA, and DHA. Between groups, the plasma concentrations of linoleic
acid in the horses fed the (n-3) fatty acid supplement are significantly decreased compared to
the concentrations in the horses fed the (n-6) fatty acid supplement af6 weeks (P::;;O.0001),
12 weeks (P=O.004) and 18 weeks (P=O.03).
63
Changes in Total PUFA, linoleic acid and the rest of the PUFA
60
~----------------------------------------
50
+-- -- - - -- ---1
40
IJ)
"'C
·u
«
i'
30
LL 0> 8
~
20
10
oweeks
DL PUFA
12 weeks
6weeks
DL PUFA
~
LA
LA
18 weeks
lSI rest
o
rest
Figure 6. Effect of feeding horses diets that differed in the (n-6) and (n-3) fatty acid content
on the concentration of fatty acids in plasma. Total plasma PUFA is subdivided into plasma
linoleic acid (LA) and the sum of the remaining PUFA (rest). These are shown for each
group of horses at 0, 6, 12 and 18 weeks. The fatty acid concentrations are expressed in
g/100 g fatty acids (mean ± SEM). Hatched bars represent those horses fed the (n-6) fatty
acid supplement; solid bars represent those horses fed the (n-3) fatty acid supplement.
64
Immunological Measurements
De/ayed-type hypersensitivity skin test
The cell-mediated immune response was evaluated using a delayed-type
hypersensitivity skin test. The neck and ear were the two sites used for judging the horses
response to keyhole limpet hemocyanin (KLH). Wheal diameters of three injection sites on
the neck were averaged for each horse and then the five horses in each group were
averaged for a group mean. Both groups of horses had severe reactions to the KLH at 24
hours with the horses fed the (n-3) fatty acid supplement showing a greater response than
the horses fed the (n-6) fatty acid supplement (P=O.06). At 48 hours, the response in both
groups of horses subsided somewhat, however, the reaction was now more marked in the
horses fed the (n-6) fatty acid supplement. The response in both groups of horses continued
to decline at 72 and 96 hours with the horses receiving the (n-3) fatty acid supplement
showing a decreased reaction compared to horses receiving the (n-6) fatty acid supplement
(see Appendix).
The DTH response on the ear was measured at the base and the tip of the ear.
Wheal diameter and ear thickness was used in determining the response.
The
measurements at the base of the ear were evaluated separately from the measurements
made at the tip of the ear. The wheal diameter was multiplied by the change in ear thickness
(i.e., the ear thickness was determined by subtracting thickness at time 0 from thickness at
the specified time). Results were expressed in mm2. There were no significant differences
between groups of horses for the reaction at the base of the ear as the responses at each
65
timepoint were similar for both groups of horses. Measurements made at the tip of the ear
showed a significantly larger reaction in the horses fed the (n-6) fatty acid supplement at 30
minutes (P=0.02) and at 24 hours (P=0.04) compared to the horses fed the (n-3) fatty acid
supplement. The reaction at 48 hours, however, was opposite that seen at 24 hours, i.e., the
horses fed the (n-3) fatty acid supplement had a larger reaction compared to horses fed the
(n-6) fatty acid supplement. This trend continued at 72 and 96 hours but was not significant
(see Appendix).
Keyhole limpet hemocyanin antibody titer
The humoral response was measured four weeks after horses were first sensitized to
KLH protein. The antibody titers, which were transformed to a log scale before comparisons
were made between groups, revealed no significant difference between the two groups of
horses (see Appendix).
Leukotriene 84 and 85 quantification
The production of LT84 and LT85 by peripheral blood neutrophils was compared
between the horses fed the (n-6) fatty acid supplement and the horses fed the (n-3) fatty acid
supplement at 0 and 12 weeks (see Appendix and Figure 7). Leukotriene 84 or 85
production by stimulated peripheral blood neutrophils was not significantly different between
the two groups of horses at 0 weeks. At 12 weeks, the horses consuming the (n-6) fatty acid
supplement produced significantly less LT85 than horses fed the (n-3) fatty acid supplement
(P:::;0.01). The production of LT84 by peripheral blood neutrophils was also significantly
66
different between these two groups of horses, and was lower in the horses fed the (n-6) fatty
acid supplement (PSO.02). The ratio of LTB5 to LTB4 concentrations was significantly higher
in horses fed the (n-3) fatty acid supplement compared to the horses fed the (n-6) fatty acid
supplement at 12 weeks (PsO.002).
Leukotriene production by stimulated peripheral blood neutrophils within the group of
horses consuming the (n-6) fatty acid supplement did not change significantly from baseline
to week 12 of the feeding trial (P=O.09).
The horses consuming the {n-3} fatty acid
supplement however, did show a significant increase in production of LTB4 {PSO.003} and
LTB5 (PsO.01) at 12 weeks compared to their predietary levels. The ratio of LTB5 to LTB4
was also significantly higher at 12 weeks compared to baseline values (psO.0001) within the
group of horses consuming the (n-3) fatty acid supplement.
Because EPA metabolism leads to the production of LTB5 and AA metabolism leads
to production of LTB4, the relationship between the levels of these fatty acids and leukotriene
production in both groups of horses were compared. The levels of EPA and AA in the
plasma of the horses appear to correlate with the levels of LTB4 and LTBs produced by
equine neutrophils. Both groups of horses had similar baseline concentrations of plasma AA,
EPA and DHA. At 12 weeks, the levels of AA, EPA and DHA in the plasma fatty acids of the
horses fed the (n-3) fatty acid supplement were significantly higher compared to the horses
fed the (n-6) fatty acid supplement (PsO.0001, see Figure 8).
67
*
+
2 .0 , - - - - --;:; - - - - - - - - - - - - - - - - ­
>.
.0
*+
C
o
~ ~
g~
*
1.S +-----;Tr------~~-----~r_--
"00..
e
0
o...!:>
-~
C)O>
C
C
1.0
+ - -­
---1
O.S
+ - -­
----1
.....
0>0
C"­
.~ x
~..~
~
0 .0
LTBS
L TB4
L TBS/L TB4
lZ {n-6)fllttYllcid dietgroup litO wk
o(n -3)fattyac id d ietgroup atO wk
D (n-6) fatty acid diet group at 12 wk
D (n -3) fatty acid d iet group at 12 wk
Figure 7. Effect of feeding horses diets that differed in the (n-6) and (n-3) fatty acid content
on the production of LTB4 and LTBs by stimulated peripheral blood neutrophils at 12 weeks
compared to baseline (0 weeks). The ratio of LTB5 to LTB4 is compared at 0 and 12 weeks
and is expressed as mean ± SEM. Concentrations of leukotrienes are expressed in
nanograms per 1 x 107 cells (mean ± SEM). * Denotes a significant difference (P~0 .05)
between group means at that timepoint. + Denotes a significant change (P~0.05) from week
owithin a group of horses.
Plasma levels of AA, EPA, and DHA did not change significantly within the horses
receiving the (n-6) fatty acid supplement at 12 weeks compared to baseline levels.
Significant changes in plasma AA, EPA, and DHA levels did occur within the horses fed the
(n-3) fatty acid supplement. At 12 weeks, the plasma levels of AA, EPA, and DHA increased
significantly in this group of horses compared to their baseline levels (PSO.0001).
The plasma ratios of AA to EPA also appear to correlate to the ratios of LTBs to LTB4
concentrations produced by peripheral blood neutrophils. At week zero, the horses fed the
(n-6) fatty acid supplement had an EPA to AA ratio of 0.14 and a LTBs to LTB4 ratio of 0.07,
68
while the horses fed the (n-3) fatty acid supplement had an EPA to AA ratio of 0.44 and a
LTBs to LTB4 ratio of 0.12. There were no significant differences between plasma ratios of
the two groups at this timepoint (P=O.OB). At 12 weeks, in horses fed the (n-6) fatty acid
supplement, the EPA to AA ratio was 0.24 and the LTBs to LTB4 ratio was 0.2B. In the group
of horses fed the (n-3) fatty acid supplement, the ratios of EPA to AA and LTBs to LTB4 were
2.04 and 1.13, respectively. The ratios of EPA to AA and LTBs to LTB4 were Significantly
higher in the horses fed the (n-3) fatty acid supplement compared to the horses fed the (n-6)
fatty acid supplement at this timepoint
(P~0.0001
and P=0.002, respectively). Only the
horses receiving the (n-3) fatty acid supplement showed a Significant increase in the EPA to
AA ratio (P=0.02) and LTBs to LTB4 ratio (P~0.0001) from 0 to 12 weeks (see Figure 8).
69
A
o weeks
10
8
.!!2
:c
a.
«
~6
ij1
~
'0
e
c
o,...
u..
84 x
2
o ~
AA
~
EPA
DHA
-
-
~
LTB4
LTBS
EPAfAA
LTBSILTB4
B
10 ,--_ _
en
"0
*
8 + - - - ---/
~
~ 6 +--*
- ---1
u..
~
r----------------~ .!!2
:c
a.
*+
e
r----------------~ ij1
+
Ol
e;,
12weeks
+
c
....
4 +---r-:!:'1----/
*
*
*
0
~-~----------~
x
,...
+
iii
+
*+
+
2
r------~-_;~---~
!:i
Ol
c
AA
EPA
~ (n-6)
fatty acid diet
LTB4
LTB5
EPNM
LTB5ILTB4
o (n-3) fatty acid diet
Figure 8. Effect of feeding horses diets that differed in the (n-6) and (n-3) fatty acid content
on the concentration of AA, EPA, and DHA in plasma is compared to LTB4 and LTB5
production by stimulated neutrophils at 0 weeks in panel A and 12 weeks in panel B. The
ratios of EPA to AA and LTB5 to LTB4 concentrations are included. Plasma concentrations of
AA, EPA and DHA are expressed in g/100g fatty acids. LTB4 and LTB5 production is
expressed in nanograms per 1 x 107 neutrophils. + Denotes a significant change (p~0.05)
from week 0 within a group of horses. * Denotes a significant difference (p~0.05) between
groups of horses at 12 weeks.
70
Tumor Necrosis Factor-a production
Evaluation of TNF-a production by peripheral blood mononuclear cells revealed no
significant differences between groups of horses at 0, 6, 8 or 12 weeks (see Appendix).
Changes in TNF-a production by peripheral blood mononuclear cells within each group of
horses varied between timepoints and no trends in the data could be inferred. There was
also no significant difference in TNF-a production by pulmonary alveolar macrophages
between groups of horses during the dietary intervention period (see Appendix and Figure
9). TNF-a production by PAM from horses within each group were also compared to
predietary levels. The production of TNF-a increased in both groups of horses at 6 weeks
with a significance level of P=0.01 for the horses fed the (n-6) fatty acid supplement and
P=0.0003 for the horses fed the (n-3) fatty acid supplement. Horses fed the (n-3) fatty acid
supplement also had significantly higher TNF-a levels (P=0.04) at 8 weeks compared to
week zero. At 12 weeks, TNF-a levels were not significantly higher compared to week zero
for these horses. However, one horse that had very low levels of TNF-a compared to the
rest of the group also had a very low percentage of alveolar macrophages in the BALF cells.
When this outlier was removed, and the comparison was made to the predietary period, the
increase at 12 weeks was significant (P<0.0001).
71
Tumor Necrosis Factor-a. production
+
100 J!l
Q)
80
(.) 0) N
-
0)
60
.-J 0
en
'00
~
0~
40
20
0
o
8
6
12
week
I ~ (n-6) fatty acid diet
(n-3) fatty acid dietl
Figure 9. Effect of feeding horses diets that differed in the (n-6) and (n-3) fatty acid content
on the production of TNF-a. by pulmonary alveolar macrophages at 0, 6, 8, and 12 weeks. +
Denotes a significant change (P~0 . 05) from week 0 within a group of horses compared to the
specified timepoint.
Phagocytosis
The phagocytic activities of pulmonary alveolar macrophages from the two groups of
horses were not significantly different from each other before or during the diet
supplementation period (see Appendix). The percent of macrophages that engulfed latex
beads was higher in the horses fed the (n-6) fatty acid supplement compared to those fed the
(n-3) fatty acid supplement for all evaluations. When comparisons were made within each
group of horses for changes in alveolar macrophage phagocytic activities compared to their
baseline values, only the horses fed the (n-6) fatty acid supplement showed a significant
increase in activity at 12 weeks (P=0.03). The horse in the (n-3) fatty acid supplemented­
72
group that had a low percentage of alveolar macrophages in BALF was excluded from the 12
week data. However, this did not significantly impact the findings in this group of horses at 12
weeks (see Figure 10).
~
rn
.9
>­
(,.')
+
30
0
C)
ro
a.
~
c
25
20
Q)
~
Q)
15
S
>­ 10
·s
=(,.') 5
ro
(,.')
~
(,.')
0
C)
ro
a...
0
o
8
6
12
week
~
12 (n-6) fatty acid diet
CJ (n-3) fatty acid diet
Figure 10. Effect of feeding horses diets that differed in the (n-6) and (n-3) fatty acid
content on phagocytic activity of alveolar macrophages at 0, 6, 8, and 12 weeks. Percent
activity = population of cells engulfing 1 or more fluorescent beads + total population of
cells X 100 (mean ± SEM). + Denotes a significant change (P~0.05) from week 0 within a
group of horses compared to the specified timepoint.
Biochemical Measurements
Plasma lipids (cholesterol and triglyceride)
Plasma cholesterol concentrations were not significanHy different between horses
consuming the (n-6) fatty acid supplement and the horses consuming the (n-3) fatty acid
supplement at any time during the feeding period. Cholesterol/eve/s increased significantly
73
in the horses consuming the (n-6) fatty acid supplement at 6 weeks compared to the
predietary period (P=0.03). Cholesterol levels decreased in this group of horses at 8 and 12
weeks and were not significantly different from the predietary period. Similar changes in
cholesterol occurred in the horses fed the (n-3) fatty acid supplement. There was an
increase in cholesterol at 6 weeks (P=0.003). This was followed by a decrease at 8 weeks
that was still different from week zero (P=0.01). Cholesterol levels at 12 weeks in this group
of horses were not Significantly different from prestudy levels (see Appendix and Figure 11).
1 40
E
1 2 0
o
o 1 0 0
8 0
6 0
4 0
2 0
o
o
6
8
1 2
8
1 2
We e k
40
~
30
<D
2 0
~
10
:g
.g>
I-
o
o
6
Wee k
~
( n - 6 ) fa tty ac id d ie t
( n - 3 ) fa tty ac id d ie t
I
Figure 11 . Effect of feeding horses diets that differed in the (n-6) and (n-3) fatty acid content
on plasma lipid concentrations (cholesterol and triglyceride) at 0, 6, 8 and 12 weeks. Values
are expressed in mg/100 ml ± SEM. + Denotes a significant change (P:$;0.05) from week 0
within a group of horses.
74
Triglyceride levels were not significantly different between the two groups of horses
throughout the study. Changes in triglyceride levels occurring within each group of horses
over time also were non-significant (see Appendix and Figure 11).
Plasma a-tocopherol
Both groups of horses demonstrated parallel changes in their plasma a-tocopherol
levels over the course of the study (see Appendix and Figure 12). Although horses
consuming the (n-6) fatty acid supplement had higher plasma levels of a-tocopherol than the
horses consuming the (n-3) fatty acid supplement at all timepoints, they were not significant
at the
p~O.05
level. The only significant changes seen in plasma a-tocopherol levels
occurred within the group of horses consuming the (n-3) fatty acid supplement, which
showed an increase at 6 weeks (P=O.02). The levels of a-tocopherol in the plasma of horses
consuming the (n-3) fatty acid supplement remained significantly elevated at 8 weeks
(P=O.01) and 12 weeks (P=O.01) when compared to predietary levels.
75
(j)
"0
8 .0
:§..
J!!
.9 6 .0
0
E
E
-­
0
4 .0
E
3
e
2 .0
Q)
..c:
c..
80
-
0 .0
o
,
~
ro
E
en
ro
a::
8
6
12
week
~
( n-6) fa tty acid die t
( n-3) fa tty acid die t
Figure 12. Effect of feeding horses diets that differed in the (n-6) and (n-3) fatty acid
content on plasma a.-tocopherol concentrations in horses at 0, 6, 8 and 12 weeks. a.­
Tocopherol is expressed in /-tmol/mmol of total lipids (mean ± SEM); total lipids = the sum
of cholesterol and triglyceride. + Denotes a significant change (P~0 . 05) from week 0
within a group of horses.
Complete blood count (CaC) and white cell differential count
There was a significant difference in the white blood cell counts between the two
groups of horses at week zero (P=0.04) with the horses in the (n-6) fatty acid diet gorup
being higher than those in the (n-3) fatty acid diet group. This is attributable to the neutrophil
constituent of the white blood cell count since neutrophils were significantly higher in the
group of horses in the (n-6) fatty acid diet group at this timepoint. There were no other
significant differences between groups of horses in the CSC, white cell differential counts or
red blood cell indices at any timepoint. Significant changes occurred within a group of horses
consuming the same fatty acid supplement compared to the predietary period. The white
76
blood cell count was significantly lower at 8 weeks in the horses fed the (n-6) fatty acid
supplement compared to week zero (P=O.009). The neutrophil and monocyte counts were
also significantly lower in this group of horses at 8 weeks compared to week zero (P=O.03).
The white blood cell count was significantly lower in the group of horses fed (n-3) fatty acid
supplement at 6 weeks (P=O.04) and 12 weeks (P=O.01) compared to week zero. The
neutrophil count in the horses fed the (n-3) fatty acid supplement was lower at 12 weeks
compared to the predietary period (P=O.009). Plasma proteins increased in the horses fed
the (n-6) fatty acid supplement at 6 weeks (P=O.05) and 12 weeks (P=O.002) compared to
week zero. Horses fed the (n-3) fatty acid supplement showed a significant increase in
plasma proteins at 12 weeks (P=O.009) compared to zero weeks. Horses fed the (n-3) fatty
acid supplement had a significant increase in the mean cell hemoglobin concentration at 6
weeks (P=O.04) compared to the predietary period. It should be noted that even though
significant changes occurred in the CSC, white cell differential counts, and RSC indices as
outlined above, all values remained within normal limits for the horse (see Appendix).
Serum biochemistries
Serum biochemistries revealed no significant differences between the two groups of
horses during the study except for magnesium and calcium levels. Magnesium was higher in
the horses fed the (n-6) fatty acid supplement compared to the horses fed the (n-3) fatty acid
supplement at zero weeks (P=O.03) and 12 weeks (P=O.01). The normal laboratory range for
magnesium in horses is 2 to 3 mg/dl and all the horses had serum levels less than 2 mg/dl for
all timepoints measured. (This is not an uncommon clinical finding in healthy horses.)
77
Calcium was higher in the horses fed the (n-3) fatty acid supplement than in those fed the (n­
6) fatty acid supplement at 8 weeks (P:s;O.0001), however, horses from both groups remained
within normal limits for this parameter (see Appendix). Significant changes occurred within a
group of horses consuming the same fatty acid supplement over the course of the study.
Horses consuming the (n-6) fatty acid supplement had lower magnesium levels at 8 and 12
weeks compared to week zero (P=O.01), and the horses consuming the (n-3) fatty acid
supplement had lower magnesium levels at 12 weeks (P=O.05).
Additionally, horses
consuming the (n-3) fatty acid supplement had lower phosphorus levels at 8 weeks (P=O.03).
At 12 weeks this group of horses also had lower glucose (P=O.02), lower creatinine kinase
(P=O.05), and higher potassium (P=O.05) levels compared to week zero. It should be noted
that even though significant changes occurred as outlined above, all values remained within
normal limits at all timepoints except for creatinine kinase levels, which were elevated in both
groups of horses prior to the feeding period. This was not a concern to the clinical
veterinarian.
78
DISCUSSION
Researchers have used both humans and animals to investigate the immune
modulating effects of dietary (n-6) and (n-3) fatty acids. In particular, dietary supplementation
with fish oil, an (n-3) fatty acid, has been shown to have beneficial effects on inflammatory
disorders. It was the purpose of this study to investigate whether dietary (n-3) PUFA and/or
(n-6) PUFA can affect the immune response of normal horses. Another goal of this study
was to determine if (n-3) PUFA are more immunosuppressive than (n-6) PUFA on equine
immune function.
In the current study, all horses readily consumed either the com oil or the fish oil fed
to them in presoaked beet pulp. Food consumption remained
~
95% throughout the 14
weeks of feeding the dietary supplements. However, one horse in the group supplemented
with fish oil had decreased food consumption (25-50%) during the last four weeks of the trial.
This horse also had neutrophil counts and BALF cytology that were extremely different from
the rest of the horses and so this mare was excluded from the 12 week data analysis.
Physical examination by the clinical veterinarian revealed nothing remarkable about this
mare. Body weight changes of all horses remained <10%. Except for magnesium, all
changes noted in serum biochemistries were within normal limits and no Significant
differences between horses of the two diet groups were noted. Although magnesium was
low in the horses before and during the feeding trial, the clinical veterinarian was not
concerned because this is a common finding in clinically normal horses. Complete blood
counts and white cell differential counts revealed some Significant but transitory differences
79
between and within horses of the two diet groups, however, all parameters were within
normal limits for the horse. The lack of significant effects on general health parameters in the
horse (food consumption, body weight, complete blood counts and white cell differential
counts, and serum biochemistries) is consistent with what was found in a similar feeding trial
with dogs.70
As expected, the plasma fatty acid profiles of the horses reflected the PUFA provided
in the diet. Total plasma (n-6) PUFA increased in the horses fed the diet enriched in (n-6)
PUFA. Total plasma (n-3) PUFA increased in the horses fed the diet enriched in (n-3) PUFA,
with a corresponding decrease in plasma (n-6) PUFA. Additionally, plasma EPA and DHA
levels increased in horses fed the (n-3) PUFA supplement. Interestingly, although the horses
that consumed the (n-6) PUFA diet had an increase in linoleic acid, they did not have a
corresponding increase in plasma AA. On the other hand, the horses that consumed the (n­
3) PUFA diet had an increase in AA, which appeared to reflect the presence of AA in the fish
oil supplement. The increase in plasma AA in horses fed the (n-3) PUFA supplemented diet
was inconsistent with results of other studies, using other animal species, which have shown
that diets high in (n-3) PUFA typically lower membrane phospholipid AA levels compared to
diets high in (n-6) fatty acids.
In a study involving Syrian hamsters, composition of
phospholipids of the liver, lung, spleen, kidney and heart were analyzed after they were fed
diets of linoleic acid, an (n-6) fatty acid, or EPA, an (n-3) fatty acid. The hamsters fed linoleic
acid had higher phospholipid concentrations of AA, whereas the hamsters fed the EPA
supplement showed decreased phospholipid concentrations of AA and increased
phospholipid concentrations of EPA concentrations.52
Cleland et al. 60 fed rats a diet of
80
sunflower oil, fish oil or linseed oil. The fatty acid profile of the diets showed that the
sunflower oil diet was rich in linoleic acid, whereas the fish oil diet was rich in EPA. Linseed
oil contained both a-linolenic acid, an (n-3) fatty acid, and linoleic acid, an (n-6) fatty acid.
The fatty acid profiles of the rat peritoneal exudate cells revealed that the EPA content was
greater and the AA was lower in the rats fed the fish oil diet compared to rats fed the
sunflower oil diet. The rats fed linseed oil, which contained both (n-6) and (n-3) fatty acids,
had lower levels of phospholipid AA in their cells compared to rats fed the sunflower oil.
In the study repeated here, the lower plasma AA content in the horses consuming
the (n-6) fatty acid supplement was accompanied by higher levels of plasma linoleic acid.
One possible explanation is that linoleic acid was not metabolized (elongated and
desaturated) to AA by these horses. In support of this notion, another study revealed similar
results when horses were fed an (n-6) fatty acid supplement. Harris et al29 analyzed plasma
phospholipid profiles of horses after 10 and 16 months of dietary supplementation with (n-6)
PUFA. Interestingly, the amount of linoleic acid was 36 to 45 times higher compared to AA in
the phospholipids. Additionally, analysis of cholesterol esters, triglycerides and free fatty acid
revealed no detectable AA levels. In the present study, the amount of plasma linoleic acid
was 50 to 95 fold higher than AA in horses fed the (n-6) fatty acid supplement. This suggests
that AA is not readily synthesized from linoleic acid in horses.
The enzymes involved in the conversion of linoleic acid to AA include L\ 6
desaturase, an elongase and 85 desaturase (see Figure 2). Horses fed the (n-6) fatty acid
supplement had very low plasma concentrations of the fatty acids derived from the enzymatic
action of these enzymes. The high plasma linoleic acid content in this group of horses
81
reflects the large amount of this fatty acid that was contained in the corn oil supplemented
diet (see Table 1). Paradoxically, horses fed the (n-3) fatty acid supplement had an increase
in plasma AA after consuming the fish oil supplemented diet. It is unlikely that the horses fed
fish oil were able to manufacture AA from the small amount of linoleic acid present in the fish
oil, because the other horses consuming large amounts of linoleic acid (provided in corn oil)
could not. The increase in plasma AA concentrations noted in the horses fed the (n-3) fatty
acid supplements was more likely a result of endogenous AA contained in the fish oil (see
Table 1). The small amount of AA present in the fish oil may have been sufficient to cause
the increase in plasma AA noted in horses fed the (n-3) fatty acid supplement.
It is possible that horses lack one or more of the enzymes involved in the conversion
of linoleic acid to AA. This phenomenon has previously been documented in wild and
domestic cats,?1-73 The cat lacks the fl6 desaturase enzyme, which is the first enzyme in
the metabolic pathway from linoleic acid to AA. It is also the rate-limiting enzyme in this
synthetic reaction and so limits conversion of linoleic acid even though there may be an over
abundance of this fatty acid in plasma. Activity of the fl 6 desaturase enzyme is also affected
by concentrations of (n-6) and (n-3) PUFA. Fatty acids that are substrates for this enzyme,
i.e., 18:2(n-6) and 18:3(n-3), can competitively inhibit the metabolism of the other fatty
acid,?4
Linoleic acid remained elevated in the plasma of horses fed the diets enriched in (n­
6) fatty acids throughout the 12 week dietary supplementation period. Similarly, horses
supplemented with (n-3) fatty acids continued to have high plasma concentrations of EPA
and AA throughout the 12 week dietary supplementation period. Four weeks after the diets
82
were discontinued, the plasma fatly acid profiles of all horses returned to levels similar to
those observed before dietary intervention.
The production of leukotriene B4 and B5 from equine neutrophils reflects the plasma
concentration of the substrates, AA and EPA, respectively, from which they are derived.
Based on the results depicted in Figure 8, dietary supplementation of horses with fish oil
results in high plasma concentrations of EPA and DHA and unexpectedly, high plasma
concentrations of AA. Consequently, those horses supplemented with (n-3) fatty acids had
higher levels of the corresponding leukotrienes (LTB5 and LTB4). Dietary supplementation of
horses with corn oil resulted in high plasma concentrations of linoleic acid, but not AA. It is
apparent from the data represented in the graph that horse neutrophils stimulated with
calcium ionophore do not readily produce leukotriene B4 in the absence of AA.
A study performed by Lindberg et al. 75 also indicates that exogenous arachidonic
acid is necessary for cultured equine neutrophils to produce leukotrienes when stimulated
with calcium ionophore. Investigators incubated neutrophils alone, neutrophils plus AA, or
neutrophils plus platelets with calcium ionophore to stimulate leukotriene production. Results
showed that equine neutrophils cultured alone do not produce measurable amounts of
leukotrienes. When neutrophils were cultured with AA, they produced significantly higher
amounts of LTB4 compared to neutrophils alone. The neutrophils cultured with platelets
produced more LTB4 than neutrophils alone but less than neutrophils that were cultured with
AA. The authors suggested that the presence of platelets (which lack 5-lipoxygenase
activity) contributed AA substrate to the neutrophils after calcium ionophore stimulation.
83
The results of the current study suggest that a diet rich in (n-3) PUFA causes an
increase in the production of LTB4 probably because AA from the fish oil diet is incorporated
into membrane phospholipids. However, a diet rich in (n-3) PUFA also increases the
production of LTB5, which is less biologically active than LTB4 and may have inhibitory effects
on the function of LTB4. 59 Thus, it is unclear to what extent the increased production of LTB4
in the horse may be harmful, based on the concurrent increased production of LTB5.
Other studies suggest that the ratio of LTB5 to LTB4 concentrations is more important
than the absolute amounts of either eicosanoid. 53,59,60 For example, Kragballe et al. 59
evaluated the inhibitory effects of LTB5 on LTB4-induced DNA synthesis by human
keratinocytes and LTB4-induced chemotaxis of human neutrophils and demonstrated that
LTBs inhibited the ability of LTB4 (which was present in optimal concentrations for activity) to
stimulate DNA synthesis and chemotaxis of neutrophils in a dose-dependent fashion. At a
LTBs to LTB4 ratio of 1000:1, keratinocyte activation was inhibited by 54% and neutrophil
chemotaxis by 18%. Additionally, it was shown that LTB5 failed to inhibit structurally
unrelated compounds (i.e., fMLP and thrombin) from inducing neutrophil chemotaxis or DNA
synthesis. This led the authors to suggest that the inhibitory effect of LTBs on LTB4 activity is
related to competition for receptors.
Cleland et al. 60 demonstrated a linear relationship between the ratio of EPA to AA in
cell phospholipids and the ratio of LTBs to LTB4 produced by rat peritoneal exudate cells in
vitro. Two of the oils fed to the rats in their study were linseed oil, a source of a-linolenic
acid, and fish oil, a source of EPA. EPA was detected in the phospholipids of exudative cells
from rats consuming either linseed oil or fish oil. EPA phospholipid content of cells positively
84
correlated with LTB5 synthesis and negatively correlated with LTB4 synthesis. There was a
positive correlation between AA content in phospholipids of exudative cells and LTB4
production. The ratio of EPA to AA from rats fed linseed oil and fish oil ranged from 0.23 to
1.22 and the ratio of LTB5 to LTB4 ranged from 0.21 to 1.01. The close linear relationship
between these ratios led the authors to suggest that EPA and AA were equally active
substrates for 5 lipoxygenase.
In this study, the horses consuming the PUFA supplements showed a similar
relationship between the ratios of EPA to AA in plasma and LTB5 to LTB4 production by
stimulated neutrophils. There was a linear relationship between the ratios of plasma EPA to
AA and LTB5 to LTB4 production in the horses consuming the (n-3) fatty acid supplement.
Both ratios were higher in the horses receiving the (n-3) fatty acid supplement than the
horses receiving the (n-6) fatty acid supplement. If it is the ratio of LTB5 to LTB4 that is
important in the horse, then a diet high in (n-3) PUFA is preferable to a diet high in (n-6)
PUFA in terms of controlling proinflammatory leukotrienes.
The relative amounts of fatty acids present in the plasma of horses may also play an
important role in regulating leukotriene production. There is some evidence that substrate
concentration can enhance or inhibit the production of these eicosanoids. Saku et al. 76
showed that LTB5 production was enhanced when rat alveolar macrophages were cultured in
low concentrations of EPA (2.5f.!M-15 f.!M), but when the concentration of EPA increased
(20f.!M), LTB5 production decreased. LTB4 production decreased in a dose dependent
fashion when cells were incubated with EPA.
85
While dietary supplementation with (n-6) fatty acids and (n-3) fatty acids did not
affect TNF-a production by stimulated peripheral blood mononuclear cells, supplementation
with these fatty acids did enhance the production of TNF-a by stimulated pulmonary alveolar
macrophages in the current study. Feeding both (n-6) PUFA and (n-3) PUFA appeared to
equally affect the production of TNF-a by PAM. Compared to baseline, however, the horses
supplemented with (n-3) fatty acids had significantly higher levels of TNF-a production by
stimulated PAM at 6, 8 and 12 weeks of the trial. The horses fed the (n-6) fatty acid
supplement had a significant increase in TNF-a production by stimulated PAM above
baseline values at 6 weeks only.
These results are in contrast to another study performed in horses which showed a
decrease in TNF-a production by stimulated peritoneal macrophages after horses were fed a
diet enriched in a-linolenic acid for 8 weeks. 48 The investigators in the latter study obtained
peritoneal macrophages from horses before and after they were supplemented with dietary
a-linolenic acid. Cells were cultured for 6 and 24 hours with 50 ng/ml LPS. There were no
differences in TNF-a production at 6 and 24 hour incubation times.
TNF-a activity
(expressed in pg/ml) was 13,803.8 pg/ml for cells obtained before a-linolenic acid
supplementation and 1,513.6 pg/ml for cells obtained after dietary supplementation for 8
weeks. This was approximately a 10-fold decrease in activity after supplementation with a­
linolenic acid. Interestingly, when cells were cultured in media alone, i.e., nonstimulated, the
cells obtained from the horses after consuming the a-linolenic acid diet for 8 weeks had
significantly more TNF-a activity than cells obtained before initiation of the diet.
86
In the present study, alveolar macrophages were cultured with 30 ug/ml of LPS for
40 hours. The endotoxin concentration and incubation time were chosen based on results of
a previous study in dogs42 in which PGE2 production was measured in stimulated peripheral
blood mononuclear cells. The 40 hour incubation time was chosen so that PGE2 production
could be determined simultaneously with measurement of TNF-a activity from the LPS­
stimulated mononuclear cells. TNF-a activity in horses has been shown to reach maximal
levels at 24 hours after alveolar macrophages are incubated with LPS, with no additional
activity seen at 48 and ?2 hours'?? Additionally, TNF-a activity begins to plateau after
incubation of alveolar macrophages with 10 /-tg/ml of LPS, and is similar to TNF-a activity
when LPS is increased to 100 /-tg/ml. 77 Therefore, differences in incubation time and
concentration of LPS used in the present study, compared to that used by Morris et al. 48
were not considered to be important factors in the comparison of results.
One explanation for the difference in TNF-a activity between the study reported here
and the Morris et al. study48 may be related to the level of LPS-induced PGE2 production by
the pulmonary alveolar macrophages. High levels of PGE2 can diminish TNF-a production by
affecting cellular levels of cAMP, whereas low levels of PGE2 stimulate cGMP production,
which leads to an increase in TNF-a production. 39 Feeding dietary {n-3} PUFA has been
shown to decrease production of PGE2 by peripheral blood mononuclear cells in dogs. 42
Peripheral blood mononuclear cells from three groups of dogs that were fed diets with a low,
medium or high content of (n-3) fatty acids for 12 weeks were stimulated with 30 /-tg/ml of
LPS and incubated for 40 hours. Dogs consuming the diet high in (n-3) fatty acids showed a
87
significant decrease in PGE2 production (P=O.04) compared to dogs consuming the diet low
in (n-3) PUFA. PBMC from dogs fed the high (n-3) fatty acid supplement also produced less
PGE2 than PBMC from the dogs consuming the medium dietary level of (n-3) fatty acids
(P=O.07). In the current study, the LPS-stimulated pulmonary alveolar macrophages in the
horses fed the (n-3) fatty acid supplement may have produced lower levels of PGE2 such that
TNF-a production was enhanced. Analysis of PGE2 production by the stimulated pulmonary
alveolar macrophages is pending, and data from this assay will help answer this question.
Horses fed the (n-6) PUFA supplement showed enhanced (although not significant,
P=O.09) phagocytosis of latex beads by pulmonary alveolar macrophages compared to
horses fed the (n-3) PUFA supplement at 12 weeks. At 12 weeks, horses fed the (n-6) fatty
acid supplement also showed a significant increase (P=O.03) in phagocytic activity compared
to week zero, whereas the horses fed the (n-3) fatty acid supplement did not. These results
agree with results from a study done by Calder et al. 10 in which they showed that the degree
of unsaturation of fatty acids in culture with macrophages affected subsequent phagocytic
activity of those cells. Phagocytic activity of murine macrophages increased after being
cultured with highly polyunsaturated fatty acids. Cells cultured in linoleic acid plus bovine
serum albumin (BSA) had increased phagocytic activity by 45% compared to control cells
cultured in BSA alone. Phagocytic activity of cells cultured in BSA plus linolenic acid or BSA
plus AA (both more highly unsaturated than linoleic acid) were 48% and 54% greater than
control cells cultured in BSA alone. Although EPA and DHA have a higher degree of
unsaturation compared to AA, macrophages cultured with these fatty acids had lower
88
phagocytic activity than AA cultured cells (26% and 35% greater than control cells cultured in
BSA alone, respectively).
In the present study, a similar relation existed between the type of PUFA fed to
horses and phagocytic activity of pulmonary alveolar macrophages. The horses fed the (n-3)
fatty acid supplement had higher plasma levels of EPA and DHA and lower phagocytic
activity of PAM than horses fed the (n-6) fatty acid supplement. Additionally, horses fed the
(n-6) fatty acid supplement had higher plasma levels of linoleic acid and higher phagocytic
activity of PAM than the horses fed the (n-3) fatty acid supplement.
The horses' response to a DTH skin test following KLH administration, after
consuming an (n-6) PUFA or an (n-3) PUFA supplement for 12 weeks, was unexpected.
Wander et al.42 had previously shown in dogs that high dietary intake of (n-3) PUFA
suppresses the DTH response to intradermal injection of KLH antigen. The diameter of
induration was significantly smaller in dogs supplemented with a high level of (n-3) PUFA at
24 and 48 hours compared to dogs consuming medium or low levels of an (n-3) PUFA
supplement. In this study, the DTH skin test following KLH administration was suppressed in
horses fed the (n-3) PUFA supplement (at only one of the three sites measured) at 30
minutes and 24 hours. The horses fed the (n-6) fatty acid supplement showed a significantly
increased response to intradermal injection of KLH when measured at the tip of the ear
(P=0.02) 30 minutes after the injection was given. This group of horses continued to have an
increased response at this site at 24 hours compared to the horses fed the (n-3) fatty acid
supplement (P=0.04). Because this reaction began at 30 minutes and subsided after 48
89
hours, it was not considered a typical DTH response. These results were more consistent
with an inflammatory response to KLH.
In a previous study by Hodgin et a1. 78 , KLH was used to evaluate DTH responses in
horses. Results of that study were similar to the present study in that the horses' reactions to
KLH began early (4 hours) and subsided after 24 hours. The investigators of that study
suggested that the DTH skin response of horses to KLH may be a combination of both
humoral and cellular mechanisms.
In the current study, the horses' humoral immune
response was evaluated by measuring the production of antibodies to KLH. IgG antibody
titers were not significantly different between groups of horses in this study. A more definitive
test of humoral immunity would be to measure additional classes of immunoglobulin. In
particular, measurement of IgE antibody titers would help define whether the horses'
reactions to KLH was related to an immediate hypersensitivity reaction, because this
antibody plays a role in mediating this type of reaction. Others have shown that unsaturated
fatty acids can decrease the production of IgG, IgM and IgA while enhancing production of
IgE.14 In their study, Yamada et al. 14 compared the effects of culturing rat lymphocytes with
different PUFA on the production of IgE antibodies and found that AA enhanced IgE
production by rat mesenteric lymph node lymphocytes more so than EPA but less so than
DHA.
It is usually argued that increased intake of PUFA should be accompanied by an
increased intake of a-tocopherol in order to prevent increased lipid peroxidation. The horses
in this study received a vitamin/mineral supplement that provided 800 IU of a-tocopherol/day
to each horse. Plasma a-tocopherol measurements in the horses showed that they had
90
plasma concentrations of a-tocopherol that exceeded 2 J.lg/ml, which is considered an
adequate level for the horse.79 The plasma a-tocopherol concentrations were expressed in
both J.lg/ml (see Appendix) and J.lmol/mmol of cholesterol and triglyceride as the latter
provides the most accurate measure of plasma a-tocopherol concentrations. BO Interestingly,
a-tocopherol concentrations increased in these horses even though the effects of the dietary
PUFA supplements on total lipid concentrations was taken into account.
Dietary
0,­
tocopherol supplementation of BOO IU/day increased plasma a-tocopherol in both groups of
horses regardless of the changes in total lipids caused by dietary supplementation with
PUFA.
The increase in plasma cholesterol levels noted in the horses fed the (n-6) fatty acid
supplement can be explained by the high concentrations of plasma linoleic acid in these
horses.
Genera"y, dietary (n-6) PUFA lower plasma cholesterol concentrations.
For
example, AA has greater cholesterol-lowering properties than linoleic acid.74 Because
linoleic acid remained in high concentrations in the plasma and was not converted to AA,
plasma cholesterol was elevated in the horses.
The horses fed the (n-3) fatty acid
supplement tended to have elevated plasma cholesterol levels as well. Because 1B:3(n-3)
fatty acids inhibit the metabolism of 1B:2(n-6) fatty acids by decreasing the activity of the L\ 6
desaturase, which converts linoleic acid to AA, here again the increase in linoleic acid may
be the reason why an elevation in plasma cholesterol was seen in horses consuming the (n­
3) fatty acid supplement. 74 Plasma triglyceride concentrations were not Significantly different
between the two groups of horses nor were there any significant changes in triglyceride
concentrations within either group of horses.
91
In summary, the horses readily ate the corn oil and fish oil supplements and no
significant side effects were noted. The plasma fatty acid profiles of the horses were
significantly altered by dietary PUFA supplementation by 6 weeks and changes disappeared
within 4 weeks of discontinuing the PUFA supplements. Dietary supplementation with (n-6)
and (n-3) fatty acids modulated the inflammatory response of normal horses in several ways.
Both fatty acid supplements increased the production of the proinflammatory cytokine TNF-a,
whereas only the (n-3) PUFA increased the production of the proinflammatory eicosanoid
LTB4. Production of the less inflammatory eicosanoid LTB5 was also increased by dietary
(n-3) PUFA supplementation. The ratio of plasma EPA to AA concentrations corresponded
to the ratio of LTB5 to LTB4 produced by equine neutrophils. Phagocytic activity was
increased by dietary (n-6) PUFA but not by dietary (n-3) PUFA. The immediate inflammatory
response to a DTH skin test was suppressed by dietary (n-3) PUFA supplementation, but not
by dietary (n-6) PUFA supplementation, and neither PUFA had an effect on the antibody
response to KLH.
Thus, this study showed that dietary PUFA can modulate the inflammatory response
of normal horses. However, it is not clear whether supplementation with (n-3) PUFA is more
anti-inflammatory compared to supplementation with (n-6) PUFA. The cell-mediated immune
response and humoral immunity to KLH does not appear to be affected by PUFA
supplementation in the horse. Additional work needs to be done in order to answer some of
the questions that have arisen from unexpected results in the current study. Firstly, because
horses supplemented with a diet high in linoleic acid do not convert much of this substrate to
AA, investigation into how the horse metabolizes PUFA (elongates and desaturates) is
92
warranted. Secondly, the relationship between EPA and AA as substrates for LTB5 and LTB4
production by equine neutrophils needs to be examined further. If the ratio of LTB5 to LTB4
concentrations is important in determining how inflammatory processes are mediated, then
investigations should be conducted that will answer such questions as: Is there an optimal
ratio of 5-series LT to 4-series LT? and What dietary sources of PUFA contain sufficient
quantities of EPA and AA to produce these ratios in the horse?
93
REFERENCES
1. Robinson NE. Current Therapy in Equine Medicine. 4th ed. ed. Philadelphia: WB
Saunders, 1997.
2. Smith BP. Large Animal Internal Medicine: diseases ofhorses, cattle, sheep and goats.
2nd ed. ed. St. Louis: Mosby-Yearbook Inc., 1996.
3. Calder PC. N-3 polyunsaturated fatty acids and immune cell function. Adv Enzyme
Regu/1997;37:197-237.
4.Calder PC. Dietary fatty acids and the immune system. Nutr. Rev. 1998;56:s70-s83.
5. James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and
inflammatory mediator production. Am J Clin Nutr 2000;71 :343S-8S.
6. Peck M. Interaction of lipids with immune function I: Biochemical effects of dietary lipids
on plasma membranes. Journal ofNutritional Biochemistry 1994;5:466-478.
7. Endres S, Meydani SN, Dinarello CA. Effects of omega 3 fatty acid supplements on ex
vivo synthesis of cytokines in human volunteers. Comparison with oral aspirin and
ibuprofen. World Rev Nutr Diet 1991 ;66:401-6.
8. Meydani SN, Endres S, Woods MM, et al. Oral (n-3) fatty acid supplementation
suppresses cytokine production and lymphocyte proliferation: comparison between
young and older women. J Nutr1991;121:547-55.
9. Sasaki T, Kanke Y, Kudoh K, et al. Dietary n-3 Polyunsaturated fatty acid and status of
immunocompetent cells involved in innate immunity in female rats. Annals of Nutrition
and Metabolism 2000;44:38-42.
10. Calder PC, Bond JA, Harvey DJ, et al. Uptake and incorporation of saturated and
unsaturated fatty acids into macrophage lipids and their effect upon macrophage
adhesion and phagocytosis. Biochem J 1990;269:807-14.
11. Calder PC. Dietary fatty acids and lymphocyte functions. Proc Nutr Soc 1998;57:487­
502.
12. Fritsche K, Johnston P. Effect of dietary omega-3 fatty acids on cell-mediated cytotoxic
activity in BALB/C mice. Nutrition Research 1990;10:pp.S77-588.
94
13. Morrow WJ, Ohashi Y, Hall J, et al. Dietary fat and immune function. I. Antibody
responses, lymphocyte and accessory cell function in (NZB x NZW)F1 mice. J Immunol
1985;135:3857-63.
14. Yamada K, Hung P, Yoshimura K, et al. Effect of unsaturated fatty acids and
antioxidants on immunoglobulin production by mesenteric lymph node lymphocytes of
Sprague-Dawley rats. J Biochem (Tokyo) 1996;120:138-44.
15. Bang HO, Dyerberg J, Nielsen AB. Plasma lipid and lipoprotein pattern in Greenlandic
West-coast Eskimos. Lancet 1971;1:1143-5.
16. Kremer JM, Robinson DR. Studies of dietary supplementation with omega 3 fatty acids
in patients with rheumatoid arthritis. World Rev Nutr Diet 1991 ;66:367-82.
17. Kremer JM, Bigauoette J, Michalek AV, et a/. Effects of manipulation of dietary fatty
acids on clinical manifestations of rheumatoid arthritis. Lancet 1985;1 :184-7.
18. Schwartz J. Role of polyunsaturated fatty acids in lung disease. Am J Clin Nutr
2000;71 :393S-6S.
19. Schwartz J, Weiss ST. Dietary factors and their relation to respiratory symptoms. The
Second National Health and Nutrition Examination Survey. Am J Epidemiol
1990;132:67-76.
20. Koch T, Duncker HP, Klein A, et al. Modulation of pulmonary vascular resistance and
edema formation by short-term infusion of a 10% fish oil emulsion [see comments].
Infusionsther Transfusionsmed 1993;20:291-300.
21. Murray MJ, Kumar M, Gregory TJ, et al. Select dietary fatty acids attenuate
cardiopulmonary dysfunction during acute lung injury in pigs. Am J Physiol
1995;269:H2090-9.
22. Harris PA. Developments in equine nutrition: comparing the beginning and end of this
century. J Nutr 1998;128:2698S-2703S.
23. Thomas HS. Flax: An aid in preventing founder. Anvil Magazine, 2000;pp 28-31.
24. Neelley KA, Herthel DJ. Essential fatty acid supplementation as a preventative for
carbohydrate overload-induced laminitis. American Association of Equine Practioners
Proceedings 1997;367-369.
25. Hintz HF, Schryver HF. Digestibility of Various Sources of Fat by Horses. Cornell
Nutrition Conference for Feed Manufacturers 1989;144-148.
95
26. Julen TR, Potter GD, Greene LW, et al. Adaptation to a fat-supplemented diet by
cutting horses. Proceedings of the Fourteenth Equine Nutrition and Physiology
Symposium 1995;56-61.
27. Meyer H, Flothow C, Radicke S. Preileal digestibility of coconut fat and soybean oil in
horses. Proceedings of the Fourteenth Equine Nutrition and Physiology Symposium
1995;78-79.
28. Swinney DL, Potter GD, Greene LW, et al. Digestion of fat in the equine small and
large intestine. Proceedings of the Fourteeth Equine Nutrition and Physiology
Symposium 1995;30-35.
29. Harris PA, Pagan JD, Crandell KG, et al. Effect of feeding thoroughbred horses a high
unsaturated or saturated vegetable oil supplemented diet for 6 months following a 10
month fat acclimation. Equine Vet J Supp/1999;30:468-74.
30. Geelen SN, Sioet van Oldruitenborgh-Oosterbaan MM, Beynen AC. Dietary fat
supplementation and equine plasma lipid metabolism. Equine Vet J Suppl
1999;30:475-8.
31. Henry MM, Moore IN, Feldman EB, et al. Effect of dietary alpha-linolenic acid on
equine monocyte procoagulant activity and eicosanoid synthesis. Circ Shock
1990;32:173-88.
32. Morris DD, Henry MM, Moore JN, et al. Effect of dietary linolenic acid on endotoxin­
induced thromboxane and prostacyclin production by equine peritoneal macrophages
[published erratum appears in Circ Shock 1990 Feb;30(2}:183]. Circ Shock
1989;29:311-8.
33. Janeway CA, Travers P, Walport M, et al. Immunobiology: The immune system in
health and disease. 4th ed. ed. New York: Garland Publishing, 1999.
34. Tizard IR. Veterinary Immunology: An Introduction. 6th ed. ed. Philadelphia: WB
Saunders, 2000.
35. Reed SM. Equine Internal Medicine. Philadelphia: WB Saunders Co., 1998.
36. Fairbairn SM, Lees P, Page CP, et al. Duration of antigen-induced
hyperresponsiveness in horses with allergic respiratory disease and possible links with
early airway obstruction. J. vet. Pharmacal. Therap. 1993;16:469-476.
37. Halliwell RE, McGorum BC, Irving P, et al. Local and systemic antibody production in
horses affected with chronic obstructive pulmonary disease. Vet Immunol
Immunopatho/1993;38:201-15.
96
38. Mathews CK, Van Holde KE. Biochemistry. 2nd ed. ed.
Benjamin/Cummings Publishing Co. Inc., 1995.
Menlo Park:
39. Calder PC. n-3 polyunsaturated fatty acids and cytokine production in health and
disease. Ann Nutr Metab 1997;41 :203-34.
40. Peck M. Interactions of lipids with immune function II: Experimental and clinical studies
of lipids and immunity. Journal of Nutritional Biochemistry 1994;5:514-521.
41. Virella G, Kilpatrick JM, Rugeles MT, et at Depression of humoral responses and
phagocytic functions in vivo and in vitro by fish oil and eicosapentanoic acid. Clin
Immunollmmunopatho/1989;52:257-70.
42. Wander RC, Hall JA, Gradin JL, et al. The ratio of dietary (n-6) to (n-3) fatty acids
influences immune system function, eicosanoid metabolism, lipid peroxidation and
vitamin Estatus in aged dogs. J Nutr 1997;127:1198-205.
43. Lim BO, Yamada K, Hung P, et al. Effects of n-3 polyunsaturated fatty acids and lectins
on immunoglobulin production by spleen Iympocytes of sprague dawley rats. Biosci.
Biotech. Biochem. 1996;60:1025-1027.
44. Fritsche K, Cassity N, Huang S-C. Effect of dietary fat source on antibody production
and lymphocyte proliferation in chickens. Poultry Science 1990;70:611-617.
45. Jolly CA, Jiang YH, Chapkin RS, et al. Dietary (n-3) polyunsaturated fatty acids
suppress murine Iymphoproliferation, interleukin-2 secretion, and the formation of
diacylglycerol and ceramide. J Nutr 1997;127:37-43.
46. Wallace FA, Yaqoob P, Miles EA, et al. Dietary fat influences the production of Th1­
but not Th2-derived cytokines. Lipids 1999;34:s141.
47. Endres S, Ghorbani R, Kelley VE, et al. The effect of dietary supplementation with n-3
polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor
by mononuclear cells. NEngl J Med 1989;320:265-71.
48. Morris DD, Henry MM, Moore IN, et al. Effect of dietary alpha-linolenic acid on
endotoxin-induced production of tumor necrosis factor by peritoneal macrophages in
horses. American Journal of Veterinary Research 1991 ;52:528-532.
49. Vance DE, Vance J. Biochemistry of Lipids, Lipoproteins and Membranes In: G.
Bernardi, ed: Elsevier, 1996.
50. Marr KA, Lees P, Page CP, et al. Inhaled leukotrienes cause bronchoconstriction and
neutrophil accumulation in horses. Res Vet Sci 1998; 64:219-24.
97
51. Wu 0, Meydani SN, Meydani M, et al. Immunologic effects of marine- and plant­
derived n-3 polyunsaturated fatty acids in nonhuman primates. Am J Clin Nutr
1996;63:273-80.
52. Whelan J, Surette ME, Hardardottir I, et al. Dietary arachidonate enhances tissue
arachidonate levels and eicosanoid production in Syrian hamsters. J Nutr
1993;123:2174-85.
53. Broughton KS, Johnson CS, Pace BK, et al. Reduced asthma symptoms with n-3 fatty
acid ingestion are related to 5-series leukotriene production. Am J Clin Nutr
1997;65:1011-7.
54. Samuelsson B, Haeggstrom JZ, Wetterholm A. Leukotriene biosynthesis. Ann N Y
Acad Sci 1991 ;629:89-99.
55. Lewis RA, Austen KF, Soberman RJ. Leukotrienes and other products of the
5-lipoxygenase pathway. NEngJ J Med 1990;323:645-655.
56. Sperling RI. The effects of dietary n-3 polyunsaturated fatty acids on neutrophils. Proc
Nutr Soc 1998;57:527-34.
57. Terano T, Salmon JA, Moncada S. Effect of orally administered eicosapentaenoic acid
(EPA) on the formation of leukotriene B4 and leukotriene B5 by rat leukocytes.
Biochem PharmacoJ1984;33:3071-6.
58. Vaughn 0, Reinhart G, Swaim S, et al. Evaluation of effects of dietary n-6 to n-3 fatty
acid ratios on leukotriene B synthesis in dog skin and neutrophils. Veterinary
Dermatology 1994;5:163-173.
59. Kragballe K, Voorhees JJ, Goetzl EJ. Inhibition by leukotriene B5 of leukotriene B4­
induced activation of human keratinocytes and neutrophils. J Invest Dermatol
1987;88:555-8.
60. Cleland LG, James MJ, Gibson RA, et al. Effect of dietary oils on the production of n-3
and n-6 metabolites of leukocyte 5-lipoxygenase in five rat strains. Biochim Biophys
Acta 1990;1043:253-8.
61. NRC. Guide for the Care and Use of Laboratory Animals. National Institutes of Health.
Bethseda, 1985.
62. Song J, Wander RC. Effects of dietary selenium and fish oil (MaxEPA) on arachidonic
acid metabolism and homeostatic function in rats. J Nutr 1991 ;121 :284-92.
98
63. Exon JH, Bussiere JL, Mather GG. Immunotoxicity testing in the rat: an improved
multiple assay model. Int J Immunopharmacol 1990;12:699-701.
64. Amalsadvala TM, Vaughn OM. Characterization of leukotriene B4 synthesis in canine
polymorphonuclear leukocytes. Prostaglandins Leukot Essent Fatty Acids
1992;45:283-8.
65. Coligan JE, Kruisbeek AM, Margulies DH, et al. Current Protocols in Immunology. New
York: John Wiley & Sons, 1992.
66. Krakowka S, Ringler SS, Lewis M, et al. Immunosuppression by canine distemper
virus: modulation of in vitro immunoglobulin synthesis, interleukin release and
prostaglandin E2 production. Vet Immunollmmunopatho/1987;15:181-201.
67. Branch DR, Shah A, Guilbert LJ. A specific and reliable bioassay for the detection of
femtomolar levels of human and murine tumor necrosis factors. J Immunol Methods
1991 ;143:251-261.
68. Wander RC, Du SH, Ketchum SO, et al. Effects of interaction of RRR-alpha-tocopheryl
acetate and fish oil on low-density-lipoprotein oxidation in postmenopausal women with
and without hormone-replacement therapy. Am J Clin Nutr 1996;63:184-93.
69. Wander RC, Du SH, Ketchum SO, et al. alpha-Tocopherol influences in vivo indices of
lipid peroxidation in postmenopausal women given fish oil. J Nutr 1996;126:643-52.
70. Tooley KA. Dietary (n-3) and (n-6) Fatty Acids and Vitamin E: Their Effects on the
Immune Response of Healthy Geriatric Beagles. Department of Biomedical Sciences,
Col/ege of Veterinary Medicine. Corvallis: Oregon State University, 1999;140.
71. Rivers JP, Sinclair AJ, Crawford MA. Inability of the cat to desaturate essential fatty
acids. Nature 1975;258:171-173.
72. MacDonald ML, Rogers OR, Morris JG. Role of Linoleate as an essential fatty acid for
the cat independent of arachidonate sythesis. J. Nutr 1983;113:1422-1433.
73. MacDonald ML, Anderson BC, Rogers OR, et al. Essential fatty acid requirements of
cats: Pathology of essential fatty acid deficiency. Am J Vet Res 1984;45:1310-1317.
74. Horrobin OF, Manku MS. Clinical Biochemistry of Essential Fatty Acids In: A. R. Liss,
ed. Omega-6 Essential Fatty Acids: Pathophysiology and Roles in Clinical Medicine,
1990;21-53.
-------
---
--
-
99
75. Lindberg A, Tornhamre S, Mugnai S, et al. lonophore A23187-induced leukotriene
biosynthesis in equine granulocytes - neutrophils, but not eosinophils require
exogenous arachidonic acid. Biochimica et Biophysica Acta 1998;1391 :247-255.
76. Saku N, Kobayashi J, Kitamura S. Eicosapentaenoic acid modulates arachidonic acid
metabolism in rat alveolar macrophages activated by silica. Prostaglandins,
Leukotrienes and Essential Fatty Acids 1999;61 :51-54.
77. MacKay RJ, King RR, Dankert JR, et al. Cytotoxic tumor necrosis factor activity
produced by equine alveolar macrophages: preliminary characterization. Vet. Immunol
Immunopatho/1991 ;29:15-30.
78. Hodgin EC, McGuire TC, Perryman LE, et al. Evaluation of delayed hypersensitivity
responses in normal horses and immunodeficient foals. Am J Vet Res 1978;39:1161-7.
79. Craig AM, Blythe LL, Lassen ED, et al. Variations of serum vitamin E, cholesterol, and
total serum lipid concentrations in horses during a 72-hour period. Am. J. vet. Res.
1989;50:1527-1532.
80. Thurnham 01, Davies JA, Crump BJ, et al. The use of different lipids to express serum
tocopherol: lipid ratios for the measurement of vitamin E status. Ann Clin Biochem 186;
23:514-20.
100
APPENDIX Table 1. Effect of feeding diets that differed in the (n-6) and (n-3) fatty acid content on plasma fatty acid profiles of horses after 6, 8, and 12 weeks compared to baseline (0 weeks).1 oweeks
14:1
16:0
16: 1(n-7)
18:0
18:1 (n-9)
18:2(n-6)
20:0
18:3{n-3)
20:1 (n-9)
20:2(n-6)
22:0
20:4{n-6)
22: 1(n-9)
20:3(n-6)
20:5(n-3)
24:0
6 weeks
(n-6)
(n-3)
0.17 ± 0.09
15.16±0.45
1.21 ±0.14
15.55 ± 0.45
16.34 ± 0.98
37.74 ± 2.65
0.39 ±0.06
2.44 ±0.65
0.39 ±0.03
0.30 ±0.08
0.37±0.12
0.75 ±0.31
0.49 ± 0.29
0.04 ±0.02
0.21 ± 0.06
0.19 ± 0.02
0.13 ± 0.05
15.41 ±0.47
1.06±0.12
16.02 ± 0.48
15.57 ± 2.38
39.72 ± 2.30
0.46 ± 0.07
2.84 ± 0.85
0.16 ± 0.07*
0.32 ±0.03
0.44 ± 0.09
0.76 ±0.28
0.19 ± 0.16
0.26 ±0.24
0.38 ±0.14
0.18 ±0.05
(n-6)
8 weeks
(n-3)
(n-6)
0.99 ±0.37
0.76 ±0.04
2.28 ± 1.23
0.11 ± 0.05
0.09 ±0.04
0.11 ± 0.04
11.92 ± 0.25+ 14.91 ± 0.77* 11.29 ± 0.46 t
0.60±0.10'f 1.96±0.07*l' 0.48 ± 0.08 t
16.06 ±0.46
15.43 ± 0.60
16.02 ± 0.55
8.14 ±0.39 + 9.30 ± 0.16*+ 7.50 ± 0.21 +
49.04 ± 1.01 + 26.84 ± 1.04*+ 48.97 ± 0.67 +
0.40 ± 0.04
0.34 ±0.02
0.35 ± 0.01
0.85 ± 0.11+
1.19±0.12
0.91 ± 0.22
0.24 ± 0.03 'f
0.12±0.07
0.33 ±0.07
0.32 ± 0.03
0.30 ±0.05
0.18 ± 0.04*+
0.32 ± 0.03
0.28 ± 0.06
0.70 ± 0.074
0.68 ± 0.11
0.98 ±0.32
3.85 ± 0.23*+
0
0.05 ±0.03
0.04 ±O.O1
0.10±0.02
0.09 ± 0.04
0.39±0.11*
0.38 ± 0.12
0.35 ±0.06
9.06 ± 0.46 *l'
0.44 ± 0.20
0.46±0.13
0.31 ±0.10
12 weeks
(n-3)
(n-6)
(n-3)
0.80 ± 0.04
0.16 ±0.07
15.01 ± 0.93 *
1.99±0.11*+
14.80 ± 0.51
9.11 ±0.10*+
26.80 ± 0.77*+
0.27 ± 0.07
0.96 ± 0.13
0.40 ± 0.03 +
0.34 ± 0.06
0.37 ±0.17
0.14 ±0.07
13.75 ± 2.35
0.44 ± 0.07 +
18.62 ± 1.68
9.77 ± 1.82 +
47.47 ± 4.05
0.33 ±0.09
0.50 ± 0.27 l'
0.25 ±0.03
0.49 ± 0.03 +
0.17±0.10
0.49 ± 0.23
0.34 ± 0.22
0.03 ±0.02
0.31 ± 0.08
0.22 ±0.04
0.87 ± 0.19
0.22 ± 0.10
16.66 ± 0.74
0.73 ± 0.07*+
3.68±0.16*l'
0.11 ± 0.07
0.23 ±0.06
8.61 ± 0.33*+
0.49±0.14
2.18 ± 0.27*+
17.48 ± 0.97
10.61 ± 0.53
29.88 ± 1.59*+
0.72 ± 0.27
0.73 ± 0.33 +
0.27 ± 0.09
0.27 ± 0.08*
0.67 ± 0.18*
4.09 ± 0.25*+
0.22 ± 0.16
0.15 ± 0.12
8.48 ± 1.11*+
0.38 ± 0.12
­
-
o
Table 1Continued. Effect of feeding diets that differed in the (n-6) and (n-3) fatty acid content on plasma fatty acid profiles of horses after 6, 8
and 12 weeks compared to baseline (0 weeks).1
oweeks
(n-6)
6 weeks
(n-3)
fatt acids
0.30 ± 0.03
0.26 ± 0.06
8 weeks
12 weeks
(n-6)
(n-3)
(n-6)
(n-3)
(n-6)
(n-3)
0.39 ± 0.02 t
0.65 ± 0.06*1
0.47 ± 0.09
0.93 ±0.13*1
0.29 ±0.09
0.60 ± 0.17
0.16 ± 0.02
0.17 ± 0.03
30.13 ± 1.7 'f
0.86 ± 0.06*1
5.62 ± 0.22*1
0.16±0.03
0.30 ± 0.15
0.05 ± 0.03
0.15 ± 0.06
0.52 ± 0.21
32.60 ± 0.53*
12.38 ± 0.10*
30.52 ± 1.75
0.88 ± 0.03*1
5.97 ± 0.28*1
32.12±0.74
22:6{n-3)
0.10 ± 0.04
0.11 ± 0.02
0.15 ± 0.04
0.11 ± 0.02
1:SFA2
1:MUFA3
33.99 ± 1.57
18.90 ± 1.01
33.73 ± 0.83
17.36 ± 2.40
1:PUFA4
1:n-65
41.68 ± 2.24
38.82 ± 2.46
44.53 ± 1.49
41.06 ± 212
1:n-36
L(n-6): L(n-3)
,
2.86 ±0.73
13.57 ± 4.11
3.47 ± 0.86
1.57 ± 0.23
11.82 ± 2.00
32.02 ±0.52
9.48 ± 0.43 t
51.70 ± 0.80 1
48.00 ± 0.89*
50.14 ± 0.98 + 31.27 ± 1.16*+
16.73 ± 0.66*1
8.93 ± 0.34 1
52.05 ± 0.47 +
50.33 ± 0.48 +
1.72 ±0.26
29.31 ± 0.46
5.11 ± 0.37*1
36.79 ± 1.10
14.10±0.82
11.23 ± 1.88 +
49.48 ±4.25
49.23 ± 2.36
47.47 ± 0.53*
31.06 ± 0.734 48.48 ±4.15
33.52 ± 1.76*1
12.70 ± 0.27*
16.41 ± 0.66*'f
1.87 ± 0.55 *'f
1.89 ± 0.55*1
Values are means ± SEM
2Sum of the saturated fatty acids: 14:0 + 16:0 + 18:0 + 20:0 +22:0 + 24:0
3Sum of the monounsaturated fatty acids: 14:1 + 16:1(n-7) + 18:1(n-9) + 20:1 (n-9) + 22:1(n-9) + 24:1
4Sum of the polyunsaturated fatty acids: 18:2(n-6) + 20:2(n-6) + 20:3(n-6) + 20:4(n-6) + 18:3(n-3) + 20:5(n-3) + 22:5(n-3) + 22:6(n-3)
5 Sum of the (n-6) fatty acids
5 Sum of the (n-3) fatty acids
* Denotes asignificant difference between group means at that timepoint (P-value s 0.05)
T Denotes aSignificant change from week zero in the (n-6) fatty acid supplemented horses (PSO.05)
T Denotes aSignificant change from week zero in the (n-3) fatty acid supplemented horses (PSO.05)
1
33.46 ±3.83
1.00 ± 0.20 t
48.36 ± 1.00t
14.84 ± 1.59*f
2.26 ± 0.84*'f
Table 2. Effect of feeding diets that differed in the (n-6) and (n-3) fatty acid content on plasma fatty acid profiles of horses after 12 weeks
compared to baseline (0 weeks) and 4 weeks after discontinuing the fatty acid supplement (18 weeks).1
12 weeks
o weeks
(n-6)
18 weeks
(n-3)
(n-6)
(n-3)
(n-6)
(n-3)
1.24 ± 0.56
0.37 ±0.17
0.87 ± 0.19
3.36 ± 0.57
0.14 ±0.07
13.75 ±2.35
0.22 ± 0.10
16.66 ± 0.74
0.44 ±0.07f
18.62±1.68
2.18 ± 0.27*f
17.48 ± 0.97
10.61 ± 0.53
0.15 ± 0.07
14.08±0.15
1.13±0.11
4.94 ± 1.D4'f
0.26 ±0.05
14.94 ±0.88
1.20 ± 0.05
14:1
16:0
16:1{n-7)
0.17 ± 0.09
15.16 ± 0.45
1.21 ± 0.14
0.13 ± 0.05
15.41 ± 0.47
1.06 ± 0.12
18:0
18: 1(n-9)
15.55 ± 0.45
16.34 ± 0.98
16.02 ± 0.48
15.57 ± 2.38
18:2(n-6)
37.74 ± 2.65
39.72 ± 2.30
20:0
18:3{n-3)
0.39 ±0.06
2.44 ± 0.65
0.46 ± 0.07
2.84 ±0.85
20:1 (n-9)
0.39 ±0.03
0.16±0.07*
20:2(n-6)
0.30 ± 0.08
0.32 ± 0.03
22:0
20:4(n-6)
0.37 ± 0.12
0.75 ± 0.31
22: 1(n-9)
20:3(n-6)
20:5{n-3)
24:0
9.77 ± 1.82T
47.47 ±4.05
0.33 ±0.09
29.88 ± 1.59*T
0.72 ±0.27
0.50 ±0.27f
0.25 ± 0.03
0.73 ± 0.33T
0.27 ± 0.09
0.27 ±0.08*
0.44 ± 0.09
0.76 ± 0.28
0.49 ± 0.03 f
0.17±0.10
0.49 ±0.23
0.49 ± 0.29
0.04 ±0.02
0.21 ± 0.06
0.19 ± 0.16
0.26 ± 0.24
0.38 ±0.14
0.34 ± 0.22
0.03 ±0.02
0.31 ± 0.08
0.19 ± 0.02
0.18±0.05
0.22 ±0.04
0.67 ± 0.18*
4.09 ± 0.25 *f
0.22±0.16
0.15±0.12
8.48 ± 1.11*f
0.38±0.12
16.56 ± 0.36
16.18 ± 0.61
14.24 ± 1.44
13.32 ± 0.8ff
39.85 ± 1.29
34.14 ± 1.77*
0.44 ± 0.03
2.99 ± 0.55
0.46 ± 0.01
2.89 ± 0.38
0.32 ± 0.05f
0.31 ± 0.05
0.09 ±0.02f
0.31 ± 0.01
1.29 ± 0.14
0.07 ±0.01f
0.25 ±0.03
1.85 ± 0.59
0.22±0.12
0.09 ± 0.04
0.24 ±0.07
0.18±0.05
0.07 ±0.03
0.20 ±0.07
0.33 ±o.of
0.26 ± 0.02*
­
0
w
Table 2 Continued. Effect of feeding diets that differed in the (n-6) and (n-3) fatty acid content on plasma fatty acid profiles of horses after 12
weeks compared to baseline (0 weeks) and 4 weeks after discontinuing the fatty acid supplement (18 weeks).1
oweeks
12 weeks
18 weeks
(n-6)
(n-3)
(n-6)
(n-3)
(n-6)
(n-3)
0.10 ± 0.04
0.11 ± 0.02
0.26 ± 0.06
0.15 ± 0.04
0.11 ± 0.02
0.29 ±0.09
0.05 ± 0.03
0.15 ± 0.06
0.60 ±0.17
0.52 ± 0.21
0.19 ± 0.06
0.15±0.04
5.11 ±0.37*T
0.24 ± 0.04 T
0.24 ± 0.03
0.23 ± 0.14
0.34 ± 0.13
ISFA2
IMUFA3
33.99 ± 1.57
18.90 ± 1.01
33.73 ± 0.83
17.36 ± 2.40
33.46 ± 3.83
36.79 ± 1.10
14.10±0.82
35.08 ± 0.88
IPUFA4
41.68 ± 2.24
44.53 ± 1.49
In-65
38.82 ±2.46
In-36
~(n-6): ~(n-3)
11.23 ± 1.88T
49.23 ±2.36
15.33 ± 0.84 f
37.03 ± 1.57
16.44 ± 1.45
39.78 ± 1.38 *
36.12±1.56*
41.06 ± 212
49.48 ±4.25
48.48 ±4.15
33.52 ± 1.76 *T
44.93 ± 1.03
41.32 ± 1.33
2.86 ± 0.73
3.47 ± 0.86
1.00 ± 0.20 f
14.84 ± 1.59 *T
3.61 ± 0.49
3.66 ±0.37
13.57 ±4.11
11.82 ± 2.00
48.36 ± 1.00 "f
2.26 ± 0.84*T
11.45 ± 1.01
9.87 ± 1.38
1 Values are means ± SEM
2Sum of the saturated fatty acids: 14:0 + 16:0 + 18:0 + 20:0 +22:0 + 24:0
3 Sum of the monounsaturated fatty acids: 14:1 + 16:1(n-7) + 18:1(n-9) + 20:1 (n-9) + 22: 1(n-9) + 24:1
4Sum of the polyunsaturated fatty acids: 18:2(n-6) + 20:2(n-6) + 20:3(n-6) + 20:4(n-6) + 18:3(n-3) + 20:5(n-3) + 22:5(n-3) + 22:6(n-3)
5 Sum of the (n-6) fatty acids
6 Sum of the (n-3) fatty acids
* Denotes asignificant difference between group means at that timepoint (p-value s 0.05)
T Denotes asignificant change from week zero in the (n-6) fatty acid supplemented horses (pSO.05)
T Denotes asignificant change from week zero in the (n-3) fatty acid supplemented horses (pSO.05)
-
~
Table 3. Effect of feeding diets that differed in the (n-6) and (n-3) fatty acid content on the delayed type hypersensitivity skin test in horses at
30 minutes, 24, 48, 72 and 96 hours.1
Diets enriched in (n-6)
or (n-3) fatty acids
Location
(n-6)
(n-3)
Neck (mm)2
PvalueS
I
(n-6)
(n-3)
Ear base (mm2)3
P value 5
(n-6)
(n-3)
Ear tiR (mm2}4
Pvalue5
Time: 30 minute
16.9 ± 0.6
14.8±1.0
0.10
95.0±11.3
85.1±10.5
0.55
92.8 ± 7.3
66.1 ±4.0
0.02
Time: 24 hour
69.7 ±4.2
89.2 ± 8.1
0.06
147.0 ± 12.7
140.9 ±9.0
0.72
101.2 ± 5.6
79.5 ±6.2
0.04
Time: 48 hour
52.1 ± 9.9
40.3 ± 11.1
0.46
161.3 ± 7.6
162.6±4.7
0.89
104.6 ± 6.1
124.6 ± 7.6
0.08
Time: 72 hour
37.0 ± 13.2
17.0 ± 0.8
0.22
154.2 ± 7.9
164.0 ± 11.3
0.49
104.8 ± 7.8
114.3±14.0
0.55
0.54
0.43
Time: 96 hour
0.58
15.5 ± 0.8
14.6 ± 6.0
127.7 ± 14.0
141.1 ±2.0
98.7 ± 18.9
113.1 ± 14.7
1 Values are means ± SEM
2 Neck wheal diameter measured in millimeters (mm); values represent the average of three sites/animal; n=5 animals in the (n-6) FA enriched diet group; n=4 animals in
the (n-3) FA enriched diet group
3Ear wheal diameter (mm) at the base of the ear multiplied by the ear thickness in mm (the ear thickness was determined by subtracting thickness at time 0from
thickness at the specified time)
4 Ear wheal diameter (mm) at the tip of the ear multiplied by the ear thickness in mm (the ear thickness was determined by subtracting thickness at time 0from thickness
at the specified time)
5 p values for comparison between group means at each timepoint
o
Ul
Table 4. Effect of feeding diets that differed in the (n-6) and (n-3) fatty acid content on the keyhole limpet hemocyanin (KLH)
antibody log titers in horses after vaccination with KLH.1
Log TIter
1
(n-6) Fatty acid enriched diet
(n-3) Fatty acid enriched diet
P value
3.75±O.16
3.97 ±O.17
0.408
Values are means ± SEM
o
0'\
Table 5. Effect of feeding diets that differed in (n-6) and (n-3) fatty acid content on leukotriene B4 and leukotriene Bs production
by horse peripheral blood neutrophils stimulated with calcium ionophore A23187; and the ratio of LTBs to LTB4 at 12 weeks
compared to baseline (0 weeks).1
oweeks
12 weeks
(n-6)
(n-3)
p-value3
(n-6)
(n-3)
p value3
LTB5 in pg/m12
23.4 ± 13.4
19.5 ± 11.3
0.83
86.9 ±31.1
1528 ± 448.0 T
0.01
LTB4 in pg/m12
207.1 ± 76.0
131.8 ±4S.S
0.42
374.4 ± 136.6
1246 ± 264.3 T
0.02
LTB5:LTB4 ratio
0.07 ±O.03
0.12 ±O.O5
0.44
0.28±O.12
1.13±O.14T
0.002
Values are means ± SEM
2 Endogenous leukotriene production by unstimulated cell samples was subtracted from each stimulated cell sample.
3 Pvalue for comparison between group means at that timepoint
1
f Denotes asignifICant difference from week 0 in the horses supplemented with (n-3) fatty acids (psO.05)
-
o
-.I
Table 6. Effect of feeding diets that differed in the (n-6) and (n-3) fatty acid content on TNF-a production by pulmonary alveolar macrophages
(PAM) and peripheral blood mononuclear cells (PBMC) from horses after 6,8, and 12 weeks compared to baseline (0 weeks).1
oweeks
(n-6)
6 weeks
(n-3)
Pvalue2
(n-6)
8 weeks
(n-3)
Pvalue 2
(n-6)
12 weeks
(n-3)
Pvalue2
(n-6)
(n-3)
P value2
% L sis of L929 cells
PAM
78.6 ±2.2
74.9 ± 1.0
0.1
88.2 ± 1.91 83.9 ± 1.01
0.08
80.9 ± 3.7 81.1 ± 2.31
PBMC
0.91
0.23 41.1 ± 11.0 42.8 ± 8.6
14.4 ± 6.6 26.7 ± 6.8
20.0 ±4.3
Values are means ± SEM 2 Pvalue for the comparison between group means at that timepoint 1 Denotes asignificant change from week zero in the (n-6) fatty acid supplemented horses (pSO.OS) 1 Denotes asignificant change from week zero in the (n-3) fatty acid supplemented horses (pSO.OS) 13.1 ± 5.3
0.96
84.0 ± 2.1 81.9±5.0
0.71
0.34
28.5 ± 10.5 35.7 ± 4.8
0.55
1
­
oOQ
Table 7. Effect of feeding diets that differed in the (n-6) and (n-3) fatty acid content on fluorescent bead engulfment by pulmonary alveolar
macrophages (PAM) from horses after 6,8 and 12 weeks compared to baseline (0 weeks).1
Time
Diets enriched in (n-6)
or n-3 fa acids
12 weeks
(n-6)
(n-3)
Pvalue3
(n-6)
(n-3)
Pvalue3
(n-6)
(n-3)
Pvalue3
(n-6)
(n-3)
Pvalue3
%
PAM
17.6±1.7 16.6±2.5
0.74
15.0±2.314.3±2.0 0.81
20.0±1.5 18.1 ±1.9
1 Values are means ± SEM
2 Percent phagocytosis =cell population that engulfed fluorescent beads + total population X 100
3 Pvalue for the comparison between group means at that timepoint
f Denotes asignificant change from week zero in the (n-6) fatty acid supplemented horses (p-value S 0.05)
0.45
26.6±3.0i' 19.2±2.5
0.09
­
o
1.0
Table 8. Effect of feeding diets that differed in the (n-6) and (n-3) fatty acid content on plasma lipid concentrations (cholesterol and triglyceride) of
horses after 6,8, and 12 weeks compared to baseline (0 weeks). 1
Time
Diets enriched in (n-6) or
(n-3) fatty acids
oweeks
(n-6)
(n-3)
6 weeks
Pvalue2
Cholesterol (mg/100 ml) 79.8 ± 7.7 75.8 ± 3.3
0.64
Triglyceride (mg/100 ml) 25.6 ±4.7 28.4 + 9.9
0.35
(n-6)
(n-3)
114.4±10.0t 108.0±6.8l
15.4 ± 3.1
16 + 1.0
8 weeks
P value2
(n-6)
(n-3)
12 weeks
Pvalue 2
0.61
103.4 ± 9.8 94.2 + 4.6l
0.42
0.86
20.0 + 2.4
0.59
18.2 + 2.2
(n-6)
(n-3)
102.2 ± 9.7 90.6 ± 7.7
17 + 3.7
19.4 + 4.0
Pvalue 2
0.38
0.67
1Values are means ± SEM
2p value for comparison between group means at each timepoint
f Denotes asignificant change from week zero in the (n-6) fatty acid supplemented horses
f Denotes asignificant change from week zero in the (n-3) fatty acid supplemented horses
-
,...
o
Table 9. Effect of feeding diets that differed in the (n-6) and (n-3) fatty acid content on plasma a-tocopherol concentrations of horses after 6,
8 and 12 weeks compared to baseline (0 weeks).1
Time
Diets enriched in (n-6) or
(n-3) fatty acids
oweeks
(n-6)
(n-3)
6 weeks
Pvalu&
(n-6)
(0-3)
12 weeks
Bweeks
Pvalue2
(n-6)
(n-3)
Pvalue2
(n-6)
(n-3)
P value2
4.3±0.7 2.8 ±OA
0.11
7.7±1.4 5.0±0.31
0.10
7.5±1.3 5.2±0.5 t
0.14
7.0 ± 1.0 4.B ±0.4 1
0.07
a-tocopherol (fJ.ffiolfmmol)3 4.4 ±0.7 2.B±0.3
0.11
5.6±0.B 3.9±O.21
0.08
5.B±O.7 4.5 ±OA 1
0.14
5.7 ± 0.6 4.4 ±O.3l'
O.OB
a-tocopherol (f.lg/ml)
1 Values are means ± SEM
2p value for comparison between group means at each timepoint
3 a-tocopherol
concentrations expressed relative to total plasma lipids (cholesterol and triglyceride)
T Denotes asignificant change from week zero in the (n-3) fatty acid supplemented horses
.....
.....
.....
Table 10. Effect of feeding diets that differed in the (n-6) and (n-3) fatty acid content on complete blood counts (CSC) of horses after 6,8, and 12
weeks compared to baseline (0 weeks). 1
oweeks
(n-6)
8~s
6 weeks
(n-3)
P
value2
(n-6)
(n-3)
P
value2
(n-6)
12 weeks
(n-3)
P
valu&
(n-6)
(n-3)
P
value2
Parameter
HGB3 (g/dl)
12.9 ± 0.72 12.8 ± 0.30
0.92
12.6 ± 1.39
13.1 ± 0.34
0.71
12.8±0.74
12.6 ±O.2
0.76
13.0 ± 0.24
12.4 ± 0.39
0.20
HCT4(%)
41.2± 2.46 40.0±0.84
0.64
38.9±4.61
40.0± 1.22
0.83
39.1 ±2.20
38.1 ±1.05
0.69
40.1 ±0.93
38.6± 1.02
0.31
RBGS (cells x106/ul)
8.57 ±0.53 8.14±0.20
0.46
8.09 ± 1.00
8.28 ± 0.38
0.86
8.11 ±0.48
7.87±0.25
0.66
8.22±0.18
7.95 ± 0.25
0.42
WBC6 (cellS/ul)
8740±273 7360 ± 507
0.04
10020 ± 620 8760 ± 266 T
0.09
7560 ± 211 T 8140 ± 376
0.21
10500 ± 764 10100 ±631 T
0.69
Neutrophils (cells/,...t)
5400 ±448 4089 ± 298
0.04
6054± 507
5228 ± 548
0.30
3967±271 T 4789 ± 302
0.07
6834 ±456
6347±589T
0.53
Lymphocytes (cells/JlI) 2926±474 2782± 174
0.78
3526 ± 451
3076 ± 369
0.46
3343± 363
3038 ± 173
0.47
3209± 337
3326± 473
0.84
Monocytes (celis/JlI)
232 ± 31
273 ±70
0.64
159.0±38
247±55
0.22
136± 16T
146±32
0.79
149± 28
306±122
0.30
Eosinophils (cells/Ill)
Plasma proteins (g/dl)
172± 30
218.3±64
0.28
196 ± 29
254±57
0.90
386 ± 270
239±153
0.89
187 ± 59
1oo±0
ND10
6.2 ± 0.12
6.1 ± 0.12
0.81
6.6±0.12T
6.3±0.13
0.22
6.2 ±0.13
6.0±0.18
0.43
7.0±0.141
6.9±0.20T
0.74
Fibrinogen (mgldl)
220 ± 37.4
180 ±37.4
0.47
260.0±24.5
260±24.5
1.00
280±66.3
400 ± 126.5 0.42
240 ± 24.5
260 ±51.0
0.73
MCV? (fl)
48.2±0.44 49.2 ± 1.10
0.41
48.3 ± 0.33
48.4 ± 0.93
0.92
48.3±0.45
48.5 ± 1.00
0.83
48.8 ± 0.45
48.7 ± 1.06
0.92
MCHCS (g/d!)
31.3±0.64 32.0±0.24
0.31
32.4±0.39 32.8±0.21 T
0.37
32.7 ±0.28
33.0±0.50
0.63
32.5±0.17
32.0 ± 0.36
0.28
MCH9(pg)
15.1 ±0.41 15.7±0.35
0.24
15.6 ±0.29
15.9±0.35
0.64
15.8 ±0.14
16.0 ± 0.51
0.65
15.8 ± 0.13
15.6 ± 0.48
0.64
Protein:flbrinogen
ratio
32.7 ± 7.38 40.4±8.04
0.50
26.1 ±2.23
25.2 +2.41
0.80
29.5 ±9.05
25.6 ±9.28
0.77
30.1 ±2.54
32.4± 7.89
0.78
T Denotes a significant change from week zero in the (n-6) fatty acid supplemented horses (p~0.05)
T Denotes aSignificant change from week zero in the (n-3) fatty acid supplemented horses (p~0.05)
1 Values are means ± SEM
2 Pvalue for comparison between group means at that timepoint
3 Hemoglobin
4 Hematocrit
5 Red
Blood Cells
aWhite Blood Cells
7 Mean
Corpuscular Volume
8 Mean Corpuscular Hemoglobin Concentration
9 Mean Corpuscular Hemoglobin
10 Not determined due to missing data values
- _..
_----­
.­
.­
tv
Table 11. Effect of feeding diets that differed in the (n-6) and (n-3) fatty acid content on serum biochemistries of horses after 8 and 12
weeks compared to baseline (0 weeks).1
oweeks
12 weeks
8weeks
(n-6)
(n-3)
P value2
(n-6)
(n-3)
P value2
(n-6)
(n-3)
P value2
12.0 ± 1.22
1.1 ±0.13
0.30
0.29
10.0 ± 2.30
1.2 ± 0.05
12.0 ±2.61
1.1 ±0.15
0.23
0.37
13.0±1.46
1.1 ± 0.06
14.0 ± 3.36
1.1 ± 0.11
0.58
0.34
Glucose (mgldl)
11.0 ± 0.71
1.2 ±0.13
97 ± 2.55
98± 1.94
0.86
93 ± 1.22
94 ± 2.18
0.76
94 ± 2.80
89 ± 2.44 T
1.00
Total protein (g/dl)
Albumin (g/dl)
6.3 ±0.07
2.9±0.12
6.1 ±0.11
0.07
0.72
6.2 ±0.15
3.1 ±0.07
6.1 ± 0.14
3.2 ± 0.07
0.15
0.11
6.6 ± 0.10
3.1 ± 0.12
6.5 ± 0.13
3.2 ± 0.08
0.10
0.51
Bilirubin (mgldl)
CK4 (lUlL)
1.2 ±0.15
951 ± 673
1.6 ± 0.36
250± 23.3
1.8±0.22
210 ± 16.4
0.63
0.20
1.2 ± 0.51
239 ± 19.9
1.6 ±0.25
416 ± 95.2
0.24
0.45
186± 22.4 1
0.27
0.12
GGT5(IU/L)
15.0±3.37
11.0 ±2.40
0.38
20.0 ±6.73
10.0±2.13
0.22
25 ± 5.91
14.0±2.50
0.14
ASTs (lUlL)
Sodium (mEqll)
476± 197.7
139 ± 0.51
344±59.52
138± 0.68
0.54
0.27
269 ± 11.69
138± 0.55
3.7±0.10
252 ± 13.39
138 ± 0.49
0.37
0.79
265 ± 7.10
139 ± 0.55
249 ± 21.18
139 ± 0.60
0.51
0.64
3.9 ± 0.05
0.18
4.0 ± 0.08
4.2±0.12f
0.36
101 ± 0.87
11.2±0.04
100 ± 0.66
12.0 ±0.02
0.72
0.00
102 ± 0.58
11.9 ±0.21
101 ± 0.58
12.2 ± 0.20
0.26
0.24
3.3 ±0.12
3.3 ± 0.23
0.82
Parameter
BUN3 (mgldl)
Creatinine (mg/dl)
3.0 ±0.11
1.4±0.12
Potassium (mEqll)
3.7 ± 0.27
3.7±0.16
0.90
Chloride (mEq/l)
Calcium (mgldl)
102 ± 1.17
11.7±0.26
1OO± 0.58
11.7±0.17
0.31
0.80
Phosphorus (mg/dl)
3.3 ±0.29
3.8 ± 0.07
0.10
3.0 ± 0.05
3.2±0.22f
0.55
Magnesium (mgldl)
1.9 ±0.04
1.7 ±0.04
0.03
1.7 ±0.02f
1.7±0.02
0.20
1 Denotes aSignificant change from week: zero in the (n-6) fatty acid supplemented horses
f Denotes aSignifICant change from week: zero in the (n-3) fatty acid supplemented horses
1 Values are means ± SEM
2 p value for comparison between group means at that timepoint
1.7±0.021 1.6 ±0.021
3 Blood Urea Nitrogen
4 Creatine kinase
5 Gamma glutamyl transpeptidase
6 Aspartate Aminotransferase
O.OOS
.....
--
w
-
- - - - - -