Immunology Published by TSRI Press . Copyright 2005,
advertisement
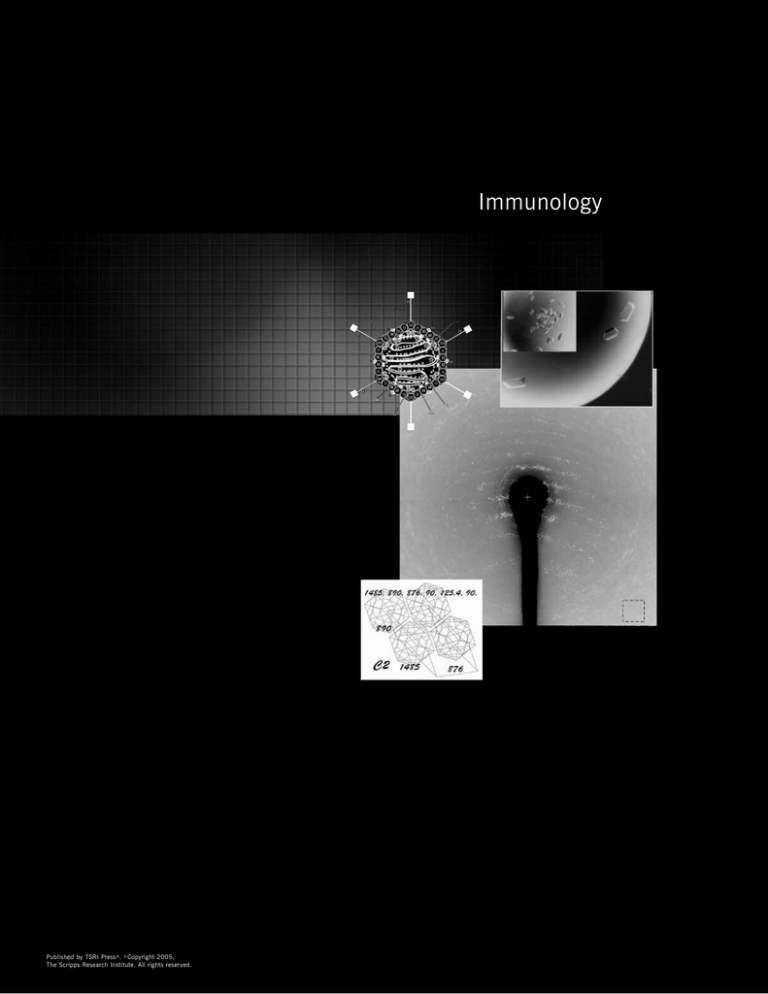
Immunology Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. Kerri A. Mowen, Ph.D., Assistant Professor, Department of Immunology Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. IMMUNOLOGY 2005 97 Ann J. Feeney, Ph.D. Associate Professor Jiing-Dwan Lee, Ph.D. Associate Professor Wolfram Ruf, M.D. Associate Professor Philippe Gallay, Ph.D. Associate Professor Erguang Li, Ph.D. Assistant Professor Daniel R. Salomon, M.D. Adjunct Associate Professor Richard J. Ulevitch, Ph.D. Professor and Chairman Nicholas R.J. Gascoigne, Ph.D. Professor Cheng Liu, M.D., Ph.D. Assistant Professor Erica Ollmann Saphire, Ph.D. Assistant Professor Roberto Baccala, Ph.D. Assistant Professor Peter Ghazal, Ph.D. Adjunct Associate Professor Nigel Mackman, Ph.D. Associate Professor Nora Sarvetnick, Ph.D. Professor Bruce A. Beutler, M.D. Professor Howard Gray, Ph.D. Adjunct Professor Michael McHeyzer-Williams, Ph.D. Associate Professor David Schlaepfer, Ph.D. Associate Professor Gary M. Bokoch, Ph.D.* Professor Jiahuai Han, Ph.D. Professor Dianne McKay, M.D. Assistant Professor Allesandro Sette, Ph.D. Adjunct Assistant Professor Dennis R. Burton, Ph.D.** Professor Wendy L. Havran, Ph.D. Associate Professor Donald E. Mosier, M.D., Ph.D. Professor Linda A. Sherman, Ph.D. Professor David A. Cheresh, Ph.D.*** Professor Moores Cancer Center La Jolla, California Shuang Huang, Ph.D. Assistant Professor Kerri A. Mowen, Ph.D. Assistant Professor Jonathan Sprent, M.D., Ph.D. Professor David Nemazee, Ph.D. Professor Charles D. Surh, Ph.D. Associate Professor Glen R. Nemerow, Ph.D. Associate Professor Luc Teyton, M.D., Ph.D. Associate Professor Per A. Peterson, M.D., Ph.D. Adjunct Professor Argyrios N. Theofilopoulos, M.D. Professor DEPAR TMENT OF IMMUNOLOGY S TA F F Robert W. Chesnut, Ph.D. Adjunct Associate Professor Tsung-Hsien Chuang, Ph.D. Assistant Professor Charles G. Cochrane, M.D. Professor Emeritus Neil R. Cooper, M.D. Professor Emeritus Linda K. Curtiss, Ph.D. Professor Edward A. Dennis, Ph.D. Adjunct Professor Henrik Ditzel, M.D., Ph.D. Adjunct Professor Frank J. Dixon, M.D. Professor Emeritus Director Emeritus, Scripps Research Jonathan G. Kaye, Ph.D. Associate Professor Hidehiro Kishimoto, M.D., Ph.D.*** Associate Professor Tokyo University of Science Tokyo, Japan Richard Klemke, Ph.D. Associate Professor Mary Laurie Phillips, Ph.D. Adjunct Assistant Professor Peter S. Tobias, Ph.D. Associate Professor Norman R. Klinman, M.D., Ph.D. Professor Pascal Poignard, M.D. Adjunct Assistant Professor Ulla Gissi Knaus, Ph.D. Associate Professor Ralph A. Reisfeld, Ph.D. Professor Daniel Von Seggern, Ph.D.**** Assistant Professor Dwight Kono, M.D. Associate Professor Matthias Riewald, M.D. Assistant Professor Susan R. Webb, Ph.D. Associate Professor Vladimir V. Kravchenko, Ph.D. Assistant Professor Hugh Rosen, M.D., Ph.D. Professor R. Anthony Williamson, Ph.D. Associate Professor S E C T I O N C O V E R F O R T H E D E P A R T M E N T O F I M M U N O L O G Y : X-ray diffraction Thomas S. Edgington, M.D. Professor Colleen Fearns, Ph.D. Assistant Professor analyses of intact adenovirus. Top right panel, Adenovirus crystals. Top left panel, Schematic diagram of adenovirus particles and the location of protein subunits. Middle panel, Diffraction pattern obtained at a synchrotron beamline. Bottom left panel, Unit cell dimensions and space group of adenovirus particles in the crystals. The work represents a collaboration between the laboratories of Glen R. Nemerow, Ph.D., Department of Immunology, and Vijay Reddy, Ph.D., Department of Molecular Biology. Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. 98 IMMUNOLOGY 2005 Curtis B. Wilson, M.D. Professor Emeritus Rong Xiang, M.D., Ph.D. Assistant Professor Kasper Hoebe, Ph.D. Christopher Alfonso, Ph.D. Roshni Chintalapati, Ph.D. Julie Jameson, Ph.D. Sandrine Arnaud-Dabernat, Ph.D. Jae Ho Cho, Ph.D. Elisabeth Jeanclos, Ph.D.**** David Chodniewicz, Ph.D. Caroline Aylott, Ph.D. Michael Zwick, Ph.D. Assistant Professor Cecile King, Ph.D.**** Liguo New, Ph.D.**** S TA F F S C I E N T I S T S Ben Croker, Ph.D. Ann Bellon, Ph.D. Qilin Pan, Ph.D. Gourab Bhattarcharjee, Ph.D. Dafang Bian, Ph.D. Jianhua Cui, Ph.D.*** Moores Cancer Center La Jolla, California Udayan Chatterji, Ph.D. Christine Pastore, Ph.D.**** Xin Du, Ph.D. Ramona Petrovan, Ph.D. Onur Boyman, Ph.D. Eleuterio De La Camara, Ph.D. John Mathison, Ph.D. Andrew Saphire, Ph.D. Carlos Cantu, Ph.D. Violane Delorme, Ph.D. Anil Munshi, Ph.D. Dwayne Stupack, Ph.D.*** Moores Cancer Center La Jolla, California Sophie Chabot, Ph.D.**** Celine Der Mardirossian, Ph.D. Jianming Chen, Ph.D. Sara Maree Weis, Ph.D.*** Moores Cancer Center La Jolla, California Zhong Chen, Ph.D **** Becky Diebold, Ph.D.*** Emory University Atlanta, Georgia Marie Cherrier, Ph.D. Anthony Don, Ph.D. Rafal Pawlinski, Ph.D. M. Germana Sanna, Ph.D. Deborah Witherden, Ph.D. SENIOR RESEARCH A S S O C I AT E S Jasimuddin Ahamed, Ph.D. Joao da Silva Correia, Ph.D. Joerge Birkenfeld, Ph.D. Aimee de Catherlineu, Ph.D. Yan Wu, Ph.D. Tomasz Zal, Ph.D.*** M.D. Anderson Cancer Center University of Texas Houston, Texas Amr Abdelhamid El Sheikh, Ph.D. R E S E A R C H A S S O C I AT E S Amanda Gavin, Ph.D. Djemel Ait-Azzouzene, Ph.D. Peter Goebel, Ph.D.*** IgE Therapeutics, Inc. San Diego, California Seyed Alavizadeh, Ph.D.*** Moores Cancer Center La Jolla, California A D M I N I S T R AT I V E A S S I S TA N T S , D E PA R T M E N T O F A D M I N I S T R AT I V E A S S I S TA N T S , D E PA R T M E N T O F I M M U N O L O G Y : Left to right: Amanda Moore, Min Lim, Joan Gausepohl, Gloria Jones, Lois Yamada, and Theresa Villalpando. A D M I N I S T R AT I V E A S S I S TA N T S , D E PA R T M E N T O F I M M U N O L O G Y : Left to right: Lindsay Smith, Katrina Schreiber, I M M U N O L O G Y : Left to right: Patty Rutledge, Jill Crowe, Sharon Tami Koth, Rachel Hanley, and Nancy Humphries. Weston, Susan Ramey, and Dian Caudebec, Administrative Manager. Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. IMMUNOLOGY 2005 99 Helen Donners, Ph.D. Neil John Hime, Ph.D. Hee Ok Kim, Ph.D. Jeff Lee, Ph.D. Stefan Dunzendorfer, Ph.D.**** Shihe Hou, Ph.D. Sungwoo Kim, Ph.D. Sang-Un Lee, Ph.D. Hong Hua, Ph.D. Rachel Kohler, Ph.D. Sung-Hyung Lee, Ph.D. Timothy Huang, Ph.D. Elizabeth Kompfner, Ph.D.**** Young Kyung Lee, Ph.D.**** Christoph Huber, Ph.D. Veera Reddy Konda, Ph.D.*** Metagenics Inc. Gig Harbor, Washington Cheng Li, Ph.D. Mike Eisenbraun, Ph.D.**** Celia Espinoza, Ph.D. Nicolas Fazilleau, Ph.D. Naaozumi Ishimaru, Ph.D.*** University of Tokushima Tokushima, Japan Marek Kovar, Ph.D. Hongbin Li, Ph.D.*** Harvard Medical School Boston, Massachusetts Jirina Kovarova, Ph.D. Jiali Li, Ph.D. Hassan Issafras, Ph.D. Carsten Kreig, Ph.D. Xiang Li, Ph.D. Zhengfan Jiang, Ph.D. Joerge Krueger, M.D. Yilei Li, Ph.D. Hyun-Bae Jie, Ph.D.*** Harvard University Cambridge Massachusetts Toru Kurakawa, Ph.D. Ssang-Taek Lim, Ph.D. Yumi Kurokawa, Ph.D. Yang Mi Lim, Ph.D. Euijung Jo, Ph.D. Rui Lin, Ph.D. Valeria Judkowski, Ph.D.**** Sabine Kurz, Ph.D.*** Invitrogen Co. Carlsbad, California Wong Soon Justin, Ph.D. Young Back Kwon, Ph.D. Milena Iacobelli, Ph.D. Clemens Feistritzer, M.D. Christofer Flood, Ph.D. Linda Frederick, Ph.D. Stefan Freigang, Ph.D. Guo Fu, Ph.D. Philippe Georgel, Ph.D. Cristina Gil-Lamaignere, Ph.D. Jonathan Gitlin, Ph.D.*** University of Kentucky Lexington, Kentucky Ting-Kun Lin, M.D., Ph.D. Young Jun Kang, Ph.D. Cheng Yu Lai, Ph.D. Jeffrey Lindquist, Ph.D.*** Moores Cancer Center La Jolla, California Emma Hamilton-Williams, Ph.D. Yu-Ya Kao, Ph.D. Jennifer Lamoureux, Ph.D. Guoxun Liu, Ph.D. Masaaki Hayashi, M.D., Ph.D. Charles Kaplan, Ph.D. Mansun Law, Ph.D. Yuan Liu, Ph.D. Natasha Hill, Ph.D. Ayse Kayali, Ph.D. Hyun-Ku Lee, Ph.D. Jeng-Fen Lo, Ph.D.**** Antje Gohla, Ph.D.**** A D M I N I S T R AT I V E A S S I S TA N T S , D E PA R T M E N T O F A D M I N I S T R AT I V E A S S I S TA N T S , D E PA R T M E N T O F I M M U N O L O G Y : Left to right: front row: Kathy Cairns and Bonnie I M M U N O L O G Y : Left to right: front row: Karen Cerveny and Towle; back row: Carol Wood, Anna Meyers, Barbara Marchand, and Betsy Layton; back row: Ellen Klahn, Shelly Gassert, and Kat Occhipinti. Theresa McCarthy. Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. 100 IMMUNOLOGY 2005 Markus Loeffler, M.D.**** Jared Purton, Ph.D. Konstantin Stoletov, Ph.D. Kenji Yoshida, Ph.D. Carina Lotz, Ph.D. Christopher Ramsey, Ph.D. Shibing Su, Ph.D.**** Jiangiang Yu, Ph.D. Christine Louis dit Sully, Ph.D. Koichi Tabeta, Ph.D.**** Hui Zhang, Ph.D. Yunping Luo, Ph.D. Jill Ricono, Ph.D.*** Moores Cancer Center La Jolla, California Joyce Tan, Ph.D. You Qing Zhang, Ph.D. Michael Lyman, Ph.D. Mark Rubenstein, Ph.D. Rachel Tilley, Ph.D. Tieming Zhao, Ph.D. Chitladda Mahanivong, Ph.D. Monica Ruse, Ph.D. Joie Trifilo, Ph.D. He Zhou, Ph.D. Presanta Maiti, Ph.D.**** Sophie Rutschmann, Ph.D. David Valenta, Ph.D. Huamin Zhou, Ph.D. Laurent Malherbe, Ph.D. Prabhakar Salunkhe, Ph.D. Sebastian Vallee, Ph.D. Zuping Zhou, Ph.D.**** Annette Marleau, Ph.D. Yuichiro Sato, Ph.D.*** University of California San Diego Medical Center San Diego, California Laurent Verkoczy, Ph.D. Beatriz Maroto, Ph.D. Annica Martensson, Ph.D.**** Gernot Schabbauer, Ph.D.**** Javier Martinez, Ph.D. Terrence Meehan, Ph.D. Monica Schaller, Ph.D.*** Basel Hospital Basel, Switzerland Satyajit Mitra, Ph.D. Jorge Luis Schettini, Ph.D. Johann Mols, Ph.D. Nicolas Schrantz, Ph.D. Adam Mullick, Ph.D. Alim Seit-Nebi, Ph.D. Eric Murphy, Ph.D.*** Moores Cancer Center La Jolla, California Suganya Selvarajah, Ph.D. Perihan Nalbant, Ph.D. Doinita Serban, Ph.D.*** Moores Cancer Center La Jolla, California Bishnu Nayak, Ph.D.**** S C I E N T I F I C A S S O C I AT E S Hendrik Versteeg, Ph.D. Katharina Von Lohneysen, Ph.D. Meng Wang, M.D. Yingchun Wang, Ph.D. Zhao Wang, Ph.D. Chenghong Wei, Ph.D. Christopher Wiethoff, Ph.D. Justin Soon Boon Wong, Ph.D. Chia Cheng Wu, Ph.D. Linda Sharp, Ph.D.**** Dong Wu, Ph.D.**** Xuifei Shen, Ph.D.**** Wenyuan Wu, Ph.D. Fu Dong Shi, Ph.D.**** Yue Xu, Ph.D. Pia Yachi, Ph.D. Motoyuki Otsuka, Ph.D. David Shields, Ph.D.*** Moores Cancer Center La Jolla, California Sandrine Pacquelet, Ph.D. Shigeki Shimada, Ph.D. Oliver Pertz, Ph.D. Jason Smith, Ph.D. Jian Ming Yang, Ph.D.*** Department of Cell Biology, Scripps Research Helle Petersen, Ph.D. Laura Solforosi, Ph.D. Michael Ye, Ph.D. Matthew Potter, Ph.D.*** Moores Cancer Center La Jolla, California Michelle Solomon, Ph.D. Jinseong Yi, Ph.D.**** Gabriel Sternik, Ph.D. Sun-Hee Yoon, Ph.D. Ron Nepomuceno, Ph.D. Nathalie Niederberger, Ph.D.**** Frank Karl Niessen, Ph.D. Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. Deepak Yadav, Ph.D. Rosana Gonzales-Quintal, Ph.D. Marcie Rose Kritzik, Ph.D. Nora Leaf Ralph Pantophlet, Ph.D. Susan D. Revak**** Dongyuan Xia, Ph.D. * Joint appointment in the Department of Cell Biology ** Joint appointment in the Department of Molecular Biology *** Appointment completed; new location shown **** Appointment completed IMMUNOLOGY Richard Ulevitch, Ph.D. Chairman’s Overview he faculty of the Department of Immunology continues its long-standing commitments to the highest level of scientific achievement, the training of graduate and postgraduate students, and participation in numerous activities outside Scripps Research. But first an update on the demographics of the department. The department consists of 46 full-time faculty members; 37 of the members are less than 60 years old, and the majority are 50 years old or less. This age distribution helps ensure the long-time vitality and success of the department and is important to the overall excellence of Scripps Research. The scientific successes of our younger faculty members are well recognized as indicated by the receipt of highly competitive career development awards such as the Burroughs Wellcome Career Award in Biomedical Science received by Erica Ollmann Saphire and the highly coveted Established Investigator Award awarded to David Schlaepfer by the American Heart Association. Although a number of faculty members have retired, most recently Charles Cochrane, and more are planning to retire soon, an active recruitment program since 1996 has added 9 new faculty members. T Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. 2005 101 Another interesting statistic is that 18% of the faculty are women, all of whom are laboratory heads and most of whom are tenured. In a recent letter in Nature Immunology, the importance of Immunology as a career for young female scientists was highlighted. Clearly, the Department of Immunology far exceeds all averages quoted in the letter. We are proud of our record. Outside honors and appointments to review committees and top editorial boards accrue to all of the faculty. One important activity is serving on National Institutes of Health (NIH) review committees, and members of our department are well represented. Serving as a permanent member on an NIH study section is a major time commitment; currently 5 of our faculty serve in this capacity: Drs. Bokoch, Curtiss, Gascoigne, Kono, and Nemazee. Their service is critical to maintaining the integrity of the NIH review process, and their willingness to do so is appreciated by all of the members of the department. Large-scale research grants have become a major focus at NIH. Consequently, it is critical that scientists in the department be able to respond to these new funding mechanisms. Today the department has several such grants. The most recent is a multimillion dollar grant to establish the Scripps Research Institute Molecular Screening Center. The goal of this pilot program is to discover small-molecule tools for translating basic biomedical discoveries more quickly into medically relevant applications. This grant also marks a key collaboration between Scripps Research in La Jolla and Scripps Florida. This funding is part of the NIH strategic funding plan, the Roadmap Initiative. Hugh Rosen is the principal investigator on the grant. His intention is to conduct high-throughput screens against various biological targets to uncover “proof of concept” molecules useful in studying human health and in developing new treatments for human diseases. As Dr. Rosen remarked, “With this grant, the NIH has recognized the unique capabilities of our established researchers in La Jolla with our newest investigators and equipment in Palm Beach County.” During the past year, the output of seminal advances was high. Members of the department were authors of more than 30 articles that appeared in high-profile journals, including Science, Nature, Cell, Nature Immunology, Nature Cell Biology, and Nature Medicine. Most of the research described in these articles is given in detail in the reports of the individual laboratories, but the following are a few of the highlights. 102 IMMUNOLOGY 2005 This department, long recognized for its continued contributions to studies of the adaptive immune system, is now recognized as one of the leaders in advancing our understanding of innate immunity. One reason for this recognition is the continued productivity of Bruce Beutler and his group. During the past year, they made several important advances. In an article published in Nature, they describe a key role for the transmembrane protein known as CD36 in sensing diacyglycerides. The researchers showed that a nonsense mutation of Cd36 causes a recessive immunodeficiency in which macrophages are insensitive to the R-enantiomer of MALP-2 (a diacylated bacterial lipopeptide) and to lipoteichoic acid. Mice homozygous for the mutation are hypersusceptible to Staphylococcus aureus infection. Studies on macrophages from mice with the mutation revealed that some, but not all, ligands for Toll-like receptor (TLR) 2 are CD36 dependent. Already known as a receptor for endogenous molecules, CD36 is also a selective and nonredundant sensor of microbial diacylglycerides that signal via the TLR2/6 heterodimer. This work provides new data on the role of innate immunity during infection and during chronic inflammatory diseases such as atherosclerosis in which endogenous diacyglycerides may be generated and trigger TLR2. A second report by Dr. Beutler and colleagues published in Nature Immunology provides new information on CD14 and its role in TLR4 signaling. This research is particularly interesting because it was nearly 15 years ago that the studies of other members of the department published in Science provided the first molecular insights into the function of CD14 as a sensor for bacterial lipopolysaccharides. Gary Bokoch and the members of his laboratory continue to provide new insights into the molecular physiology of phagocytic cells. During the past year, in an article in Nature Cell Biology, they reported the biochemical isolation of chronophin, a unique cofilin-activating phosphatase of the haloacid dehalogenase superfamily. Chronophin directly dephosphorylates cofilin with high specificity and colocalizes with cofilin in motile and dividing cells. Loss of chronophin activity blocks phosphocycling of cofilin, stabilizes F-actin structures, and causes massive defects in cell division. These findings identify a physiologic phosphoserine protein substrate for a mammalian haloacid dehalogenase–type phosphatase and indicate that chronophin is an important novel regulator of cofilin-mediated actin reorganization. Researchers in Jiahuai Han’s group recently made a major contribution to understanding the mechanisms Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. involved in stability of mRNA species that encode key molecules in innate immunity and inflammation. Adenosine-uridine–rich elements (AREs) in the 3′ untranslated region of unstable mRNAs dictate degradation of the mRNAs. An RNA interference–based screen in Drosophila S2 cells revealed that in TNF-α, components involved in microRNA processing and function are required for the rapid decay of mRNA that contains AREs. The requirement for the component Dicer in the instability of mRNA with AREs was confirmed in HeLa cells. The researchers further observed that miR16, a human microRNA containing a sequence complementary to the ARE sequence is required for the turnover of RNA that contains AREs. The role of miR16 in the decay of mRNA that contains AREs is sequence specific and requires the ARE-binding protein tristetraprolin. Tristetraprolin does not directly bind to miR16; rather it interacts through association with other components involved in microRNA processing to form a complex with miR16 and assists in the targeting of ARE. The targeting of ARE by microRNA therefore appears to be an essential step in ARE-mediated mRNA degradation. These findings provide an entirely new understanding of the function of microRNA and a new model for studies of AREs during a variety of host responses. Other publications by members of the department include reports of the pioneering research of Luc Teyton and his group, in collaboration with A. Bendelac, University of Chicago, on natural killer T cells and the cells’ key membrane sensor CD1. Specifically, in articles published in Science, Nature, and Nature Immunology, the following was noted. Natural killer T cells are a distinct lineage of T cells that coexpress a conserved αβ T-cell receptor (TCR) and natural killer receptors. Although the TCR of natural killer T cells is characteristically autoreactive to CD1d, a lipid-presenting molecule, endogenous ligands for these cells have not been identified. Dr. Teyton and his group showed that isoglobotrihexosylceramide (iGb3), a lysosomal glycosphingolipid of previously unknown function, is recognized by both mouse and human natural killer T cells. Impaired generation of lysosomal iGb3 in mice lacking β-hexosaminidase B results in a severe deficiency in natural killer T cells, suggesting that this lipid also mediates development of these T cells in mice. The findings suggest that expression of iGb3 in peripheral tissues may be involved in controlling the responses of natural killer cells to infections and malignant neoplasms and in autoimmunity. Further, the scientists have shown microbial, antigen-specific activation of natural killer T cells against IMMUNOLOGY gram-negative, lipopolysaccharide-negative α-Proteobacteria such as Ehrlichia muris and Sphingomonas capsulata. Glycosylceramides from the cell wall of Sphingomonas act as direct targets for mouse and human natural killer T cells, controlling both septic shock and bacterial clearance in infected mice. In contrast, gram-negative, lipopolysaccharide-positive Salmonella typhimurium activates natural killer T cells through recognition of iGb3 presented by lipopolysaccharide-activated dendritic cells. These findings identify 2 novel antigenic targets of natural killer T cells in antimicrobial defense and show that glycosylceramides are an alternative to lipopolysaccharide for innate recognition of the gram-negative, lipopolysaccharide-negative bacterial cell wall. Finally, in collaborative studies with Ian Wilson and his group, members of the Teyton group documented the crystal structure of CD1d in complex with a short-chain synthetic variant of α-galactosylceramide at a resolution of 2.2 Å. This structure indicates the basis for the high specificity of these microbial ligands and explains the restriction of the α-linkage as a unique pathogen-specific pattern recognition motif. Comparison of the binding of altered lipid ligands to CD1d and TCRs suggested that the differential helper T cell–like properties of natural killer T cells may originate largely from differences in the “loading” of the ligands in different cell types and hence in the tissue distribution of the ligands in vivo. Overall, this research provides a remarkably broad set of advances in understanding the functions of natural killer T cells and the structure of one of the key sensor molecules. In addition, it further illustrates the ability of members of the department to collaborate with other leaders in this field inside and outside of Scripps Research. Members of the Department of Immunology have a long-standing interest in the functions of T cells in various biological models. Studies from Wendy Havran and her group are an example of this interest. A fine balance between rates of proliferation and apoptosis in the skin provides a defensive barrier and a mechanism for tissue repair after damage. Vγ3+ dendritic epidermal T cells are primary modulators of skin immune responses. Dr. Havran and her group showed that these cells both produce and respond to insulin-like growth factor 1 (IGF-1) after TCR stimulation. Mice deficient in the cells had a notable increase in epidermal apoptosis that was abrogated by the addition of Vγ3+ dendritic epidermal T cells or IGF-1. Furthermore, mice deficient in the cells had reduced activation of IGF-1 receptors at wound sites. These findings indicate critical functions for Vγ3 + dendritic epiPublished by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. 2005 103 dermal T cell–mediated IGF-1 production in regulating skin homeostasis and repair. Linda Sherman and members of her laboratory also provided important new insights into the role of T cells in tumor immunity. Studies published in Immunity, done in collaboration with M. Theobald, Johannes Gutenberg University, Mainz, Germany, revealed that efficient immune attack on malignant disease requires the concerted action of both CD8+ cytotoxic T lymphocytes (CTLs) and CD4+ T helper cells. The researchers used HLA-A*0201 (A2.1) transgenic mice, in which the mouse CD8 molecule cannot efficiently interact with the α3 domain of A2.1, to generate a high-affinity, CD8-independent TCR specific for a commonly expressed, tumor-associated CTL epitope derived from p53, a human tumor suppressor protein. Introduction of this TCR into human T cells resulted in CD8+ T lymphocytes with broad tumor-specific cytotoxic activity and CD4 + T cells with potent tumor-reactive, p53A2.1-specific helper activity. Both T-cell subsets interacted synergistically with dendritic cell intermediates and tumor targets. The intentional redirection of both CD4 + helper T cells and CD8+ CTLs by the same high-affinity, CD8-independent, tumor-specific TCRs could provide the basis for novel broad-spectrum cancer immunotherapeutic agents. Other contributions of note during the past year include studies done by David Nemazee and members of his laboratory on B-cell development. In developing B cells, expression of immunoglobulin on the cell surface is an important signal to terminate expression of recombinase activator gene (RAG) and V(D)J recombination. However, autoreactive antigen receptors promote continued gene rearrangement and receptor editing. Regulation of RAG expression and editing by B-cell receptor signaling is poorly understood. Dr. Nemazee and his colleagues found that in editing-competent cells, RAG mRNA expression induced by B-cell receptor ligands is regulated at the level of RAG transcription. In immature B cells carrying innocuous receptors, RAG expression appears to be under rapidly reversible negative regulation. Research with transduction of a superrepressive IκBα protein indicated that NF-κB/Rel proteins promote RAG transcription. Interestingly, cells deficient in NF-κB overexpress RAG and undergo an exaggerated receptor editing response. These results implicate NF-κB transcription factors in the regulation of RAG transcription mediated by B-cell receptors. Rapidly activated NF-κB pathways may facilitate prompt antigen receptor–regulated changes in RAG expression important for editing and haplotype exclusion. 104 IMMUNOLOGY 2005 In closing, I can say without reservation that writing this report was a great pleasure, because it gave me a chance to review the accomplishments of the faculty of the Department of Immunology. These accomplishments span all aspects of 21st century science: cutting-edge contributions to knowledge of the immune system; education of undergraduate, graduate, and postgraduate students; and participation in important outside activities that include serving on NIH review committees and journal editorial boards and organizing and participating in important national and international scientific meetings. Each member of the faculty contributes in all these aspects of science and makes the Department of Immunology, to me, the model that many others aspire to emulate. Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. IMMUNOLOGY INVESTIGATORS’ R EPORTS The Genetic Basis of Resistance to Infection B. Anchonda, K. Benson, B. Croker, K. Crozat, X. Du, P. Georgel, C. Gil-Lamaignere, K. Hoebe, Z. Jiang, N. Mann, S. Mudd, S. Rutschmann, L. Shamel, S. Sovath, K. Tabeta, H. Uy, Z. Zhou, B. Beutler o “universal pathogens” exist, and even the most virulent human pathogens (e.g., HIV and smallpox virus) are innocuous in most mammalian species. Conversely, no human has ever died of mouse cytomegalovirus (MCMV) infection. In general, interspecies differences in susceptibility to infectious diseases cannot be explained on the basis of differences in adaptive immunity. The combinatorial system for generating diversity in T- and B-cell receptors is similarly effective in all mammals. Rather, innate immunity is the characteristic that differs remarkably among species. And even among members of a single species, differences in innate immunity may foretell life or death in the event of an infection. Evidently, a large number of genes confer resistance to infection. For this reason, in humans, susceptibility to death caused by infection has greater heritability than susceptibility to death from any other cause. Our broad goal is to identify all the genes required for a strong innate immune response. Most genes that serve this function in mice are also required for innate immunity in humans, and, indeed, conservation of core innate immune processes is such that many of the same genes are even used for innate defense by fruit flies. To find nonredundant components of the innate immune system, we create inherited innate immunodeficiency states through the use of a randomly acting germ-line mutagen, N-ethyl-N-nitrosourea. Those exceptional animals that have compromised immunity are detected by phenotypic screening. The mutations are brought to homozygosity and mapped by using classical genetic methods. DNA sequencing is then applied to pinpoint the culpable mutation and hence find the gene (and protein) with an indispensable role in disease resistance. We use 2 general types of phenotypic screens. N COMPONENTS OF THE TOLL-LIKE RECEPTOR SIGNAL T R A N S D U C T I O N PAT H WAY S We earlier determined that the mammalian Tolllike receptors (TLRs) are responsible for perceiving Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. 2005 105 diverse infections, ranging from those caused by bacteria and fungi to those caused by protozoa and viruses. TLRs alert the host to the presence of conserved molecules that are synthesized by these microbes, such as lipopolysaccharide, DNA with unmethylated cytosine-guanine dinucleotides, double-stranded RNA, and various diacylglycerides and triacylglycerides. We have implemented a focused screen for defects in signaling from TLRs to the level of TNF production. Using this screen, we identified 11 mutations, which affect 10 genes. Of the 10 genes, 5 encode “known” components of the TLR signaling apparatus, and 5 encode components that were not previously known. Among the new elements are an adapter protein for TLR signaling (TRIF), a coreceptor for sensing microbial diacylglycerides (CD36), a protein kinase (feckless) that links the sensing of double-stranded RNA to the activation of NF-κB (a key inflammatory transcription factor), and a component of the endoplasmic reticulum (3d) that is required for signaling via 4 of the TLRs. On the basis of the number and types of mutations found, we calculated that about 50 proteins are required for signaling from the TLRs to the level of TNF production. The protein 3d is remarkable because it is required not only for TLR signaling but also for the processing of antigens for activation of adaptive immune responses. Thus, 3d links the innate and adaptive immune systems. Further investigation of the mechanism of action of 3d may reveal much about how autoimmune diseases are initiated and sustained. SUSCEPTIBILITY TO MCMV C57BL/6 mice normally are strongly resistant to infection by MCMV. However, we found that a substantial fraction of the mouse genome is devoted to the creation of resistance. Mutations in approximately 300 genes can cause profound susceptibility to MCMV. Approximately 10% of these genes have now been altered by mutation, and several of the mutations have been brought to homozygosity, a necessary precondition for positional cloning. The MCMV “resistome,” those genes that serve nonredundant functions in resistance to this pathogen, will gradually be defined through a forward genetic approach. Many of the mutations so far that diminish resistance to MCMV have broad effects on resistance to infection overall. We therefore think that the MCMV resistome is not much smaller than the “universal resistome,” the complement of genes required for resistance to all microbes. 106 IMMUNOLOGY 2005 N O V E L PAT H WAY S F O R T H E A C T I VAT I O N O F ADAPTIVE IMMUNE RESPONSES The TLRs mediate most infection-related phenomena, including “bad” effects such as fever and shock and “good” effects such as activation of the adaptive immune response. This last effect has been of particular interest to immunologists, because it is an important factor in the development of vaccines. However, we discovered that TLR-independent pathways exist for initiation of an adaptive immune response. One pathway impels a highly efficient CD8 T-cell response to foreign proteins expressed by cells undergoing apoptosis (programmed death). This “death-driven” adjuvant pathway is the most efficient means of CD8 activation ever described. It presumably evolved to permit the detection of pathogens that trigger an apoptotic response. Our assumption is that the host has retained pathogen-specific pathways for cell death and uses them to encourage an adaptive immune response. Aberrant activation of these pathways may be important in the development of dysfunctional adaptive immune responses to host proteins. PUBLICATIONS Anfossi, N., Robbins, S.H., Ugolini, S., Georgel, P., Hoebe, K., Bouneaud, C., Ronet, C., Kaser, A., DiCioccio, C.B., Tomasello, E., Blumberg, R.S., Beutler, B., Reiner, S.L., Alexopoulou, L., Lantz, O., Raulet, D.H., Brossay, L., Vivier, E. Expansion and function of CD8+ T cells expressing Ly49 inhibitory receptors specific for MHC class I molecules. J. Immunol. 173:3773, 2004. Hoebe, K., Janssen, E., Beutler, B. The interface between innate and adaptive Immunity. Nat. Immunol. 5:971, 2004. Jiang, Z., Georgel, P., Du, X., Shamel, L., Sovath, S., Mudd, S., Huber, M., Kalis, C., Keck, S., Galanos, C., Freudenberg, M., Beutler, B. CD14 is required for MyD88independent LPS signaling. Nat. Immunol. 6:565, 2005. Mattner, J., DeBord, K.L., Ismail, N., Goff, R.D., Cantu, C. III, Zhou, D., SaintMezard, P., Wang, V., Gao, Y., Yin, N., Hoebe, K., Schneewind, O., Walker, D., Beutler, B., Teyton, L., Savage, P.B., Bendelac, A. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 434:525, 2005. Rietschel, E.T., Rietschel, M., Beutler, B. How the mighty have fallen: fatal infectious diseases of divine composers. Infect. Dis. Clin. North Am. 18:311, 2004. Smythe, I., Du, X., Taylor, M.S., Justice, M.J., Beutler, B., Jackson, I.J. The extracellular matrix gene Frem1 is essential for the normal adhesion of the embryonic epidermis. Proc. Natl. Acad. Sci. U. S. A. 101:13560, 2004. Theofilopoulos, A.N., Baccala, R., Beutler, B., Kono, D.H. Type I interferons (α/β) in immunity and autoimmunity. Annu. Rev Immunol. 23:307, 2005. Zhou, Z., Hoebe, K., Du, X., Jiang, A., Shamel, L., Beutler, B. Antagonism between MyD88- and TRIF-dependent signals in B7RP-1 up-regulation. Eur. J. Immunol. 35:1918, 2005. Regulation of Cell Function by Rho GTPases G.M. Bokoch, C. Aylott, J. Birkenfeld, M. Crawford, V. Delorme, C. DerMardirossian, A.M. DeCathlineau, B.A. Diebold, T. Huang, Y.-Y. Kao, P. Nalbant, K. Pestonjamasp, Y. Wu, T. Zhao, B.P. Bohl, A. Fowler, Beutler, B. SHIP, TGF-β, and endotoxin tolerance. Immunity 21:134, 2004. J. Neuberg Beutler, B. The Toll-like receptors. In: Genetic Susceptibility to Infection. Kaslow, R.L., McNicholl, J., Hill, A.V.S. (Eds.). Oxford University Press, New York, in press. ho GTPases control the assembly of the actin cytoskeleton, the production of reactive oxygen species (ROS), and the activity of kinase cascades that mediate cell growth, death, and motility. This spectrum of activities makes Rho GTPases key components of such physiologic and pathologic processes as tumor growth and metastasis, wound healing, neuronal connectivity, inflammatory responses, angiogenesis, and development. We use cellular, molecular, biophysical, and biochemical approaches to understand how the activities of Rho GTPases are regulated, to identify the proteins they interact with to control cell function, and to ascertain how these regulatory processes are abnormal in various disease states. Beutler, B., Crozat, K., Koziol, J.A., Georgel, P. Genetic dissection of innate immunity to infection: the mouse cytomegalovirus model. Curr. Opin. Immunol. 17:36, 2005. Beutler, B., Hoebe, K., Georgel, P., Du, X. Forward genetic analysis of TLR pathways: a shared system for the detection of endotoxin and viral infection. In: Toll and Toll-Like Receptors: An Immunologic Perspective. Rich, T. (Ed.). Kluwer Academic/Plenum, New York, 2005, p. 168. Molecular Biology Intelligence Unit. Beutler, B., Hoebe, K., Georgel, P., Tabeta, K., Du, X. Genetic analysis of innate immunity: TIR adapter proteins in innate and adaptive immune responses. Microbes Infect. 6:1374, 2004. Georgel, P., Crozat, K., Lauth, X., Makrantonaki, E., Seltmann, H., Sovath, S., Hoebe, K., Du, X., Rutschmann, S., Jiang, Z., Bigby, T., Nizet, V., Zouboulis, C.C., Beutler, B. A Toll-like receptor 2-responsive lipid effector pathway protects mammals against skin infections with gram-positive bacteria. Infect. Immun. 73:4512, 2005. Hawn, T.R., Verbon, A., Janer, M., Zhao, L.P., Beutler, B., Aderem, A. Toll-like receptor 4 polymorphisms are associated with resistance to Legionnaires’ disease. Proc. Natl. Acad. Sci. U. S. A. 102:2487, 2005. Hoebe, K., Beutler, B. LPS, dsRNA and the interferon bridge to adaptive immune responses: Trif, Tram, and other TIR adaptor proteins. J. Endotoxin Res. 10:130, 2004. Hoebe, K., Beutler, B. Unraveling innate immunity using large scale N-ethyl-Nnitrosourea mutagenesis. Tissue Antigens 65:395, 2005. Hoebe, K., Georgel, P., Rutschmann, S., Du, X., Mudd, S., Crozat, K., Sovath, S., Shamel, L., Hartung, T., Zähringer, U., Beutler, B. CD36 is a sensor of diacylglycerides. Nature 433:523, 2005. Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. R RHO GTPASES AND HUMAN LEUKOCYTES We previously established that the GTPase Rac regulates the formation of ROS that are used by human phagocytic cells for microbial killing and that result in inflammatory responses. Using a cell-free recombinant system, we established the molecular interaction of Rac with cytochrome b, a component of the mem- IMMUNOLOGY brane-bound NADPH oxidase, independent of p67phox, leading to a 2-step mechanism for regulation of electron transfer to form superoxide anion (Fig. 1). We showed that the Rac-related GTPase Cdc42 acts as a competitive inhibitor of Rac binding to cytochrome b. This inhibition results in an antagonistic cross talk between Rac and Cdc42 that modulates the formation of ROS in leukocytes and, potentially, nonphagocytic cells. This unique mechanism may coordinate formation of ROS with cytoskeletal dynamics during chemotaxis and phagocytosis. 2005 107 inhibitor and modulate its ability to bind Rac GTPase. We discovered a potential positive-feedback Rac activation cycle that involves phosphorylation of Rho GDP-dissociation inhibitor by p21-activated kinase 1 (PAK1), a downstream effector of Rac and Cdc42 signaling. We are using live-cell imaging in combination with fluorescent methods to determine the spatial and temporal localization of Rho GTPase activation. We are beginning to determine the molecular signals that govern the chemotactic responses of human leukocytes and the biochemical pathways that lead to assembly of the cytoskeleton and motility. We established a primary link between the actin and microtubule cytoskeletons that involves regulation of Rho GTPase via physical sequestration of the Rho GEF-H1 by microtubules. We recently discovered that GEF-H1 is a signaling link between microtubules in the mitotic spindle and the initiation of Rho-dependent formation of cleavage furrows in dividing cells (Fig. 2). F i g . 1 . Two-step activation mechanism for Rac GTPase–mediated regulation of oxidant formation by phagocyte NADPH oxidase. We found that an inhibitory cross talk between leukocyte adhesion receptors and NADPH oxidase activation occurs via modulation of Rac2 GTPase activity. Rac2 acts as a critical “molecular switch” that regulates formation of ROS in adherent cells. Integrin signaling inhibited activation of Vav1, the upstream guanine nucleotide exchange factor (GEF) that regulates Rac2GTP formation and thus NADPH oxidase activity. We have now identified a mechanism by which TNF-α and certain other cytokines can overcome adhesion-induced inhibition to allow rapid formation of ROS at inflammatory sites. This mechanism requires the activity of proline-rich tyrosine kinase 2 to initiate signaling from Vav1 to Rac2. These studies (1) address the important physiologic question of how leukocytes migrate to inflammatory sites without perpetuating continuous oxidative damage to underlying tissue and (2) provide additional mechanistic insight into the inflammatory actions of TNF-α. R E G U L AT I O N O F R H O G T PA S E S The regulatory protein GDP-dissociation inhibitor is a critical control point for Rho GTPase function. We are investigating the action of kinases that phosphorylate this Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. F i g . 2 . Immunofluorescent images show colocalization of endog- enous GEF-H1 with microtubules (tubulin) in the mitotic spindle. R E G U L AT I O N O F I N N AT E I M M U N I T Y B Y A N T H R A X TOXINS Bacillis anthracis inhibits the function of immune cells by generating lethal toxin and edema toxin. As part of a program grant funded by the Centers for Disease Control and Prevention, we are investigating the molecular basis for the suppressive effects of the anthrax toxins on human neutrophil chemotaxis and formation of ROS. A potential requirement for Rho GTPases in uptake and action of the anthrax toxins in macrophages is also under study. C E L L R E G U L AT I O N B Y PA K S PAKs are cellular effectors of Rac and Cdc42. The C-terminal kinase domain of these enzymes phosphorylates substrates involved in regulating NADPH oxidase, stress responses, and the cellular actin-myosin system. PAKs are important mediators of chemotaxis, wound healing, tumor metastasis, neurite outgrowth, antigen presentation, and other processes that depend on cytoskeletal polarization. 108 IMMUNOLOGY 2005 The phosphorylation of cofilin, which depolymerizes and severs actin, by PAK1–LIM kinase is an important regulatory point in cytoskeletal dynamics. Using a biochemical screen, we identified a unique cofilin phosphatase, termed chronophin, that regulates stimulus-dependent activation of cofilin. Using small interfering RNA to reduce the expression of chronophin, we found that this phosphatase is involved in the control of cytokinesis during cell division. Chronophin is implicated in the formation of aneuploid cancers; it is overexpressed in such tumors and is an autoantigen in patients with cancer. We are investigating the regulation of cell motility and other cellular processes by this unique regulatory phosphatase. We are also investigating the requirement for PAK function in retrograde actin flow, a critical component of cell motility. These ongoing studies provide diverse avenues of investigation. PUBLICATIONS Birukov, K.G., Bochkov, V.N., Birukova, A.A., Kawkitinarong, K., Rios, A., Leitner, A., Verin, A.D., Bokoch, G.M., Leitinger, N., Garcia, J.G. Epoxycyclopentenone-containing oxidized phospholipids restore endothelial barrier function via Cdc42 and Rac. Circ. Res. 95:892, 2004. Bokoch, G.M. Regulation of Innate Immunity by Rho GTPases. Trends Cell Biol. 15:163, 2005. DerMardirossian, C., Bokoch, G.M. GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 15:356, 2005. Diebold, B.A., Bokoch, G.M. Rho GTPases and the control of the oxidative burst in polymorphonuclear leukocytes. Curr. Top. Microbiol. Immunol. 291:91, 2005. Gohla, A., Birkenfeld, J., Bokoch, G.M. Chronophin, a novel HAD-type serine protein phosphatase, regulates cofilin-dependent actin dynamics. Nat. Cell Biol. 7:21, 2005. Makino, A., Glogauer, M., Bokoch, G.M., Chien, S., Schmid-Schonbein, G.W. Control of neutrophil pseudopods by fluid shear: role of Rho family GTPases. Am. J. Physiol. Cell Physiol. 288:C863, 2005. Stofega, M., DerMardirossian, C., Bokoch, G.M. Affinity-based assay of Rho GTPase activation. Methods Mol. Biol., in press. Yuan, Z.Q., Kim, D., Kaneko, S., Sussman, M., Bokoch, G.M., Kruh, G.D., Nicosia, S.V., Testa, J.R., Cheng, J.Q. ArgBP2γ interacts with Akt and p21-activated kinase-1 and promotes cell survival. J. Biol. Chem. 280:21483, 2005. Zhao, T., Bokoch, G.M. Critical role of proline-rich tyrosine kinase 2 in reversion of the adhesion-mediated suppression of reactive oxygen species generation by human neutrophils. J. Immunol. 174:8049, 2005. Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. Human Antibodies and Design of a Vaccine to HIV Type 1 M.B. Zwick, R.A. Pantophlet, R.O. Aguilar-Sino, R. Astronomo, D. Bowley, H. Donners, A. Gakhal, A.J. Hessell, R.C. Jensen, M. Law, J.D. Nelson, E. Scherer, S. Selvarajah, M. Wang, R.A. Dwek,* P.M. Rudd,* D. Calarese, R.L. Stanfield, I.A. Wilson, D.R. Burton * Oxford Glycobiology Institute, Oxford, England IV type 1 (HIV-1) is a scourge on humanity, Nearly 42 million persons are infected with the virus, and 22 million persons have died of AIDS. It is widely recognized that a vaccine most likely is the best way to control HIV-1 infection worldwide. We wish to understand antibody responses to HIV-1 in humans and to design vaccines that will elicit protective responses to the virus. We used phage display technology to generate panels of human monoclonal antibodies to HIV-1. We are examining human antibody responses to the virus and the antiviral activities of these antibodies. In particular, we generated a human monoclonal antibody, b12, that potently neutralizes a broad array of different viruses. The existence of this antibody indicates that some features of HIV-1 are conserved and are attractive targets for vaccines. Further, b12 and a few monoclonal antibodies with similar qualities are powerful tools for exploring antibody activity against HIV-1. Among the first questions we tackled were the following: Can antibodies protect against HIV-1 infection, and, if so, under what conditions? On the basis of passive transfer studies in a number of animal model systems, the answer is clearly yes. Complete protection from infection is possible at serum titers of neutralizing antibody greater than about 1:100, although lower titers can provide benefit in terms of lowered or delayed viremia. Further, we showed that topically applied antibody can protect monkeys against vaginal challenge with HIV. Another major issue is the best method for eliciting protective neutralizing antibodies. Accumulated evidence suggests that protective neutralizing antibodies are those antibodies that bind avidly to the envelope trimer on the surface of HIV-1 virions. However, such antibodies, particularly those to conserved regions of the envelope that are most important for vaccines, are difficult to elicit. Apparently the envelope trimer, which is composed of 2 glycoproteins, gp120 and gp41, has low antigenicity and immunogenicity. Several strate- H IMMUNOLOGY gies to circumvent these problems are being investigated. One strategy is to study the interaction of the neutralizing antibodies with envelope glycoprotein at the molecular level and then use the knowledge gained to design antigens capable of eliciting the relevant antibodies. In these studies, we are collaborating with I.A. Wilson, Department of Molecular Biology. Currently, we are studying the epitopes recognized by 5 broadly neutralizing antibodies: b12, 2G12, 4E10, Z13, and 2F5. The antibody b12 recognizes a complex epitope that overlaps the CD4-binding site of gp120. We are using a hyperglycosylation-mutagenesis strategy to “immunofocus” responses to this epitope by blocking nonneutralizing epitopes on gp120. The strategy is based on our previous determination of the structure of b12, docking of b12 and gp120, and extensive mutagenesis studies of b12 and gp120. The antibody 2G12 recognizes a cluster of sugar residues on gp120. Solution of the structure of 2G12 in complex with sugars enabled us, in collaboration with C.-H. Wong, Department of Chemistry, to design synthetic sugars that are being used as immunogens to elicit 2G12-like antibodies. The antibody 4E10 recognizes a linear epitope on gp41 (Fig. 1). We determined the structure of 4E10 with peptide bound, and we are working with P. Dawson, Department of Cell Biology, to design peptide immunogens that can elicit 4E10-like antibodies. The antibody Z13 recognizes an epitope on gp41 that overlaps with that of 4E10. Both 4E10 and Z13 are providing molecular insights into the structure and function of the membrane proximal region of HIV-1 gp41. 2005 109 gating the molecular origins of this problem. Overall, HIV vaccine development has been distilled down to challenges in the design of protein, carbohydrate, and peptide immunogens. PUBLICATIONS Binley, J.M., Wrin, T., Korber, B., Zwick, M.B., Wang, M., Chappey, C., Stiegler, G., Kunert, R., Zolla-Pazner, S., Katinger, H., Petropoulos, C.J., Burton, D.R. A comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency type 1 monoclonal antibodies. J. Virol. 78:13232, 2004. Blixt, O., Head, S., Mondala, T., Scanlan, C., Huflejt, M.E., Alvarez, R., Bryan, M.C., Fazio, F., Calarese, D., Stevens, J., Razi, N., Stevens, D.J., Skehel, J.J., van Die, I., Burton, D.R., Wilson, I.A., Cummings, R., Bovin, N., Wong, C.-H., Paulson, J.C. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc. Natl. Acad. Sci. U. S. A. 101:17033, 2004. Bryan, M.C., Fazio, F., Lee, H.-K., Huang, C.-Y, Chang, A., Best, M.D., Calarese, D.A., Blixt, O., Paulson, J.C., Burton, D., Wilson, I.A., Wong, C.-H. Covalent display of oligosaccharide arrays in microtiter plates. J. Am. Chem. Soc. 126:8640, 2004. Cardoso, R.M.F., Zwick, M.B., Stanfield, R.L., Kunert, R., Binley, J.M., Katinger, H., Burton, D.R., Wilson, I.A. Broadly neutralizing anti-HIV antibody 4E10 recognizes a helical conformation of a highly conserved fusion-associated motif in gp41. Immunity 22:163, 2005. Ho, J., Uger, R.A., Zwick, M.B., Luscher, M.A., Barber, B.H., MacDonald, K.S. Conformational constraints imposed on a pan-neutralizing HIV-1 antibody epitope result in increased antigenicity but not neutralizing response. Vaccine 23:1559, 2005. Koefoed, K., Farnaes, L., Wang, M., Svejgaard, A., Burton, D.R., Ditzel, H.J. Molecular characterization of the circulating anti-HIV-1 gp120-specific B cell repertoire using antibody phage display libraries generated from pre-selected HIV-1 gp120 binding PBLs. J. Immunol. Methods 297:187, 2005. O’Connor, D.H., McDermott, A.B., Krebs, K.C., Dodds, E.J., Miller, J.E., Gonzalez, E.J., Jacoby, T.J., Yant, L., Piontkivska, H., Pantophlet, R., Burton, D.R., Rehrauer, W.M., Wilson, N., Hughes, A.L., Watkins, D.I. A dominant role for CD8+-T-lymphocyte selection in simian immunodeficiency virus sequence variation. J. Virol. 78:14012, 2004. Pantophlet, R., Wilson, I.A., Burton, D.R. Improved design of an antigen with enhanced specificity for the broadly HIV-neutralizing antibody b12. Protein Eng. Des. Sel. 17:749, 2004. Zhang, M.Y., Shu, Y., Rudolph, D., Prabakaran, P., Labrijn, A.F., Zwick, M.B., Lal, R.B., Dimitrov, D.S. Improved breadth and potency of an HIV-1-neutralizing human single-chain antibody by random mutagenesis and sequential antigen panning. J. Mol. Biol. 335:209, 2004. Zwick, M.B., Jensen, R., Church, S., Wang, M., Stiegler, G., Kunert, R., Katinger, H., Burton, D.R. Anti-human immunodeficiency virus type 1 (HIV-1) antibodies 2F5 and 4E10 require surprisingly few crucial residues in the membrane-proximal external region of glycoprotein gp41 to neutralize HIV-1. J. Virol. 79:1252, 2005. Antibodies and Emerging Viruses S.K. Kurz-Camacho, W. Oswald, E.O. Saphire, P.B. Jahrling,* P.M. Rudd,** R.A. Dwek,** H. Feldman,*** D.R. Burton F i g 1 . Model of 2F5 and 4E10, broadly neutralizing antibodies to HIV-1, bound to the membrane proximal external region of gp41. The fifth antibody, 2F5, recognizes another linear epitope on gp41 (Fig. 1). Previous attempts to present this epitope in a wide variety of immunogens did not elicit 2F5-like neutralizing antibodies. We are investiPublished by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. * U.S. Army Medical Research Institute of Infection Diseases, Frederick, Maryland ** Oxford Glycobiology Institute, Oxford, England *** Bureau of Microbiology, Health Canada, Winnipeg, Manitoba W e are interested in determining the immunogenicity of soluble vs surface glycoproteins of Ebola virus, a filovirus that is one of the 110 IMMUNOLOGY 2005 deadliest human pathogens. Results indicate strong cross-reactivity and immunogenicity of the different glycoproteins. This finding supports the hypothesis that some of the soluble forms of the glycoproteins act as decoys. Another focus of our Ebola virus research is the early molecular events that occur during infection with the virus. We found that changes in cellular gene expression occurred as early as 1 hour after Ebola virus infection of mononuclear phagocytes, the major target cell of the virus. On the basis of our microarray data, we hypothesized that binding and entry of the Ebola virus glycoprotein cause the early changes in expression levels. To test this hypothesis, we produced soluble forms of the glycoprotein and Ebola viruslike particles that present the glycoprotein on the surface. Incubation with the viruslike particles but not with the soluble glycoproteins caused the same changes in cellular gene and protein expression in Ebola virus target cells as did infection with live Ebola virus. We detected changes in gene expression for inflammatory cytokines, chemokines, molecules involved in the development of hemorrhage, and potential receptors for the virus. Using the viruslike particles, we are testing whether the indicated receptors are involved in Ebola virus binding and signaling. PUBLICATIONS Wahl-Jensen, V., Kurz, S.K., Hazelton, P.R., Schnittler, H.-J., Ströher, U., Burton, D.R., Feldmann, H. The role of Ebola virus secreted glycoproteins and virus-like particles in activation of human macrophages. J. Virol. 79:2413, 2005. Reverse Cholesterol Transport and High-Density Lipoproteins L.K. Curtiss, C. Flood, N.J. Hime, A.E. Mullick, R.J. Petrovan, D.T. Valenta n order to be removed from the body, cholesterol must be dissolved into or converted to bile acids in the liver. This biliary excretion pathway is fed by the transport of cholesterol from peripheral tissues and is referred to as reverse cholesterol transport. An early step in reverse cholesterol transport is the transfer of peripheral cell-free cholesterol to plasma high-density lipoproteins (HDLs). The HDLs serve as transport vehicles for excess cellular cholesterol through the plasma compartment to the liver. The major protein in HDL is apolipoprotein AI. Importantly, the transfer of cellular cholesterol to HDL does not occur in plasma. It occurs in extracellular spaces such as the subendothelial space or intima of a vessel within an atherosclerotic lesion. I Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. We are investigating how apolipoprotein AI promotes the efficient transfer of excess cholesterol from peripheral cells (i.e., intimal macrophage foam cells) to HDL. We have published convincing in vivo data that apolipoprotein E and apolipoprotein AI participate in the efflux of free cholesterol from atherosclerotic lesions. We found that both plasma-derived apolipoprotein AI and macrophage-produced apolipoprotein E participate in the efficient efflux of free cholesterol from aortas containing atherosclerotic lesions in atherosclerosis-prone mice. The in vivo specificity for apolipoprotein AI in efflux of cholesterol from macrophages in atherosclerotic lesions is a direct function of the ability of the apolipoprotein to dissociate from spherical HDL and to form transiently stable, lipid-poor apolipoprotein AI, which can accept macrophage ATP binding cassette A1–transported free cholesterol and phospholipid. We are studying this phenomenon in mice that lack receptors for low-density lipoprotein. Mice deficient in receptors for low-density lipoprotein are severely hyperlipidemic, and detectable atherosclerotic lesions develop in the aorta and aortic sinus within weeks if the animals are fed a high-fat diet. We are focusing on the role of multiple macrophage nuclear liver X receptor–inducible genes in reverse cholesterol transport. We are determining the role played by phospholipid transfer protein (PLTP) in the generation of lipid-poor or lipid-free apolipoprotein AI in vivo and in vitro. To examine the role of macrophage-derived PLTP in cholesterol metabolism and atherosclerosis, we performed bone marrow transplantations in mice deficient in receptors for low-density lipoprotein; the mice were lethally irradiated and were reconstituted with either wild-type or PLTP-deficient bone marrow cells. The transplanted animals were fed a high-fat diet for 16 weeks to induce atherosclerosis. We found that macrophage PLTP deficiency led to increases in the total plasma levels of cholesterol and in the extent of atherosclerotic lesions, suggesting an atheroprotective role of macrophage-derived PLTP in the intima. We are also determining the role of cholesteryl ester transfer protein (CETP) in this same process in vivo and in vitro. Both PLTP and CETP are expressed by macrophages, can generate lipid-poor apolipoprotein AI from spherical HDL, are present in atherosclerotic lesions, and are induced by ligation of liver X receptors. Finally, we are studying the role played by the lipoprotein triglyceride hydrolases lipoprotein lipase and hepatic lipase in the generation of lipid-poor apolipo- IMMUNOLOGY protein AI in vitro. Both of these lipases also are expressed by macrophages, are present in atherosclerotic lesions, and are upregulated by ligation of nuclear liver X receptors. Macrophage-derived lipoprotein lipase and hepatic lipase promote the formation of lipid-poor apolipoprotein AI from mature HDL. This remodeling of HDL facilitates a reduction in the size of HDL particles to provide free apolipoprotein AI substrate for PLTP and CETP lipid transfer. Both lipid transfer proteins and neutral triglyceride lipases participate in HDL remodeling and in macrophage-mediated efflux of cholesterol from atherosclerotic lesions. Therefore a number of nuclear liver X receptor–inducible gene products are expressed by macrophages in response to the accumulation of cholesteryl ester and participate in reverse cholesterol transport to prevent the formation of foam cells. PUBLICATIONS Bradshaw, G., Gutierrez, A., Miyake, J.H., Davis, K.R., Li, A.C., Glass, C.K., Curtiss, L.K., Davis, R.A. Facilitated replacement of Kupffer cells expressing a paraoxonase-1 transgene is essential for ameliorating atherosclerosis in mice. Proc. Natl. Acad. Sci. U. S. A. 102:11029, 2005. Jahangiri, A., Rader, D.J., Marchadier, D., Curtiss, L.K., Bonnet, D.J., Rye, K.-A. Evidence that endothelial lipase remodels high density lipoproteins without mediating the dissociation of apolipoprotein A-I. J. Lipid Res. 25:896, 2005. Mullick, A.E., Tobias, P.S., Curtiss, L.K. Modulation of atherosclerosis in mice by Toll-like receptor 2. J. Clin. Invest., in press. Schneider, M., Witztum, J.L., Young, S.G., Ludwig, E.H., Miller, E., Tsimikas, S., Curtiss, L.K., Marcovina, S.M., Taylor, J.M., Lawn, R.W., Innerarity, T.L., Pitas, R.E. High level lipoprotein (a) expression in transgenic mice: evidence for oxidized phospholipid in lipoprotein (a) but not in low density lipoprotein. J. Lipid Res. 46:769, 2005. Tobias, P., Curtiss, L.K. Paying the price for pathogen protection: Toll receptors in atherogenesis. J. Lipid Res. 46:404, 2005. Wiedmer, T., Zhao, J., Li, L., Zhou, Q., Hevener, A., Olefsky, J.M., Curtiss, L.K., Corr, M., Witztum, J.L. Adiposity, dyslipidemia and insulin resistance in mice with targeted deletion of phospholipid scramblase 3 (PLSCR3). Proc. Natl. Acad. Sci. U. S. A. 101:13296, 2004. Expression of a Novel Large Noncoding RNA in Neoplasia, Proliferation, and Differentiation R. Lin, C. Liu, T.S. Edgington, S. Maeda,* M. Karin* * University of California, San Diego, California ecent genomic and transcriptomic studies have revealed the existence of a substantial number of large noncoding RNAs. Only a few of these RNAs have been characterized, and little is known of their functional roles. Using differential display analysis of CD31-enriched tumor microvascular endothelial R Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. 2005 111 cells from a syngeneic murine colon carcinoma, we discovered a novel large noncoding RNA of approximately 360 base pairs. The full-length gene is a 7000-base mRNA-like transcript but lacks extensive or conserved open reading frames. Bioinformatics analysis suggested that the full-length gene is highly conserved among vertebrates, implying functional significance. More important, Northern blot analysis and in situ hybridization indicated that expression of the full-length transcript is highly upregulated in carcinogen-induced primary hepatomas and is a novel marker for hepatomas and for other neoplasms so far analyzed. We designated this noncoding RNA hepatomin. We found that hepatomin transcription is induced in liver undergoing regeneration, skin undergoing wound healing, hair follicles during growth of hair, and cultured cells synchronized at the mitosis stage. These results suggest that hepatomin plays roles in proliferation and differentiation. We conclude that hepatomin is a member of the new class of large noncoding RNAs and a new marker for hepatomas. This discovery can provide unique murine models for analysis of the role of this noncoding RNA in neoplasia, proliferation, and differentiation as an apparent regulator of the changes in gene expression associated with these processes. Novel Redirected Molecular Expression, Architecture, and Properties in the Microvasculature of Tumors A. El-Sheikh, G. Bhattacharjee, Z. Ruggeri,* T.S. Edgington, P. Borgstrom** * Department of Molecular and Experimental Medicine, Scripps Research ** Sidney Kimmel Cancer Center, La Jolla, California eoplastic cells induce remarkable changes in their new angiogenic microvasculature as they seek nutrients, oxygen, and the freedom to invade and metastasize. The loss of normal vascular development and architecture results in a bizarre, functionally incompetent maze of capillary-like vessels lined by endothelium with strikingly divergent gene transcription and aberrant display of cell-surface proteins. Understanding the aberrations may broaden our knowledge N 112 IMMUNOLOGY 2005 of the molecular biology of these complex angiogenic and postangiogenic vascular mazes, and some of the molecules involved may provide novel targets for therapeutic intervention. We discovered an endothelial receptor complex that appears unique for the tumor microvascular environment. Vascular endothelial growth factor receptor 2 and its coreceptor neuropilin-1 can be found elsewhere within the vascular tree, but the association of an oligosaccharide (chondroitin C sulfate) to create a ternary complex is unique to the tumor microvasculature. This complex is a novel target for therapeutic strategies. We developed a thrombogen specific for tumor vasculature that recognizes this ternary complex selectively on tumor neoangiogenic vessels. This soluble hybrid protein, HBDt-TFt, incorporates the modified exon 7–encoded heparin-binding domain of the gene for vascular endothelial growth factor at its N terminus with the extracellular domain of tissue factor at the C terminus. The hybrid can selectively dock on the surface of tumor endothelium at this trimolecular complex and initiate the coagulation protease cascade. Subsequently, the tumor microvasculature is thrombosed, resulting in local infarctive eradication of tumor. Using intravital microscopy, we optimized the dose and rate of infusion of HBDt-TFt and monitored microcirculation within the tumor as well as thrombus formation. In addition, using fluorescein-labeled platelets, we showed a central participation of platelets in tumor-specific microvascular thrombosis. Regulation of Tissue Factor–Mediated Initiation of the Coagulation Cascade by Grp78 G. Bhattacharjee, J. Ahamed, B. Pedersen, A. El-Sheikh, N. Mackman, W. Ruf, C. Liu, T.S. Edgington e used biopanning with phage-displayed peptidyl libraries to discover peptide probes that bind selectively to the surface of the endothelium in atherosclerotic plaques. EKO130, the peptide with the highest affinity among those tested, binds the 78-kD glucose-regulated protein (Grp78). This protein participates in many pathologic processes, including regulation of the coagulation cascade. To characterize W Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. how Grp78 regulates coagulation, we analyzed the effect of the protein on tissue factor–mediated procoagulant activity in mouse brain endothelial cells and in macrophage-like cells. We found that Grp78 is present on the endothelium and on monocyte/macrophage-like cells in atherosclerotic lesions. Inhibition of Grp78 increased procoagulant activity. We also found that Grp78 negatively regulates procoagulant activity by interacting directly with the extracellular domain of tissue factor on the cell surface. These findings indicate that Grp78 negatively regulates the functional activity of tissue factor by binding directly to tissue factor and subsequently inhibiting it. Determining how Grp78 regulates the function of tissue factor may provide insight into the pathobiology of atherosclerosis and associated arterial thrombosis. Control of V(D)J Recombination and Formation of the Antibody Repertoire in Normal and Autoimmune Mice A.J. Feeney, C.R. Espinoza, J. Lamoureux, M. Cherrier, L. Watson, P. Lao, R.Z. MacDonald main focus of our laboratory is the molecular analysis of factors that influence the composition of the antibody repertoire and elucidation of the mechanisms that control the V(D)J rearrangement. In each precursor B lymphocyte, a different set of V, D, and J genes recombines to form exons for the light and heavy chains of the antibody molecule. Each locus has many V, D, and J genes, but the gene segments are not used equally. One of our goals is to understand the basis of this nonrandom use of gene segments. We previously showed that much of this bias occurs because V genes undergo recombination with different intrinsic frequencies due to differences in the recombinase signal sequence, the binding site for the recombinase, flanking each gene segment. The recombinase signal sequence is composed of a relatively conserved heptamer and nonamer flanking a “spacer” of conserved length but only modestly conserved sequence. Few genes have consensus heptamers and nonamers, however, and changes in this natural variation in the recombinase signal sequence can greatly affect recombination A IMMUNOLOGY frequency in vitro and in vivo. Surprisingly, even differences in the sequence of the spacer can greatly influence recombination frequency, and these differences also contribute to nonrandom use of genes in vivo. However, other factors clearly influence recombination frequencies, and currently, we are focusing on the role of transcription factors and chromatin modifications in controlling accessibility to V(D)J recombination and recombination frequency. Genes in loci that are undergoing recombination are often associated with histones that are acetylated. We hypothesized that the extent of histone modification affects the frequency of recombination of individual genes, and indeed we observed a correlation between the relative rearrangement frequency of several individual genes in vivo and the extent of acetylation of histones H3 and H4 associated with those genes as assessed by chromatin immunoprecipitation. We also use a novel system in which certain immunoglobulin gene rearrangements can be induced in a nonlymphoid cell line after the transient transfection by vectors expressing a B cell–specific transcription factor, E2A or EBF, and the recombinases. We showed that E2A induces rearrangement of VκI genes. However, the VκII and VκIII genes, which are interspersed with the VκI genes within the IgVκ locus, seldom rearrange after E2A transfection. EBF induces VλJλ rearrangement, but mainly of only a single Vλ gene. Thus, this induction of accessibility of genes is not uniform across the locus. Neighboring genes can be differentially induced to rearrange, suggesting localized control of accessibility for rearrangement. Current studies are aimed at elucidating the mechanism of this localized gene-specific control. Transfection with E2A does not induce much acetylation of the histones associated with VκI genes, but the rearranged genes are associated with acetylated histones, suggesting that once a V gene becomes accessible, it efficiently rearranges. In other studies, we are examining the breakdown of B-cell tolerance in autoimmunity. When precursor B cells successfully recombine both heavy- and lightchain gene segments, they express a B-cell receptor for the first time. If the receptor is autoreactive, then the immature B cell normally continues to undergo lightchain V-J rearrangement until an innocuous receptor is made. This process is termed receptor editing and is an important checkpoint in B-cell tolerance. We have evidence that this process is not functioning the same in lupus-prone mice as in nonautoimmune mice, and Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. 2005 113 we are investigating why this difference occurs. Such misregulation of this key checkpoint could lead to the release of autoreactive B cells into the periphery, where they can become activated to secrete autoantibodies and cause autoimmune disease. PUBLICATIONS Li, L., Salido, E., Zhou, Y., Battacharyya, S., Yannone, S.M., Dunn, E., Meneses, J., Feeney, A.J., Cowan, M.J. Targeted disruption of the Artemis murine counterpart results in SCID and defective V(D)J recombination that is partially corrected with bone marrow transplantation. J. Immunol. 174: 2420, 2005. Syndecans and HIV Type 1 Pathogenesis M. Bobardt, U. Chatterji, A. de Parseval,* J. Elder,* P. Gallay * Department of Molecular Biology, Scripps Research yndecans are transmembrane receptors highly expressed on adherent cells (e.g., macrophages, epithelial or endothelial cells) but poorly expressed on suspension cells (e.g., T lymphocytes). The syndecan family has 4 members, syndecan-1 through syndecan-4. The ectodomain in each syndecan has linear heparan sulfate chains, which are composed of a repetition of a sulfated disaccharide motif. The sulfation pattern of the heparan sulfates dictates the ligand specificity of the syndecan. Syndecans function as receptors for HIV type 1 (HIV-1). Pretreatment of target cells with heparinase that removes heparan sulfates from syndecans dramatically reduces HIV-1 infectivity. We showed that syndecans also serve as in trans (i.e., on the surface of cells opposite each other) receptors for HIV-1. Specifically, HIV-1 binds syndecans richly expressed on the endothelium. HIV-1 bound to syndecans remains infectious for a week, whereas cell-free virus loses its infectivity after a single day. Most importantly, HIV-1 attached to the endothelium via syndecans is an in trans source of infection for circulating T cells. These findings suggest that syndecan-rich endothelium can provide a microenvironment that amplifies HIV-1 replication in T cells. Last, we showed that syndecans on brain microvascular endothelial cells play a significant role in HIV-1 transmigration through the blood-brain barrier. Altogether these observations suggest that syndecans, by acting as in cis (i.e., on the surface of the same cell) and in trans receptors, may profoundly affect HIV-1 pathogenesis. We recently found that a single conserved arginine at position 298 in the HIV envelope glycoprotein gp120 S 114 IMMUNOLOGY 2005 governs HIV-1 binding to syndecans. An amine group on the side chain of this residue is necessary for syndecan utilization by HIV-1. We showed that HIV-1 binds syndecans via a 6-O sulfation, indicating that this binding is not the result of random interactions between basic residues and negative charges but the result of specific contacts between gp120 and a welldefined sulfation in syndecans. Surprisingly, arginine at position 298 that mediates HIV-1 binding to syndecans also mediates HIV-1 binding to the major HIV-1 entry receptor CCR5. We postulated that HIV-1 recognizes similar motifs on syndecans and CCR5. In support of this hypothesis, we found that the 6-O sulfation recognized by HIV-1 on syndecans mimics the sulfated tyrosines recognized by HIV-1 in the N terminus of CCR5. Our finding that CCR5 and syndecans are exploited by HIV-1 via a single determinant echoes the mechanisms by which chemokines use these 2 disparate receptors and suggests that the gp120-chemokine mimicry may represent a common strategy in microbial pathogenesis. PUBLICATIONS de Parseval, A., Bobardt, M.D., Chatterji, A., Chatterji, U., Elder, J.H., David, G., Zolla-Pazner, S., Farzan, M., Lee, T.-H., Gallay, P.A. A highly conserved arginine in gp120 governs HIV-1 binding to both syndecans and CCR5 via sulfated motifs. J. Biol. Chem., in press. Gallay, P. Syndecans and HIV-1 pathogenesis. Microbes Infect. 6:617, 2004. Innate Intracellular Immunity and Infection With HIV Type 1 not have any idea of how this restriction factor blocks HIV-1 infection. An understanding of the nature of restrictions to HIV-1 infection after cellular entry of the virus in primates is critical for several reasons. First, information on the viral and cellular factors that modulate these processes will shed light on the poorly understood series of events that govern the fate of capsids after entry. Second, species-specific barriers to HIV-1 infection present obstacles to the development of animal models for the study of HIV-1 pathogenesis, treatment, and prophylaxis. Finally, an understanding of this critical part of the HIV-1 life cycle may suggest approaches to intervene in HIV-1 transmission to or spread within the host. PUBLICATIONS Chatterji, U., Bobardt, M.D., Stanfield, R., Ptak, R.C., Pallansch, L.A., Ward, P.A., Jones, M.J., Stoddart, C.A., Scalfaro, P., Dumont, J.-M., Besseghir, K., Rosenwirth, B., Gallay, P.A. A naturally occurring capsid motif renders HIV-1 cyclophilin A independent in human cells and TRIM-cyclophilin resistant in owl monkey cells. J. Biol. Chem., in press. Galigniana, M.D., Morishima, Y., Gallay, P.A., Pratt, W.B. Cyclophilin-A is bound through its peptidylprolyl isomerase domain to the cytoplasmic dynein motor protein complex. J. Biol. Chem. 279:55754, 2004. Molecular Interactions in T-Cell Development and Activation N.R.J. Gascoigne, J. Ampudia, G. Fu, K. Holmberg, H.-C. Hung, H.-O. Kim, C. Lotz, A. Munshi, N. Niederberger, G. Sternik, S. Vallee, P. Yachi, M.A. Zal, T. Zal, M. Gronski,* P. Ohashi,* M.Y. Lin,** S.M. Hedrick,** N. Bosco,*** R. Ceredig,*** M. Cahalan**** U. Chatterji, M. Bobardt, P. Gallay * Ontario Cancer Institute, Toronto, Ontario onhuman primate cells contain intracellular innate factors that inhibit infection by HIV type 1 (HIV-1) by targeting the incoming viral capsid core, which makes up the shell that surrounds the viral genome. These restriction factors block HIV-1 replication at steps before integration but subsequent to entry. The first intracellular primate restriction factor identified, TRIM5α, is a member of the tripartite motif (TRIM) family of proteins. The introduction of TRIM5α derived from species that highly restrict HIV-1 (e.g., Rhesus macaque, African green or owl monkeys) into cells that are normally permissive (e.g., human cells) makes the cells nonpermissive to HIV-1. However, although TRIM5α was identified more than 2 years ago and several laboratories are actively studying it, we still do ** University of California, San Diego, California N Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. *** U548 INSERM, Grenoble, France **** University of California, Irvine, California IMAGING MOLECULAR INTERACTIONS IN LIVING C E L L S I N T - C E L L A C T I VAT I O N e used live-cell fluorescence deconvolution microscopy and fluorescence resonance e nergy transfer (FRET) microscopy to investigate molecular movement and intermolecular interactions during T-cell activation. FRET between cyan and yellow fluorescent proteins is effective at ranges of less than 10 nm and is therefore ideal to investigate interactions between proteins in living cells. Using FRET, we showed that the coreceptor CD8 and the T-cell receptor (TCR) signal-transducing protein W IMMUNOLOGY CD3ζ are recruited to the “immunologic synapse,” where they interact when antigen is presented to the T cell. No FRET occurs when weaker (e.g., TCR antagonist) ligands are used. We compared formation of the immunologic synapse and the TCR-coreceptor interaction in a system in which the affinity of the interaction between TCRs and MHC-peptides is known. We found that the strength of weak agonists is more closely related to the speed at which they recruit TCRs to the synapse and start to induce FRET than it is to the affinity of the interactions between TCRs and MHC-peptides. The induction of FRET appears to explain why some agonists are stronger or weaker than would be predicted on the basis of their affinities. By controlling the level of antigenic MHC-peptide complexes presented to T cells, in the presence or absence of natural endogenous nonstimulatory MHC-peptides, we found that the endogenous complexes aided in the recognition of the antigenic complexes. The interaction between CD8 and the endogenous MHC-peptide improves the TCR recognition of the antigenic MHC-peptide, including the ability to associate with CD8. This surprising finding suggests how T cells can respond to small amounts of antigens in a “sea” of nonstimulatory MHC-peptides. In a series of experiments in collaboration with P. Ohashi, Ontario Cancer Institute, Toronto, Ontario, we found that the strength of TCR interaction with MHC peptide ligands alters the ability of an autoantigen to induce diabetes, so that a reduced affinity results in a reduced incidence of disease. We also used fluorescent tetrameric class I MHC molecules to measure binding of TCR-MHC-peptide complexes on T cells to compare binding of negative- and positive-selecting ligands. Although crystallographic studies indicate that the coreceptor CD4 exists as a dimer, biochemical or biological evidence for this finding has been weak. We made CD4 chimeras with cyan and yellow fluorescent proteins to probe dimerization on the cell surface. We found a weak constitutive association, which was greatly increased at the immunologic synapse during antigen recognition. TWO-PHOTON MICROSCOPY OF TCR AND CORECEPTOR MOVEMENT IN LIVING TISSUES Two-photon microscopy allows visualization of cells deep in tissues and thus allows observation of T cells interacting with antigen-presenting cells during an immune response or of thymocytes interacting with thymic stromal cells during development. We produced transgenic mice that express the fluorescent chimeric Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. 2005 115 CD8 and CD3ζ molecules, as well as transgenic TCRs, and are collaborating with M. Cahalan, University of California, Irvine, to use 2-photon microscopy to investigate movement and interaction of TCRs and coreceptors. We found that the cell-surface molecules form synapses within tissues, and we are investigating how the synapses form during the initiation of an immune response, during responses to a solid tumor, and during thymocyte development. ROLE OF THE PROTEIN KINASE η ISOFORM IN THE IMMUNOLOGIC SYNAPSE We found that the η isoform of protein kinase C (PKCη) is upregulated after TCR ligation in developing thymocytes and in natural positive selection. Of the PKC isoforms, only PKCθ is known to have a special role in T cells, where it is recruited to the immunologic synapse during antigen recognition. The finding that mice deficient in PKCθ have normal thymic selection suggested that PKCη could be replacing PKCθ in the developing thymocytes. We found that PKCη is also naturally recruited to the synapse in mature thymocytes and T cells. In the absence of PKCθ, PKCη is expressed at an earlier stage of thymocyte development, where it functions in place of PKCθ. G E N E E X P R E S S I O N I N E A R LY T - C E L L D I F F E R E N T I AT I O N We identified a novel protein with strongly regulated expression during thymocyte differentiation. The protein is expressed during the stages of TCR gene rearrangement. It interacts with the cell-cycle and DNA damage– repair enzyme ATM and with phospholipase Cγ1, which is important in T-cell signaling. We are now using small interfering RNA, gene ablation, and transgenic techniques to investigate the possible role of the protein in development and T-cell signaling. TCR ENDOCYTOSIS, RECYCLING, AND U B I Q U I T I N AT I O N Because allelic exclusion of the TCR α-chain is poor, many mature T cells express 2 α-chain proteins. However, expression of 2 α-chains on the cell surface is quite rare. We previously showed that functional allelic exclusion is attained in the thymus at the start of positive selection and that this exclusion is posttranslationally regulated. We found that the positively selected αβ combination remains on the surface when the TCR is stimulated, whereas the other αβ combination is endocytosed. Endocytosis and allelic exclusion are controlled by TCR signaling involving the kinase Lck and the ubiquitin ligase Cbl, which controls degra- 116 IMMUNOLOGY 2005 dation of endocytosed TCRs. To analyze ubiquitination of TCRs after endocytosis, we are using FRET between ubiquitin monomers and TCR subunits labeled with fluorescent proteins. In collaboration with R. Ceredig, INSERM, Grenoble, France, we examined the TCR α-chain repertoire of specialized CD25+CD4 + regulatory T cells. We found that the repertoire is as diverse as that of mainstream CD4+ T cells. PUBLICATIONS Bosco, N., Hung, H.-C., Pasqual, N., Jouvin-Marche, E., Marche, P.N., Gascoigne, N.R.J., Ceredig, R. Role of the T cell receptor α-chain in the development and phenotype of naturally arising CD25+CD4+ T cells. Mol. Immunol., in press. Gronski, M.A., Boulter, J.M., Moskophidis, D., Nguyen, L.T., Holmberg, K., Elford, A.R., Deenick, E.K., Kim, H.O., Penninger, J.M., Odermatt, B., Gallimore, A., Gascoigne, N.R.J., Ohashi, P.S. TCR affinity and negative regulation limit autoimmunity. Nat. Med. 10:1234, 2004. Lin, M.Y., Zal, T., Ch’en, I.L., Gascoigne, N.R.J., Hedrick, S.M. A pivotal role for the multifunctional calcium/calmodulin-dependent protein kinase II in T cells: from activation to unresponsiveness. J. Immunol. 174:5583, 2005. Niederberger, N., Buehler, L.K., Ampudia, J., Gascoigne, N.R.J. Thymocyte stimulation by anti-TCRβ, but not by anti-TCRα, leads to induction of developmental transcription program. J. Leukoc. Biol. 77:830, 2005. Yachi, P.P., Ampudia, J., Gascoigne, N.R.J., Zal, T. Nonstimulatory peptides contribute to antigen-induced CD8-T cell receptor interaction at the immunological synapse. Nat. Immunol. 6:785, 2005. ligand influences ligand-induced MAP kinase activation but has no effect on NF-κB activation. Studies are under way to define the mechanisms that selectively activate MAP kinase and NF-κB. INTRACELLULAR SIGNALING The p38 MAP kinase pathway is important in TLRinduced cellular changes. One of our interests is to determine how the signal is transduced from TLRs to the p38 pathway. We evaluated the contribution of MAP kinase kinase (MKK)–dependent and MKK-independent p38 activation. We found that both MKK-dependent and MKK-independent pathways contribute to p38 activation; the MKK-dependent pathway is the primary one in most situations. We also found that MKK-independent p38 activation can occur with or without dependence on TAB1, the activating protein for transforming growth factor-β–activated kinase 1. MyD88, TRIF, and TRAF6 are clearly the signaling molecules between the TLRs and p38 activation. However, which molecules link these adaptors and the p38 pathway is still not clear; we are identifying these proteins. We think that information on the proteins will bridge the gap in our knowledge of how the p38 pathway is activated by TLRs. R E G U L AT I O N O F G E N E E X P R E S S I O N Cellular Activation Mechanisms in the Innate Immune System J. Chen, M. Cheung, Y. Kang, S. Lee, Y. Li, J. Mols, A. Seit-Nebi, M. Otsuka, C.-C. Wu, Y. Xu, T. Zarubin, H. Zhou, J. Han acterial pathogens and inflammatory cytokines are important players in the innate immune response. Our interests are the signal transduction pathways activated by pathogens and cytokines. We study the signaling events at different cellular levels. B S I G N A L I N G AT T H E C E L L S U R FA C E Toll-like receptors (TLRs) are an important point of first contact between host and microbe. Once activated, the receptors generate signals that culminate in the induction of genes important for host defense. TLRs often need to cooperate with other proteins on the cell surface to function. For example, CD14 and MD2 are involved in TLR4-mediated activation by lipopolysaccharide. We found that 4-1BB (CD137) ligand is another protein that interacts with TLRs on the cell surface. Unlike CD14 and MD2, 4-1BB ligand affects intracellular signaling rather than ligand recognition. 4-1BB Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. The p38 pathway plays a key role in posttranscriptional regulation of cytokine gene expression. We used Drosophila melanogaster S2 cells to screen a number of genes for their requirement in mRNA decay mediated by adenine-uridine–rich elements (AREs). We found that proteins in the Dicer and Ago families are required for ARE-mediated mRNA decay. We further confirmed the requirement of Dicer in mammalian cells. Because Dicer and Ago family members are required for processing microRNA, we evaluated whether any microRNA is required for ARE-mediated mRNA degradation. Sequence analysis revealed that miR16, a human microRNA containing a sequence complementary to the ARE sequence, has complementary sequence to miR16, and we confirmed that miR16 is required for the quick ARE-mediated decay of mRNA. Because miR16 and ARE can only form a maximum of 8 pairs, most likely miR16 alone is not sufficient to guide the targeting of AREs by the RNA-induced gene-silencing complex (RISC). We hypothesized that ARE-binding proteins may have a role and further determined that the ARE-binding protein tristetraprolin is required for miR16 to target ARE-containing mRNA. Tristetraprolin does not directly interact with miR16, but via association with an Ago family member forms a complex with miR16. IMMUNOLOGY Thus, the initiation of ARE-induced mRNA decay requires miR16-containing RISC and tristetraprolin. Currently, we are focusing on the signaling mechanism between the p38 pathway and the involvement of RISC and tristetraprolin in ARE-mediated mRNA decay. M A C R O P H A G E D E AT H The activation and life span of macrophages are important in innate immunity. Although macrophage death in vivo has not been well evaluated, it can be induced by different stimuli, such as a combination of cytokines and lipopolysaccharide or anthrax lethal toxin. We used 2 systems, macrophage death induced by lipopolysaccharide plus the caspase inhibitor vZAD and macrophage death induced by anthrax lethal toxin, to study the death process of macrophages. Previously, we showed that induction of the orphan nuclear receptor Nur77 plays a role in macrophage death induced by lipopolysaccharide plus vZAD. We recently found that macrophage death induced by this combination had a phenotype of autophagy and depends on reactive oxygen species and poly(ADP-ribose) polymerase. We also found that macrophage death induced by lipopolysaccharide plus vZAD can be effectively blocked by serine protease inhibitors. Thus, multiple cellular events may be involved in this type of macrophage death. To study macrophage death induced by anthrax lethal toxin, we used random mutagenesis to identify genes that are required for lethal toxin–induced death. The identified genes are being characterized. PUBLICATIONS Cao, J., Semenova, M.M., Solovyan, V.T., Han, J., Coffey, E.T., Courtney, M.J. Distinct requirements for p38α and c-Jun N-terminal kinase stress-activated protein kinases in different forms of apoptotic neuronal death. J. Biol. Chem. 279:35903, 2004. Jing, Q., Huang, S., Guth, S., Zarubin, T., Motoyama, A., Chen, J., Di Padova, F., Lin, S.-C., Gram, H., Han, J. Involvement of microRNA in AU-rich element-mediated mRNA instability. Cell 120:623, 2005. Li, J., Li, Q., Xie, C., Zhou, H., Wang, Y., Zhang, N., Shao, H., Chan, S.C., Peng, X., Lin, S.-C., Han, J. β-Actin is required for mitochondria clustering and ROS generation in TNF-induced caspase-independent cell death. J. Cell Sci. 117(Pt. 20):4673, 2004. Qi, X., Tang, J., Pramanik, R., Schultz, R.M., Shirasawa, S., Sasazuki, T., Han, J., Chen, G. p38 MAPK activation selectively induces cell death in K-ras-mutated human colon cancer cells through regulation of vitamin D receptor. J. Biol. Chem. 279:22138, 2004. Rui, Y., Xu, Z., Lin, S., Li, Q., Rui, H., Luo, W., Zhou, H.M., Cheung, P.Y., Wu, Z., Ye, Z., Li, P., Han, J., Lin, S.C. Axin stimulates p53 functions by activation of HIPK2 kinase through multimeric complex formation. EMBO J. 23:4583, 2004. 2005 117 Shen, G., Hebbar, V., Nair, S., Xu, C., Li, W., Lin, W., Keum, Y.S., Han, J., Gallo, M.A., Kong, A.N. Regulation of Nrf2 transactivation domain activity: the differential effects of mitogen-activated protein kinase cascades and synergistic stimulatory effect of Raf and CREB-binding protein. J. Biol. Chem. 279:23052, 2004. Specificity and Function of Intraepithelial γδ T Cells W.L. Havran, G. Cauvi, M. Haynes, J. Jameson, H.K. Komori, T. Meehan, R. Mills, L. Sharp, D. Witherden e have a long-term interest in interactions between intraepithelial γδ T cells and their neighboring epithelial cells. We focus on interactions in the thymus, skin, and intestine. We are investigating the development, specificity, and function of these γδ T cells. Our results have defined unique properties of these cells and support a specialized role for epithelial γδ T cells in immune surveillance, wound repair, inflammation, and protection from malignant tumors. W M O L E C U L E S R E Q U I R E D F O R γδ T - C E L L A C T I V A T I O N In murine skin, γδ T cells express an invariant γδ T-cell receptor that recognizes an unknown antigen expressed by damaged or malignant neighboring keratinocytes. We propose that in addition to antigen, damaged keratinocytes express molecules that participate in activation of skin γδ T cells by binding to coreceptors or costimulatory molecules on the T-cell surface. Skin γδ cells do not express classical molecules, including CD4, CD8, and CD28, known to affect activation of αβ T cells. We recently identified several molecules expressed by the skin γδ T cells and keratinocytes that provide important costimulatory signals for activation of γδ T cells. One such molecule, AMICA/JAML, is uniquely costimulatory for γδ T cells. We also found that the semaphorin Sema4D (CD100) is expressed by skin γδ T cells upon activation and binds to a new member of the plexin superfamily of semaphorin receptors, plexinB2, expressed on keratinocytes. These novel receptors and receptor-ligand pair most likely play key roles in the interactions between skin γδ T cells and keratinocytes during homeostasis and in skin disorders. We will also examine the role of these molecules in interactions between intestinal intraepithelial γδ T cells and epithelial cells during colitis. A R O L E F O R I N T R A E P I T H E L I A L γδ T C E L L S I N T H E REPAIR OF EPITHELIAL TISSUE Sakai, A., Han, J., Cato, A.C., Akira, S., Li, J.D. Glucocorticoids synergize with IL-1β to induce TLR2 expression via MAP kinase phosphatase-1-dependent dual Inhibition of MAPK JNK and p38 in epithelial cells. BMC Mol. Biol. 5:2, 2004. Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. We recently showed a role for skin γδ T cells in the reepithelialization stage of wound repair. The γδ T cells 118 IMMUNOLOGY 2005 are activated at wound sites and produce cytokines, including the epithelial growth factors KGF-1 and KGF-2. In the absence of skin γδ T cells, keratinocyte proliferation and tissue reepithelialization after wounding are defective. Recent results indicated that a keratinocyteresponsive γδ T-cell receptor is necessary for activation of the T cells by damaged keratinocytes during wound healing and is also required for the maintenance of T cells in the epidermis. In addition, we found that the skin γδ T cells are necessary for the recruitment of inflammatory cells into the wound site. In a novel mechanism, γδ T cell–produced KGFs stimulate production of hyaluronan by epidermal cells, which then controls migration of macrophages into wounds. Skin γδ T cells play roles not only in the repair of damaged tissue but also in the normal maintenance of the epidermis. Insulin-like growth factor 1 is required by keratinocytes in the skin for maintenance and during wound healing. We determined that after activation skin γδ T cells produce this growth factor that affects wound healing and apoptosis in the skin. Together these results indicate a role for skin γδ T cells in multiple aspects of wound repair and for homeostasis of the epithelium. In previous studies, we showed that intestinal intraepithelial γδ T cells play a similar role in responding to tissue damage in a model of colitis. Results in both models support our hypothesis that intraepithelial γδ T cells respond to epithelial damage or disease and play important roles in tissue repair and epithelial homeostasis. Future studies should provide information that will further define the role of γδ T cells in epithelial inflammatory disorders and may be useful in designing or testing new therapies. PUBLICATIONS Baccala, R., Witherden, D., Gonzalez-Quintial, R., Dummer, W., Surh, C.D., Havran, W.L., Theofilopoulos, A.N. γδ T cell homeostasis is controlled by IL-7 and IL-15 together with subset-specific factors. J. Immunol. 174:4606, 2005. Jameson, J.M., Cauvi, G., Sharp, L.L., Witherden, D.A., Havran, W.L. γδ T cellinduced hyaluronan production by epithelial cells regulates inflammation. J. Exp. Med. 201:1269, 2005. Sharp, L.L., Jameson, J.M., Cauvi, G., Havran, W.L. Dendritic epidermal T cells regulate skin homeostasis through local production of insulin-like growth factor 1. Nat. Immunol. 6:73, 2005. Sharp, L.L., Jameson, J.M., Witherden, D.A., Komori, H.K., Havran, W.L. Dendritic epidermal T-cell activation. Crit. Rev. Immunol. 25:1, 2005. Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. Lysophosphatidic Acid–Induced Expression of Urokinase in Ovarian Cancer Cells H. Li, C. Mahanivong, D. Bian, S. Huang scites fluid and plasma of patients with ovarian cancer have high concentrations of lysophosphatidic acid (LPA). In experimental models, LPA promotes invasion and metastasis of ovarian cancer cells by enhancing cell migration, upregulating expression of angiogenic factors, and promoting cell survival and proliferation. Recently, we focused on how LPA affects the expression of urokinase, an invasion-associated protease, in ovarian cancer cells. We found that LPA can upregulate expression of urokinase in the majority of ovarian cancer cell lines but not in any normal ovary surface epithelial cells. Using an invasive ovarian cancer cell line as a model system, we explored the signaling molecules and pathways essential for LPA-induced upregulation of urokinase. Using specific inhibitors and dominant-negative forms of signaling molecules, we found that the pathway associated with the GTP-binding protein Gi, but not the pathways associated with the GTP-binding proteins G12/13 and Gq, mediates this LPA-induced event. Moreover, both constitutively active H-Ras (V12) and Raf-1 (BXB) enhanced urokinase expression, whereas dominant-negative H-Ras (N17) and Raf-1 (301A) blocked LPA-induced urokinase upregulation, suggesting that the Ras-Raf pathway works downstream of Gi to mediate this LPA-induced process. Surprisingly, dominant-negative MAP kinase kinase (MEK) 1 or extracellular signal–regulated kinase (ERK) 2 had only a marginal inhibitory effect on LPA-induced urokinase upregulation, suggesting that a signaling pathway distinct from Raf-MEK1/2-ERK is the prominent pathway responsible for this process. In other studies, we showed that LPA activates the transcription factor NF-κB in a Ras-Raf–dependent manner and that blocking NF-κB activation with either nonphophorylable IκB or dominant-negative IKKβ abolished LPA-induced upregulation of urokinase and activation of the urokinase promoter. Furthermore, introducing mutations to delete the NF-κB binding site of the urokinase promoter resulted in more than an 80% reduction in LPA-induced activation of the promoter. In contrast, LPA-induced activation of the promoter A IMMUNOLOGY was only moderately affected by introducing mutations in the AP1 binding sites of the promoter. These results suggest that the Gi–Ras–Raf–NF-κB signaling cascade is responsible for LPA-induced upregulation of urokinase in ovarian cancer cells. PUBLICATIONS Chen, L.-Y., Doerner, A., Lehmann, P.F., Huang, S., Zhong, G., Pan, Z.K. A novel protein kinase C (PKCε) is required for fMet-Leu-Phe-induced activation of NF-κB in human peripheral blood monocytes. J. Biol. Chem. 280:22497, 2005. Jing, Q., Huang, S., Guth, S., Zarubin, T., Motoyama, A., Chen, J., Di Padova, F., Lin, S.-C., Gram, H., Han, J. Involvement of microRNA in AU-rich element-mediated mRNA instability. Cell 120:623, 2005. Li, H., Ye, X., Mahanivong, C., Bian, D., Chun, J., Huang, S. Signaling mechanisms responsible for lysophosphatidic acid-induced urokinase plasminogen activator expression in ovarian cancer cells. J .Biol. Chem. 280:10564, 2005. Yu, J., Bian, D., Mahanivong, C., Chang, R.K., Zhou, W., Huang, S. p38 Mitogen-activated protein kinase regulation of endothelial cell migration depends on urokinase plasminogen activator expression. J. Biol. Chem. 279:50446, 2004. Regulators of T-Cell Development and Lymphocyte Function J. Kaye, P. Aliahmad, O. Garijo, P. Han, C. Krieg recursor cells in the thymus undergo a complex developmental program before seeding peripheral lymphoid organs as mature T lymphocytes. Developmental checkpoints in the thymus, termed β-selection, positive selection, and negative selection, narrow the repertoire of T-cell antigen specificities to those that are not overtly autoreactive but maintain weak reactivity against self-MHC-peptide complexes. We are interested in the mechanisms that determine the fate of developing T cells and the control of gene expression during these developmental processes. Our identification of a cell-surface protein that is upregulated on developing thymocytes also led to studies on regulation of the immune response and the potential of this protein as a novel therapeutic target. P A N U C L E A R P R O T E I N I N V O LV E D I N R E G U L AT I O N OF THYMOCYTE SELECTION We identified thymocyte selection–associated high mobility group (HMG) box protein (TOX) several years ago. Members of the HMG box protein superfamily share one or more copies of a sequence-related and structurally related DNA-binding domain that can recognize distorted DNA structures and modify chromatin by bending DNA. In general, HMG box proteins function as architectural factors that regulate gene expression by promoting forPublished by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. 2005 119 mation of transcriptional complexes or by acting as components of chromatin-remodeling complexes. We found that TOX belongs to a small subfamily of evolutionarily conserved proteins whose members share almost identical HMG box sequences. The HMG box sequence in TOX can recognize distorted DNA but is a relatively poor bender of DNA, because of the lack of a critical internal wedge residue. Expression of TOX in the thymus is tightly regulated. The protein is expressed in early thymocyte progenitors and then transiently upregulated during β-selection and positive selection. Using transgenic animals that express wild-type TOX or mutants of the protein, we investigated the role of this nuclear factor in positive selection and the associated commitment to the CD4 + or CD8 + T-cell lineages. Our data indicate that expression of TOX is sufficient to initiate the differentiation of immature thymocytes to the CD8+ T-cell lineage, even in the absence of signals mediated by T-cell antigen receptors. Both the DNA-binding domain and the N-terminal domain of TOX are required for this in vivo activity. Interestingly, expression of TOX coupled with IL-7–mediated signals appears to be sufficient to induce both the development and parital maturation of CD8 + lineage T cells. Signaling through the serine/threonine phosphatase calcineurin is required for positive selection of thymocytes. We found that expression of the gene for TOX is regulated by this signaling pathway. These studies led to a model in which upregulation of TOX is a critical component of cell-lineage decisions during positive selection. We are producing mice that lack the gene for TOX to shed further light on the role of TOX in vivo. A C E L L - S U R FA C E LY M P H O C Y T E A N D A N T I G E N P R E S E N T I N G C E L L P R O T E I N W I T H R E G U L AT O R Y FUNCTION The functional outcome of engagement of the T-cell antigen receptor is modulated by secondary signals, which can have costimulatory or coinhibitory functions. We isolated a gene that encodes a cell-surface protein of the immunoglobulin superfamily, now designated BTLA (B- and T-lymphocyte attenuator), that is upregulated during positive selection and that is expressed by mature lymphocytes and antigen-presenting cells. Evidence indicates that this protein can act as a negative regulator of lymphocyte activation. We produced mice that lack the gene for BTLA and panels of monoclonal antibodies specific for BTLA to analyze the in vivo function of this protein. One of our monoclonal 120 IMMUNOLOGY 2005 antibodies acts as an agonist for this coinhibitory molecule, thereby inhibiting facets of T-cell activation, including T-cell proliferation and IL-2 secretion. We are testing the ability of this antibody to modulate immune responses in several model systems. PUBLICATIONS Kreig, C., Han, P., Stone, R., Goularte, O.D., Kaye, J. Functional analysis of BTLA engagement of CD4+ and CD8+ T cells. J. Immunol., in press. Parinaz, A., Kaye, J. Commitment issues: linking positive selection signals and lineage diversification in the thymus. Immunol. Rev., in press. Mechanisms of Cell Migration and Cancer Progression R.L. Klemke, Y. Wang, K. Stoletov, O. Pertz, W. Wang, C. Green, M. Holcomb, R. Hanley ormal cell migration plays an essential role in embryonic development, wound repair, and immune function. However, aberrant cell motility contributes to cancer progression, inflammation, and developmental defects. Cell movement is a dynamic process that involves the coordinated protrusion of a leading pseudopodium (lamellipodium) followed by retraction of the rear compartment of the cell through actin-myosin– mediated events. Directed cell migration, or chemotaxis, occurs as a response to a chemokine gradient and is thought to be one of the key mechanisms by which cancer cells metastasize (spread) to distinct tissues in the body. We are investigating how complex signaling networks temporally and spatially regulate cell migration and cancer metastasis in vitro and in vivo. For our studies, we developed a method to isolate the leading pseudopodium from the rear compartment of a migrating cell. In collaboration with J. Yates, Department of Cell biology, we used multidimensional protein identification technology and large-scale proteomics to identify several hundred proteins that localized to this structure, including Lasp-1, which is an actin-binding scaffolding protein that is overexpressed in 8%–12% of metastatic breast cancers. We discovered that Lasp-1 translocates to newly forming focal adhesions and membrane ruffles in response to chemokines and that c-Abl tyrosine kinase regulates this process. Using small interfering RNA techniques, we showed that Lasp-1 is required for proper cell migration and survival. We are also investigating the role of the small GTPase RhoC in mediating pseudopodial dynamics N Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. and cancer cell invasion. Our findings indicate that overexpression of RhoC, but not its related isoform RhoA, leads to pseudopodia with a distinct morphology and increased dynamics. Using fluorescence resonance energy transfer microscopy and large-scale proteomics, we are investigating the signaling events that lead to this altered phenotype and how the phenotype is related to metastasis. Our goals are to continue characterizing RhoC and Lasp-1 and to identify and characterize novel phosphotyrosine signaling proteins that regulate pseudopodial formation during chemotaxis and cancer invasion. We are also investigating how c-Abl tyrosine kinase regulates cell migration and survival properties in normal and metastatic cells. We discovered that c-Abl negatively regulates the CAS/Crk signaling module, which is necessary for normal cell migration and survival. Interestingly, inhibition of the 26S proteasome promotes c-Abl activation. Increased c-Abl activation facilitates the phosphorylation of tyrosine 221 of Crk, leading to the disassembly of CAS/Crk complexes, the destabilization of the cytoskeleton, and the induction of apoptosis. Angiogenesis and vascular remodeling facilitate cancer progression by providing a nutrient supply to the tumor and potential pathways for tumor cell metastasis. However, studying these processes in live animals has been difficult. Therefore, we developed a novel xenograft model of human tumor formation and angiogenesis that allows high-resolution and real-time imaging of these dynamic processes in living zebra fish. Transgenic zebra fish engineered to express green fluorescent protein in all blood vessels were injected with human tumor cells expressing red fluorescent protein. Using intravital confocal microscopy, we found that the transplanted cancer cells rapidly homed to existing blood vessels and formed solid tumors. The invasive tumor cells then permeabilized the vessel wall, creating looplike structures that subdivided into channels and formed new functional vessels. These distinct structures form in proximity to tumor cells, are dynamic, and undergo continual remodeling during tumor progression. Our findings are the first high-resolution observations of tumor formation and tumor-induced vascular remodeling in a living organism. This model is also readily amendable to testing of pharmacologic agents and genetic manipulation of conserved vertebrate angiogenic processes mediated by vascular endothelial cell IMMUNOLOGY growth factor, ephrin-B2, and basic fibroblast growth factor. Our results will provide a better understanding of cancer metastasis and provide diagnostic markers of cancer progression. PUBLICATIONS Chodniewicz, D., Klemke, R.L. Guiding cell migration through directed extension and stabilization of pseudopodia. Exp. Cell Res. 301:31, 2004. Emami, S., Klemke, R.L. Regulation of cell motility by Abl family kinases. In: Abl Family Kinases in Development and Disease. Koleske, T. (Ed.). Landes Bioscience, Georgetown, TX, 2005. Available at: http://www.eurekah.com/abstract.php?chapid =2426&bookid=185&catid=56. Wozniak, M.A., Kwong, L., Chodniewicz, D., Klemke R.L., Keely, P.J. R-Ras controls membrane protrusion and cell migration through the spatial regulation of Rho and Rac. Mol. Biol. Cell 16:84, 2005. Regulation of the Innate Immune Response in Inflammation and Infection U.G. Knaus, A. Bamberg, M. Lehmann, K. von Loehneysen, S. Luxen, S. Pacquelet, M. Ruse, M. Valo, M. Ye nnate immune cells are the first line of defense in the fight against invading pathogens. We focus primarily on understanding molecular mechanisms that phagocytes and the pulmonary epithelium use to protect the host from the injury and how some responses wind up damaging the host. For example, second messengers such as reactive oxygen species (ROS) or nitric oxide that are produced during infection can have beneficial as well as detrimental effects. The overall outcome depends on precise spatial and temporal regulation of these second messengers by the affected cell populations. The intracellular signaling pathways that control these turn on–turn off mechanisms are an ideal target for intervention in disease. Almost all of the processes connected to pathogen uptake, pathogen elimination, and sustained inflammation are governed by small GTPases of the Ras superfamily. Our research centers on the Rho GTPases Rac, Cdc42, and Rho, which are essential regulators for various leukocyte functions ranging from production of ROS to chemotaxis and phagocytosis. Generation of superoxide anion is accomplished by a Rac-dependent NADPH oxidase (Nox) upon stimulation with chemotactic factors or phagocytic stimuli. We have identified several Rac effector protein kinases, p21-activated kinases (PAKs), in leukocytes, and we are investigat- I Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. 2005 121 ing the role of PAKs in the generation of superoxide anion. Additionally, we identified and are characterizing downstream targets of PAKs, which are relevant for innate immune cell functions. GTPases of the Rho family are also involved in signaling cascades, which originate from pathogen-activated Toll-like receptors. Toll-like receptors 2 and 4, stimulated by microbial products derived from gram-positive and gram-negative bacteria, activate Rac1 and RhoA, which initiate 2 independent pathways required for RelA transactivation and subsequent NF-κB–dependent gene transcription. We are studying different aspects of signaling by Toll-like receptors in several primary human cell types, including monocytes and neutrophils, and genetically altered mouse models and the impact of this signaling on innate immune cell functions such as apoptosis and upregulation of proinflammatory mediators. Another area of research is the interaction and communication between innate immune cells and the pulmonary epithelium. To this end, we established an in vitro reconstitution system for lung epithelium that we use to examine signaling mechanisms initiated by pathogens (Fig. 1). The differentiated and fully functional lung epithelium also serves as a model for studies of F i g . 1 . Transmission electron micrograph of a 3-dimensional culture of human airway epithelium grown in air-liquid interface culture for 33 days (3900X). 122 IMMUNOLOGY 2005 lung barrier function and the influence of bacteria-derived ligands and toxins on transmigration of neutrophils. In addition, we will investigate processes leading to uptake of pathogens or environmental particles and the impact of these pathogens on airway epithelial functions. Recently, ROS-generating Nox proteins have been identified in epithelial cells, and work is in progress to study the molecular basis for ROS generation by these novel proteins. Nox proteins may serve as compartmentalized signaling modules, thereby activating or inhibiting signaling cascades via superoxide, or as an epithelial host defense mechanism via hydrogen peroxide–generating Nox/Duox isoforms. Because of their tissue-specific distribution and distinct localization patterns, Nox proteins might have highly specialized functions and undergo isoform-dependent regulation. For example, Nox4, an oxidase expressed in colon tissue and melanomas, is constitutively active in certain conditions and does not require any of the known oxidase components for superoxide generation. Elucidating physiologic stimuli and control mechanisms for these Nox proteins combined with structure-function studies will help define the biological functions of Nox in health and disease. PUBLICATIONS Chan, A.Y., Coniglio, S.J., Chuang, Y.Y., Michaelson, D., Knaus, U.G., Philips, M.R., Symons, M. Roles of the Rac1 and Rac3 GTPases in human tumor cell invasion. Oncogene, in press. Martyn, K.D., Frederick, L.M., von Loehneysen, K., Dinauer, M.C., Knaus, U.G. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell. Signal., in press. Martyn, K.D., Kim, M.J., Quinn, M.T., Dinauer, M.C., Knaus, U.G. p-21 Activated kinase (Pak) regulates NADPH oxidase activation in human neutrophils. Blood, in press. Yamauchi, A., Marchal, C.C., Molitoris, J., Pech, N., Knaus, U., Towe, J., Atkinson, S.J., Dinauer, M.C. Rac GTPase isoform-specific regulation of NADPH oxidase and chemotaxis in murine neutrophils in vivo: role of the C-terminal polybasic domain. J. Biol. Chem. 280:953, 2005. Regulatory Mechanisms for Tumor Carcinogenesis M. Hayashi, J.-F. Lo, S.-W. Kim, J.-D. Lee T H E F O U R T H M A P K I N A S E PAT H WAY ig mitogen-activated kinase 1 (BMK1), also called extracellular signal–regulated kinase 5, a newer member of the mammalian MAP kinase family, is activated by angiogenic growth factors. Using a mouse model in which expression of the gene for BMK1 B Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. can be deleted, we showed that the BMK1 pathway delivers an antiapoptotic signal in endothelial cells and plays a critical role in neovascularization during development and in response to stimulation by angiogenic growth factors. Our recent data suggest that BMK1 regulates the function of endothelial cells not only at the transcriptional level but also at the translational level. Because endothelial cells are a key component in the vasculature and are important for the formation of new blood vessels, most likely the BMK1 pathway regulates angiogenesis through its function in endothelial cells. Moreover, because angiogenesis contributes to tumor growth, we hypothesize that the BMK1 pathway is involved in tumor-induced neovascularization, which is vital for sustaining tumor growth. To shed light on the role of the BMK1 pathway in various aspects of cancer development, we use multidisciplinary approaches, including molecular, cellular, genetic, and pathologic methods, to elucidate the molecular mechanisms of BMK1 in angiogenesis and oncogene-dependent tumorigenesis. The results will provide insights into new strategies for therapeutic interventions of carcinogenesis. THE TUMOR SUPPRESSOR TID1 Tid1 is the human counterpart of the Drosophila tumor suppressor Tid56. Mutations that cause loss of function of the gene for Tid56 result in tumorous imaginal discs due to continuous cell proliferation without differentiation. To date, the mechanism of tumor suppression of Tid56 in Drosophila and the cellular function of Tid1 in human tumorigenesis are poorly understood. We discovered that the signaling domain of the receptor protein-tyrosine kinase ErbB2 interacts with Tid1 protein. We also found that increased expression of Tid1 in breast cancer cells overexpressing ErbB2 promotes ubiquitinization and proteosomal degradation of ErbB2, resulting in potent inhibition of ErbB2-dependent intracellular signaling and proliferation/survival of the cells. To evaluate and characterize the role of Tid1 in breast tumorigenesis in adult animals, we have established a mouse model in which the gene for Tid1 can be deleted specifically in mammary epithelial cells. These animals can be used to closely mimic the effect in humans of Tid1 removal on the onset and progression of breast cancer associated with Tid1 dysfunction. The mice can also be used to screen and test agents used for treatment of breast tumors involving a Tid1 defect. IMMUNOLOGY PUBLICATIONS Hayashi, M.. Lee, J.-D. Role of the BMK1/ERK5 signaling pathway: lessons from knockout mice. J. Mol. Med. 82:800, 2004. Kim, S.-W, Chao, T.H., Xiang, R., Lo, J.F., Campbell, M.J., Fearns, C., Lee, J.-D. Tid1, the human homologue of a Drosophila tumor suppressor, reduces the malignant activity of ErbB-2 in carcinoma cells. Cancer Res. 24:7732, 2004. Lo, J.F., Zhou, H., Fearns, C., Reisfeld, R.A., Yang, Y., Lee, J.-D. Tid1 is required for T cell transition from double-negative 3 to double-positive stages. J. Immunol. 174:6105, 2005. Host-Pathogen Interactions: Mechanisms and Applications E. Li, S.P. Lad, E.Y. Fukuda, J. Li ur research activities focus on host responses to viral and bacterial infections. We use infections with adenovirus and the obligate intracellular bacterial pathogen Chlamydia trachomatis as model systems. Chlamydia trachomatis is the most common cause of sexually transmitted infection that can lead to pelvic inflammatory disease and infertility in females. Chlamydial infection of nonimmune cells produces inflammatory factors, including IL-8 and interferons, that play a critical role in the disease process of Chlamydia infection. IL-8 induces inflammatory responses and promotes angiogenesis, resulting in tissue damage and scarring that are characteristic of chlamydial disease. On the other hand, interferon treatment was reported to inhibit growth of Chlamydia. Unlike the inflammatory responses to most invasive bacterial pathogens that induce rapid cytokine production with endotoxins or peptidoglycans, IL-8 production during Chlamydia infection is delayed and relies on bacterial replication. Growth of Chlamydia induces host lipid remodeling for bacterial lipid uptake. We found that this process causes production of inflammatory factors. Chlamydia infection of cervical epithelial cells, the primary target of chlamydial infection, activates cytosolic phospholipase A 2 for release of arachidonic acid and upregulates cyclooxygenase 2 for conversion of arachidonic acid to prostaglandins, including prostaglandin E2. Both Chlamydia and prostaglandin E 2 induce IL-8 release through the extracellular signal–regulated kinase/ MAP kinase pathway, and inhibition of chlamydial growth or lipid remodeling reduces IL-8 release. We also examined the host antimicrobial response to Chlamydia infection. We found that cervical epithelial cells produce IFN-β in response to Chlamydia O Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. 2005 123 infection. Production of IFN-β induces signal transducer and activator of transcription 1 (STAT1), STAT2, and interferon-stimulated gene factor 3γ, components of the Janus kinase–STAT signal transduction pathway. The upregulation and activation of this pathway are critical for host clearance of chlamydial infection because Chlamydia growth is inhibited in cells with upregulated STAT1 expression; STAT1-deficient cells support Chlamydia propagation more efficiently. Using DNA and protein expression profiles and small interfering RNA, we also showed that STAT1 upregulation is critical for antimicrobial gene expression. These studies provide insights into host innate immune responses to Chlamydia infection. They also highlight the importance of maintaining a balanced habitat for parasitic pathogens as obligate intracellular organisms. We are also working on the design and modification of adenovirus for targeting gene delivery. Although widely used for gene therapy studies, adenovirus-based vectors cannot target specific tissues. We generated modified adenoviruses that selectively infect cancer cells with upregulated growth factor receptors. Our ultimate goal is to generate modified viruses by fusing tissuespecific antibody to these viruses for selectively targeting tumor cells. PUBLICATIONS Fukuda, E.Y., Lad, S.P., Mikolon, D.P., Iacobelli-Martinez, M., Li, E. Activation of lipid metabolism contributes to interleukin-8 production during Chlamydia trachomatis infection of cervical epithelial cells. Infect. Immun. 73:4017, 2005. Lad, S.P., Fukuda, E.Y., Li, J., de la Maza, L.M., Li, E. Up-regulation of the JAK/STAT1 signal pathway during Chlamydia trachomatis infection. J. Immunol. 174:7186, 2005. The Microenvironment of Tumors and Protease-Activated Prodrugs W. Wu, P. Kuo, L. Wong, C. Liu nvasion and metastasis are critical features that define a malignant tumor. The ability of cancer cells to break through tissue boundaries and penetrate into surrounding normal tissues involves the actions of diverse extracellular proteases from multiple enzymatic classes. This enrichment of proteolytic activity is one of the distinctive features of the tumor microenvironment, in contrast to the microenvironment of normal tissues. These cellular proteases also participate in a wide range of biological and pathologic processes, I 124 IMMUNOLOGY 2005 such as the formation of new blood vessels, signal transduction, and cell survival. According to recent reports, humans have 553 genes that encode proteases. We use an integrated approach to characterize the repertoire of extracellular proteases operating in the tumor microenvironment (i.e., the “cancer degradome”) and the functional modulators, target substrates, and specificity of the enzymes. We call this approach cancer degradomics. Currently, we are focusing on a subset of cell-surface anchored or associated proteases, such as legumain, type II membrane serine protease 4, prostate-specific membrane antigen, and a number of novel protease-encoding genes, that are drastically overexpressed in a high percentage of human cancers. Certain protease inhibitors (survivin) and cofactor molecules (tissue factor) are also upregulated in tumors. We showed that legumain activates both cathepsin cysteine proteases and matrix metalloprotease 2 and that overexpression of legumain in tumors increases tumor invasion and metastasis. Similarly, type II membrane serine protease 4 activates matrix metalloprotease 9 and promotes cancer cell migration and metastasis. In contrast, expression of prostate-specific membrane antigen in prostate cancers reportedly reduces invasive potentials. These findings indicate a high level of complexity involving the interactive protease network in cancers. The cellular proteases and the inhibitors that constitute the cancer degradome are valuable prognostic and diagnostic markers as well as attractive targets for cancer imaging and therapy. The proteolytic specificities of peptide substrates provide modular chemical tools for the rational design of protease-activated prodrugs. For example, a novel legumain-activated, cellimpermeable doxorubicin prodrug, LEG-3 (Fig. 1), is activated exclusively in the tumor microenvironment. After administration of LEG-3, a profound increase occurs in the end product doxorubicin in the nuclei of cells in tumors, but little increase in other tissues. This protease-activated prodrug completely arrested growth of a variety of neoplasms, including multidrugresistant tumors, in vivo and significantly extended survival without evidence of myelosuppression or cardiac toxic effects. The design of prodrugs activated by proteases in the tumor microenvironment can be extended to other proteases and chemotherapeutic compounds and therefore provides new potential for the rational development of more effectively targeted cancer therapeutic agents. Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. F i g . 1 . Chemical structure (A) and optimized structural model (B) of prodrug LEG-3. PUBLICATIONS Luo, Y., Zhou, H., Mizutani, M., Mizutani, N., Liu, C., Xiang, R., Reisfeld, R. A DNA vaccine targeting Fos-related antigen 1 enhanced by IL-18 induces long-lived T-cell memory against tumor recurrence. Cancer Res. 65:3419, 2005. Tissue Factor, Coagulation Proteases, and Protease-Activated Receptors in Hemostasis, Thrombosis, and Inflammation R. Pawlinski, G. Schabbauer, M.T. Lin, R.E. Tilley, J. Luyendyk, J. Nieva,* N. Mackman * Scripps Cancer Center, La Jolla, California issue factor (TF), the initiator of blood coagulation, is expressed by cells that surround blood vessels. After vascular injury, TF is exposed to clotting factors in the blood, and coagulation is initiated. The formation of a clot stops further blood loss from the vasculature. Aberrant expression of TF also contributes to thrombosis and inflammation in a variety of disease states, such as sepsis, cancer, and atherosclerosis. We are interested in the role of TF in hemostasis, thrombosis, and inflammation. T H E M O S TA S I S TF is essential for hemostasis because complete deficiency of TF is not compatible with life. TF-depen- IMMUNOLOGY dent clotting is counterbalanced by 3 anticoagulant molecules/pathways: the complex formed by TF and coagulation factor VIIa is inhibited by TF pathway inhibitor; the protein C pathway inactivates the clotting cofactors coagulation factors Va and VIIIa; and antithrombin inhibits all coagulation proteases, including thrombin. A complete deficiency in any of these 3 pathways leads to embryonic lethality due to thrombosis. We hypothesized that reducing the levels of TF would rescue embryos deficient in these anticoagulants. Crossbreeding of mice with low levels of TF rescued embryos deficient in TF pathway inhibitor or the protein C pathway but not those deficient in antithrombin. In adult mice, the relative levels of the 3 anticoagulant pathways were different in different tissues, indicating that the different anticoagulants acted in a tissue-specific manner. THROMBOSIS Recently, researchers have proposed a role for bloodborne TF in the propagation of a thrombus. The pool of blood-borne TF is incorporated into growing thrombi. However, different injury models have resulted in conflicting data. Using a murine laser-induced microvascular thrombosis model, we found that blood-borne TF contributes to the growth of the thrombus. In contrast, in a photochemical injury model of the common carotid artery, thrombus formation was driven primarily by TF derived from the blood vessel wall. Currently, we are collaborating with J. Nieva, Scripps Cancer Center, to investigate the hypothesis that cancer patients have elevated levels of blood-borne TF and that this elevation contributes to the increased incidence of deep-vein thrombosis. We developed a new functional assay that measures TF activity in microparticles and platelets from the blood. We compared levels of functional blood-borne TF from healthy volunteers and from cancer patients. We found low levels of TF in healthy volunteers and elevated levels in the blood of cancer patients. Preliminary data revealed that a patient with a high level of blood-borne TF also had a deep-vein thrombosis. Our discovery that elevated levels of blood-borne TF are found in cancer patients and may correlate with the occurrence of deep-vein thrombosis suggests that these patients may benefit from antiTF therapy. I N F L A M M AT I O N Another disease in which TF expression is induced and contributes to intravascular coagulation is sepsis. In sepsis, lipopolysaccharide released by gram-negative Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. 2005 125 bacteria activates the innate immune system, a situation that leads to inflammation and disseminated intravascular coagulation. The phosphatidylinositol-3′-kinase (PI3K) signaling pathway is activated by numerous stimuli and affects many cellular processes. Previously, we showed in vitro that the PI3K pathway suppresses lipopolysaccharide induction of TF and TNF-α in human monocytic cells. More recently, we showed that the PI3K pathway inhibits lipopolysaccharideinduced expression of inflammatory mediators and coagulation in a murine endotoxemia model. Insulin is a potent activator of the PI3K pathway and is used in the treatment of sepsis in humans. We found that low doses of insulin, lower than the level that affects glucose metabolism, reduce inflammation in an endotoxemia model in a PI3K-dependent manner. Further studies will determine the mechanism by which the PI3K pathway inhibits lipopolysaccharide signaling. PUBLICATIONS Chou, J., Mackman, N., Merrill-Skoloff, G., Pedersen, B., Furie, B.C., Furie, B. Hematopoietic cell-derived microparticle tissue factor contributes to fibrin formation during thrombus propagation. Blood 104:3190, 2004. Day, S.M., Reeve, J., Pedersen, B., Farris, D.M., Myers, D.D., Im, M., Wakefield, T.W., Mackman, N., Fay, W.P. Macrovascular thrombosis is driven by tissue factor derived primarily from the blood vessel wall. Blood 105:192, 2005. Frank, R.D., Schabbauer, G., Holscher, T., Sato, Y., Tencati, M., Pawlinski, R., Mackman, N. The synthetic pentasaccharide fondaparinux reduces coagulation, inflammation and neutrophil accumulation in kidney ischemia-reperfusion injury. J. Thromb. Haemost. 3:531, 2005. Mackman, N. Mouse models in haemostasis and thrombosis. Thromb. Haemost. 92:440, 2004. Morrow, D.A., Murphy, S.A., McCabe, C.H., Mackman, N., Wong, H.C., Antman, E.M. Potent inhibition of thrombin with a monoclonal antibody against tissue factor (Sunol-cH36): results of the PROXIMATE-TIMI 27 trial. Eur. Heart J. 26:682, 2005. Pawlinski, R., Mackman, N. Tissue factor, coagulation proteases, and protease-activated receptors in endotoxemia and sepsis. Crit. Care Med. 32(5 Suppl.):S293, 2004. Pawlinski, R., Pedersen, B., Erlich, J., Mackman, N. Role of tissue factor in hemostasis, thrombosis, angiogenesis and inflammation: lessons from low tissue factor mice. Thromb. Haemost. 92:444, 2004. Pedersen, B., Holscher, T., Sato, Y., Pawlinski, R., Mackman, N. A balance between tissue factor and tissue factor pathway inhibitor is required for embryonic development and hemostasis in adult mice. Blood 105:2777, 2005. Pyo, R.T., Sato, Y., Mackman, N., Taubman, M.B. Mice deficient in tissue factor demonstrate attenuated intimal hyperplasia in response to vascular injury and decreased smooth muscle cell migration. Thromb. Haemost. 92:451, 2004. Schabbauer, G., Tencati, M., Pedersen, B., Pawlinski, R., Mackman, N. PI3K-Akt pathway suppresses coagulation and inflammation in endotoxemic mice. Arterioscler. Thromb. Vasc. Biol. 24:1963, 2004. Yu, J.L., May, L., Lhotak, V., Shahrzad, S., Shirasawa, S., Weitz, J.I., Coomber, B.L., Mackman, N., Rak, J.W. Oncogenic events regulate tissue factor expression in colorectal cancer cells; implications for tumor progression and angiogenesis. Blood 105:1734, 2005. 126 IMMUNOLOGY 2005 Helper T Cell–Regulated B-Cell Immunity tion mechanism establishes and maintains functional heterogeneity among antigen-experienced helper T cells in vivo. DEVELOPMENT OF ANTIGEN-SPECIFIC B CELLS M.G. McHeyzer-Williams, L.J. McHeyzer-Williams, L.P. Malherbe, A.P. O’Connor roduction of high-affinity antibodies is considered the most effective long-term protection against reinfection by pathogens. Hence, vaccines need to promote strong, long-lasting memory in the antigen-specific B-cell compartment to ensure persistent adaptive immunity in vivo. We seek to understand the rules that govern the helper T cell–regulated development of the antigen-specific B-cell memory. We focus our efforts on well-characterized murine models of immunity to protein antigens. P ANTIGEN-EXPERIENCED DENDRITIC CELLS Dendritic cells initiate most aspects of adaptive immunity. Primarily, their capacity for antigen uptake, antigen processing, and presentation of foreign peptides recruits antigen-specific helper T cells. Using antibodies to complexes consisting of specific peptides and MHC class II molecules, we can identify antigen-experienced dendritic cells in the secondary lymphoid organs draining sites of protein vaccination. The extent and dynamics of this cellular response vary with adjuvant and dose of antigen, providing an important quantitative index of immunogenicity in vivo. We propose that these differences critically affect the fate and the function of naive antigen-specific helper T cells recruited into the adaptive phase of the immune response. This approach provides new and objective ways to evaluate the impact of vaccination regimens on the quality of adaptive immunity in vivo. DEVELOPMENT OF ANTIGEN-SPECIFIC HELPER T CELLS The specificity of clones that respond to foreign antigens is the earliest defining attribute of adaptive immunity. The rules that underpin this selection process for helper T cells have been difficult to assess experimentally. Using an adoptive transfer model and binding of T cells to complexes consisting of peptide and MHC class II molecules, we detected and quantified the selective loss of antigen-specific clonotypes that express lower affinity antigen receptors. This affinitythreshold selection is followed by the unbiased propagation of preferred clonotypes regardless of binding half-lives or affinity. We propose that this unique selecPublished by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. The cellular organization of B-cell memory affects the quality and quantity of the immune response to antigen rechallenge. We are studying the complex development and cellular organization of antigen-specific memory B cells. Using antigen binding, cell-surface phenotype, single-cell analysis of the diversity of the antigen receptor repertoire, and gene expression analysis directly ex vivo, we are determining a comprehensive and unique view of subsets of memory B cells after initial antigen priming and after challenge with the priming antigen later on in vivo. We propose a linear progression of development from typical memory phenotype B cells that exit the germinal center reaction to a compartment of distinct and persistent preplasma memory B cells that appear to be the immediate cellular precursors of high-affinity plasma cells. PUBLICATIONS Malherbe, L., Hausl, C., Teyton, L., McHeyzer-Williams, M.G. Clonal selection of helper T cells is determined by an affinity threshold with no further skewing of TCR binding properties. Immunity 21:669, 2004. McHeyzer-Williams, L.J., Malherbe, L.P., McHeyzer-Williams, M.G. Helper T cell regulated B cell immunity. Curr. Top. Microbiol. Immunol., in press. McHeyzer-Williams, L.J., McHeyzer-Williams, M.G. Analysis of antigen-specific B cell memory directly ex vivo. Methods Mol. Biol. 271:173, 2004. McHeyzer-Williams, L.J., McHeyzer-Williams, M.G. Antigen-specific memory B cell development. Annu. Rev. Immunol. 23:487, 2005. McHeyzer-Williams, M.G. Memory B cell development. In: The Autoimmune Diseases, 4th ed. Rose, N.R., Mackay, I.R. (Eds.). Elsevier, St. Louis, in press. Adaptive and Innate Responses to Alloantigens D.B. McKay, A. Shigeoka, E. Zambricki urgical and medical advances have provided an opportunity for life to patients who would otherwise succumb to end-stage organ disease. Despite remarkable technological advances, most efforts to prevent rejection of transplanted tissues and organs have relied on treatment with nonspecific immunosuppressive medications that are toxic and require life-long use. One experimental method has allowed survival of transplanted organs without the use of immunosuppressive medications: intravenous exposure of the organ recipi- S IMMUNOLOGY ent to donor antigens before transplantation. In several animal models and human clinical trials, exposure to donor antigens before transplantation downregulated the T-cell responses of recipients to donor antigens. One focus of our research is the intracellular signaling events that lead to the induction of peripheral T-cell tolerance by exposure to donor antigens. We found that intravenous infusion of semiallogeneic donor cells into recipient mice leads to a series of events that culminate in acquired unresponsiveness to donor antigens and tolerance to allografts. We discovered that several proximal T-cell receptor–coupled signaling molecules are altered in peripheral T cells of the recipient mice. We are investigating how these proximal molecules may be regulated in T cells from recipients that do not reject their transplanted organs. In addition, we are interested in the initial events that regulate activation of recipient T cells, namely the events that regulate the initial activation of the cells that present donor antigens. In other research, we are investigating the mechanisms that mediate the expansion of activated T cells in response to transplantation antigens. The long-range goal of this project is to develop effective ways to block proliferative signals and thereby prevent allograft rejection. Transplantation of allogeneic tissues induces vigorous proliferation of host T cells specific for donor alloantigens. Activation and cell division of recipient T cells are differentially regulated by intracellular signals evoked through the ligation of cell-surface receptors. A classic example is the binding of IL-2 to its receptor, which culminates in 3 distinct intracellular signaling pathways, including 2 that are important for T cell proliferation: the Janus kinase–signal transducer and activator of transcription pathway and the Ras–MAP kinase pathway. A third pathway, the phosphatidylinositol-3′-kinase–Atk kinase pathway, induces both proapoptotic and antiapoptotic signals, explaining the dual role of IL-2. Antibodies that specifically block binding of IL-2 to its receptor have been used in clinical transplantation. We are evaluating the intracellular signaling mechanisms that lead to effective blockade of the ligation of the receptors of growth factors. In the microenvironment of an allogeneic organ, several growth factors may influence the proliferation of potentially alloreactive T cells, and understanding these factors may lead to better use of therapeutic blockade of growth factors. Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. 2005 127 PUBLICATIONS McKay, D.B., Josephson, M.A., Armenti, V.T., August, P., Coscia, L.A., Davis, C.L., Davison, J.M., Easterling, T., Friedman, J.E., Hou, S., Karlix, J., Lake, K.D., Lindheimer, M., Matas, A.J., Moritz, M.J., Riely, C.A., Ross, L.F., Scott, J.R., Wagoner, L.E., Wrenshall, L., Adams, P.L., Bumgardner, G.L., Fine, R.N., Goral, S., Krams, S.M., Martinez, O.M., Tolkoff-Rubin, N., Pavlakis, M., Scantlebury, V. Women's Health Committee of the American Society of Transplantation. Reproduction and transplantation: report on the AST Consensus Conference on Reproductive Issues and Transplantation. Am. J. Transplant. 5:1592, 2005. Zambricki, E., Shigeoka, A., Kishimoto, H., Sprent, J., Burakoff, S., Carpenter, C., Milford, E., McKay, D. Signaling T-cell survival and death by IL-2 and IL-15. Am. J. Transplant. 5:2623, 2005. CCR5 and CXCR4, Receptors for HIV Type 1 D.E. Mosier, C. Pastore, A. Ramos, R. Nedellec, O. Hartley,* R. Offord,* M. Lederman** * Centre Médical Universitaire, Geneva, Switzerland ** Case Western Reserve University, Cleveland, Ohio IV type 1 (HIV-1) is the cause of the AIDS pandemic. The first step in HIV-1 infection is sequential binding of the virus to the cell-surface receptors CD4 and CCR5. Because CCR5 binding occurs after CD4 binding, CCR5 is defined as a coreceptor. The importance of CCR5 in HIV-1 infection was first appreciated because some persons have a natural mutation that prevents expression of CCR5. These persons are naturally resistant to HIV-1 infection, and they have no apparent clinical consequences of lacking CCR5. These observations led to research programs to develop CCR5-blocking agents to prevent HIV-1 infection. HIV-1 can undergo mutations that allow a second chemokine receptor, CXCR4, to replace the binding function of CCR5. We have studied the costs to viral fitness of those mutations. H A N T I V I R A L C O M P O U N D S T H AT TA R G E T C C R 5 The normal function of CCR5 is to bind chemokines and signal cell migration. RANTES is the CCR5-binding chemokine with the most potent activity against HIV-1, but it is poor at inhibiting the infection of macrophages. We prepared synthetic modifications of the N-terminal domain of RANTES. We found that the most potent of these compounds, PSC-RANTES, is 1000 times more effective than native RANTES at inhibiting HIV-1 infection and that it completely blocks infection of macrophages. A single injection of PSC-RANTES before inoculation of virus prevents HIV-1 infection of 100% of mice with severe combined immunodeficiency repopulated with 128 IMMUNOLOGY 2005 human peripheral blood leukocytes. Brief exposure of human cells to PSC-RANTES leads to prolonged internalization of CCR5. These properties led to the formulation of PSC RANTES as a topical microbicide to prevent sexual transmission of HIV-1. Recently, treatment with PSCRANTES prevented vaginal transmission of a chimeric virus consisting of simian immunodeficiency virus and HIV in the rhesus macaque model. Preclinical development of this compound is progressing toward the first trials in humans. M U TAT I O N A L C O S T S O F C O R E C E P T O R S W I T C H I N G One concern about CCR5-blocking agents such as PSC -RANTES is that they might select for resistant viruses that can infect via other chemokine receptors, such as CXCR4. Although previously we showed that such “coreceptor switch” mutants can arise during treatment, a recent detailed analysis revealed that most mutants have a loss of fitness during coreceptor switching that coincides with a period when neither CCR5 nor CXCR4 supports efficient virus infection. The mutations in the HIV-1 envelope that drive coreceptor switching occur mainly in the exposed variable loops (V1/V2 and V3), and different HIV-1 isolates require as few as 1 mutation or as many as 7 mutations to switch from use of CCR5 to use of CXCR4. Poor replication in both CCR5- and CXCR4-expressing target cells and increased sensitivity to both CCR5 and CXCR4 inhibitors were common features of viruses that were switching coreceptors. To more fully understand the cost of each mutation associated with changing coreceptor binding from CCR5 to CXCR4, we reconstructed all possible mutational pathways between a parental CCR5-using virus and a CXCR4-using descendent virus separated from the parent virus by 5 mutations. We used site-directed mutagenesis to introduce all 32 possible combinations of single and multiple mutations in the HIV-1 envelope gene. These mutated envelopes were combined with an envelope-deficient reporter virus to make HIV-1 particles capable of only a single cycle of infection. We found that mutations in variable loops 1 and 2 of the envelope improved the use of CCR5 but did not permit infection via CXCR4. Mutations in variable loop 3 led to use of CXCR4 for viral entry, but only poorly. Combinations of mutations in all 3 variable loops improved the ability of the virus to use CXCR4. The sequence in which mutations were introduced was critical. About 30% of possible mutations were noninfectious. Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. Maximum likelihood analysis indicated 1 favored mutational pathway in 120 sequential possibilities. The probability of coreceptor switching is thus constrained by having to make the right mutation at the right place at the right time. In the lottery of ongoing viral mutation, a coreceptor switch event is a rare winner. PUBLICATIONS Hartley, O., Gaertner, H., Wilken, J., Thompson, D., Fish, R., Ramos, A., Pastore, C., Dufour, B., Cerini, F., Melotti, A., Heveker, N., Picard, L., Alizon, M., Mosier, D., Kent, S., Offord, R. Medicinal chemistry applied to a synthetic protein: development of highly potent HIV entry inhibitors. Proc. Natl. Acad. Sci. U. S. A. 101:16460, 2004. Lederman, M.M., Veazey, R.S., Offord, R., Mosier, D.E., Dufour, J., Mefford, M., Piatak, M., Jr., Lifson, J.D., Salkowitz, J.R., Rodriguez, B., Blauvelt, A., Hartley, O. Prevention of vaginal SHIV transmission in rhesus macaques through inhibition of CCR5. Science 306:485, 2004. Mosier, D.E. HIV-1 envelope evolution and vaccine efficacy. Curr. Drug Targets Infect. Disord. 5:171, 2005. Control of Cytokine Expression by Arginine Methylation K.A. Mowen, J.W. Fathman elper T cells can be divided into 2 distinct populations on the basis of their immune specificity and cytokine profiles. Type 1 helper T cells produce IFN-γ and are responsible for cellmediated immunity; type 2 helper T cells secrete IL-4 and are associated with the humoral immune response. These 2 types of cells have been associated with susceptibility to malignant, infectious, allergic, and autoimmune diseases. The improper development of type 2 helper T cells can lead to allergy and asthma, and an overactive response by type 1 helper T cells can lead to autoimmune diseases such as type 1 diabetes. Because of the opposing roles of the 2 types in immune function, the development and migration of helper T cells must be tightly regulated. Indeed, the discrete subsets, type 1 and type 2, reciprocally antagonize the maturation and behavior of each other in the immune response, resulting in a population of helper T cells that is primarily type 1 or type 2. Thus, manipulating the ratio of type 1 to type 2 helper T cells provides an intriguing avenue of therapy, and understanding the molecular events that control lineage-specific cytokine expression may provide useful tools to modulate the helper T cell response. Although several lineage-specific and nonspecific transcription factors are required for the development and function of type 1 and type 2 helper T cells, less H IMMUNOLOGY is known about the events that occur after the reactivation of type 1 and type 2 effector populations and result in the disparate cytokine profiles of the 2 types of helper T cells. Signal transduction pathways use posttranslational modifications to translate changes in the extracellular milieu into environment-sensitive gene expression in a timely and efficient fashion. Phosphorylation of serine, threonine, and tyrosine residues and protein ubiquitination have been widely studied. Although methylation of arginine residues was discovered more than 30 years ago, it has only recently aroused renewed interest. Arginine methylation of proteins by members of the protein arginine methyltransferase (PRMT) family regulates the subcellular localization of the methylated proteins and modulates protein-protein interactions. We discovered a unique contribution of arginine methylation to cytokine gene expression downstream of signaling by T-cell receptors. Our goal is to investigate more broadly the role for arginine methylation in immune function, including further study of helper T cells and other immune cell types. We also plan to examine the upstream regulation of PRMT expression and activity and characterize the effects of ablation or suppression of PRMT expression. Understanding the role of posttranslational modifications, such as arginine methylation, of proteins that are key in regulating cytokine production will give us novel targets in diseases induced or exacerbated by the cytokine environment, such as inflammatory arthritis. Analysis of Immune Learning in B Lymphocytes D. Nemazee, A. Gavin, D. Aït-Azzouzene, C. Huber, L. Verkoczy, J. Vela, B. Duong, P. Skog, M. Lim he main goal of our research is to understand how lymphocytes distinguish between self and nonself antigens. Because antigen receptors on lymphocytes are assembled from component parts through an essentially random mechanism, many lymphocytes have self-reactive receptors. Regulation of such autoreactive specificities may be important to prevent autoimmune disease and to ensure efficient response to microbes. The development of B lymphocytes is a multistep process punctuated by the somatic generation of genes T Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. 2005 129 for antibody heavy and light chains through DNA recombination, which is catalyzed by the products of recombinase activator gene 1 (RAG-1) and RAG-2. Because V(D)J recombination is imperfect and error prone, pre-B and B cells are endowed with sensing mechanisms to detect protein expression of heavy chains and assembled heavy and light chains (i.e., intact surface IgM). A major function of the expression of immunoglobulin in immature B cells is signaling to downregulate recombinase activity and to stimulate developmental progression. Newly formed B-cell receptors are also screened for autoreactivity. These quality control mechanisms rely on signaling by antigen receptors. Previously, we showed that B cells with autoreactive receptors do not downregulate recombination because of excessive signaling through the antigen receptor, resulting in “receptor editing,” a process in which previously expressed genes for antibody light chains are inactivated and replaced by secondary DNA recombination. More recent data indicated that editing can also play an important role in inactivating and replacing receptor genes that are underexpressed at the protein level. In this situation, subnormal expression of unligated surface immunoglobulin does not provide a needed signal. These recent results suggest that quality control of newly formed B lymphocytes is surprisingly stringent and that through recombinase regulation, B cells are often able to “repair” unacceptable light-chain genes by replacing the unacceptable genes with new genes. Because of the apparent efficiency of the editing process, we suspect that we have uncovered a major cellular “proofreading” pathway. A key question of current interest is how signaling through the antigen receptor regulates editing. A major nuclear end point is the regulation of RAG transcription. We are assessing the biochemical signaling pathways by which the signal from antigen receptors regulates RAG transcription. Recent results suggested that NF-κB and rel transcription factors may be involved in both positive and negative regulation of the RAG genes. In addition, we are using DNA array analysis and other screening methods to look more closely at changes in gene expression during and subsequent to the receptor editing response. We found that a relatively small fraction of genes is differentially expressed, including a handful of previously uncharacterized mRNAs, which we are analyzing further. In other studies, we focused on the cues that mature B cells use to distinguish self from nonself. 130 IMMUNOLOGY 2005 Fully mature recirculating B cells can be rapidly inactivated and induced to apoptosis when confronted with tissue antigen, whereas the same cells are able to respond to antigens expressed by microbes. We are investigating both the death pathway involved in selftolerance and the nature of the signals that prevent this pathway in responses to nonself antigens. Recently, we found that the ability of B cells to distinguish self from nonself in this setting is independent of T lymphocytes and instead most likely involves a novel pathway of self-recognition. We are exploring the idea that immune tolerance in mature B cells depends on specific costimulation by self-tissue. PUBLICATIONS Aït-Azzouzene, D., Verkoczy, L., Peters, J., Gavin, A., Skog, P., Vela, J.L., Nemazee, D. An immunoglobulin Cκ-reactive single chain antibody fusion protein induces tolerance through receptor editing in a normal polyclonal immune system. J. Exp. Med. 201:817, 2005. Gavin, A., Aït-Azzouzene, D., Mårtensson, A., Duong, B., Verkoczy, L., Skog, J.L., Skog, P., Nemazee, D. Peripheral B lymphocyte tolerance. Keio J. Med. 53:151, 2004. Gavin, A.L., Duong, B., Skog, P., Aït-Azzouzene, D., Greaves, D.R., Scott, M.L., Nemazee, D. ∆BAFF, a splice isoform of BAFF, opposes full-length BAFF activity in vivo in transgenic mouse models. J. Immunol. 175:319, 2005. Peters, B., Sidney, J., Bourne, P., Bui, H.H., Buus, S., Doh, G., Fleri, W., Kronenberg, M., Kubo, R., Lund, O., Nemazee, D., Ponomarenko, J.V., Sathiamurthy, M., Schoenberger, S.P., Stewart, S., Surko, P., Way, S., Wilson, S., Sette, A. The immune epitope database and analysis resource: from vision to blueprint. PLoS Biol. 3:e91, 2005. Verkoczy, L., Aït-Azzouzene, D., Skog, P., Mårtensson, A., Lang, J., Duong B., Nemazee, D. A role for nuclear factor κB/rel transcription factors in the regulation of the recombinase activator genes. Immunity 22:519, 2005. Verkoczy, L.K., Mårtensson, A.S., Nemazee, D. The scope of receptor editing and its association with autoimmunity. Curr. Opin. Immunol. 16:808, 2004. improved gene delivery to human hematopoietic cells that express this receptor. Important new advances in data acquisition and image processing resulted in major improvements in resolution compared with the resolution in earlier studies. For example, electron cryomicroscopy density was observed for hexon residues missing from the crystal structure that included hypervariable regions and the epitope of a neutralizing antibody. On the inner capsid surface, density was revealed at the base of the hexons and below the penton base that most likely correspond to minor adenovirus proteins, including protein VI. On the basis of the new structural information, we proposed a new model for Ad35F. In particular, the model presents 2 possible orientations for protein IX, either binding on the capsid surface or extending away from the capsid, consistent with the use of the C terminus of protein IX for the insertion of exogenous ligands to redirect adenovirus vectors to alternative receptors. These studies increase our knowledge of adenovirus capsid assembly and antibody neutralization and thus may promote further improvements in gene delivery to hematopoietic cell types. Targeting a General Biochemical Pathway in Viral Infections Via Cyclic D,L-α-Peptides W.S. Horne, C.M. Wiethoff, C. Cui, K.M. Wilcoxen, Structural Analyses of an Adenoviral Vector Targeted to Hematopoietic Cells S. Saban,* R.R. Nepomuceno, G.R. Nemerow, P.L. Stewart* * Vanderbilt University Medical Center, Nashville, Tennessee espite recent advances in the uses of adenovirus vectors for vaccines and gene delivery, we still lack basic knowledge of the structure of intact adenovirus particles. In recent studies, we used electron cryomicroscopy and image reconstruction to determine the 3-dimensional structure of an adenovirus vector, Ad35F, at 9-Å resolution. This viral vector recognizes the receptor CD46, a member of the complement regulatory protein family, thereby allowing D Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. M. Amorin, M.R. Ghadiri, G.R. Nemerow iverse human viruses have coevolved to exploit the acidification of endosomal compartments to gain entry into host cells. Recently, we used a supramolecular approach to selectively target and inhibit viral infections through this central pathway. We used a high-throughput screen with an adenovirus vector encoding green fluorescent protein to select an 8-residue cyclic D ,L-α-peptide from a directed combinatorial library that specifically inhibited the development of low pH inside endocytic vesicles, thereby arresting escape of virus from these compartments. The peptide had no adverse effect on cell viability and was only able to exert its inhibitory activity when added to cells in the presence of the virus. Confocal fluorescence microscopic studies with labeled adenovirus particles indicated that the peptide did not hinder D IMMUNOLOGY viral attachment or entry but rather extinguished the pH gradient inside cell endosomes. Influenza virus that uses a mode of entry similar to that of adenovirus was also inhibited by the peptide. Our results suggest that self-assembling cyclic peptides may provide a broad-spectrum and alternative approach to the design of antiviral drugs. Inhibiting Expression of Proinflammatory Cytokines With Adenoviruses M. Iacobelli-Martinez, R.R. Nepomuceno, J. Connolly,* G.R. Nemerow * Isis Pharmaceuticals, Inc., Carlsbad, California ost adenovirus serotypes bind to host cells via the coxsackievirus-adenovirus receptor, but subgroup B and subgroup D (adenovirus 37) viruses recognize the receptor CD46. Interestingly, diverse microbial pathogens that use CD46 for infection downregulate the expression of IL-12, a cytokine involved in both the innate and the adaptive immune responses. We determined whether adenovirus serotypes that use CD46 alter the expression of proinflammatory cytokines. We found that subgroup B adenoviruses type 16 and 35 and subgroup D adenovirus type 37, but not subgroup C adenoviruses type 2 or 5, significantly reduced expression of IL-12 by peripheral blood mononuclear cells stimulated by IFN-γ and lipopolysaccharide. IL-12 mRNA, as well as mRNA encoding other mediators, such as IL-1α, IL-β, the receptor for IL-1α, and IL-6, were also downregulated upon interaction with adenoviruses that use CD46. Analysis of transcription factor activity required for cytokine expression indicated that adenoviruses that used CD46 preferentially inhibited the DNA-binding activity of the transcription factor CCAAT/enhancer-binding protein β (C/EBP-β). Expression of C/EBP-β protein induced by IFN-γ was also impaired by adenoviruses that use CD46, consistent with the reduced DNA-binding activity of C/EBP-β. Interference with IFN-γ signaling events by adenoviruses that use CD46, but not adenoviruses that use the coxsackievirus-adenovirus receptor, revealed a potentially critical difference in the host immune response against adenovirus vectors, a situation that has implications for gene delivery and vaccine development. M Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. 2005 131 PUBLICATIONS Horne, W.S., Wiethoff, C.M., Cui, C., Wilcoxen, K.M., Amorin, M., Ghadiri, M.R., Nemerow, G.R. Antiviral cyclic D,L-α-peptides: targeting a general biochemical pathway in virus infections. Bioorg. Med. Chem. 13:5145, 2005. Hsu, C., Boysen, M., Gritton, L.D., Frosst, P.D., Nemerow, G.R., Von Seggern, D.J. In vitro dendritic cell infection by pseudotyped adenoviral vectors does not correlate with their in vivo immunogenicity. Virology 332:1, 2005. Iacobelli-Martinez, M., Nepomuceno, R.R., Connolly, J., Nemerow, G.R. CD46utilizing adenoviruses inhibit C/EBPβ-dependent expression of proinflammatory cytokines. J. Virol. 79:11259, 2005. Saban, S.D., Nepomuceno, R.R., Gritton, L.D., Nemerow, G.R., Stewart, P.L. CryoEM structure at 9Å resolution of an adenovirus vector targeted to hematopoietic cells. J. Mol. Biol. 349:526, 2005. Wiethoff, C.M., Wodrich, H., Gerace, L., Nemerow, G.R. Adenovirus protein VI mediates membrane disruption following capsid disassembly. J. Virol. 79:1992, 2005. DNA Vaccine–Induced Long-Lived T-Cell Memory Against Tumor Recurrence Y. Luo, H. Zhou, J. Krueger, C. Kaplan, S.H. Lee, R. Xiang, R.A. Reisfeld ong-lived T-cell memory is a major objective of vaccination against tumors because it provides continuous protection against tumor dissemination and recurrence. Key requirements for successful protection against tumors include an increased number of tumor antigen–specific CD8+ T cells in an immune host and the capability of CD8+ T memory cells to proliferate, secrete inflammatory antitumor cytokines, and repeatedly kill recurring tumor cells more effectively than naive CD8+ T cells do. We achieved these objectives with a DNA vaccine that encodes Fos-related antigen 1 (Fra-1), a murine transcription factor. The antigen was fused to polyubiquitin, modified by cotransformation with a gene encoding secretory murine IL-18, and carried to secondary lymphoid organs (i.e., Peyer’s patches) by attenuated Salmonella typhimurium. This DNA vaccine was effective in 2 different breast carcinoma models and in a non–small lung carcinoma model. In syngeneic mice, it induced a strong antitumor immunity that could be maintained as a long-lived specific immune response and resulted in eradication of tumor metastases. In addition, IL-18 enhanced this immune response by activating both T cells and natural killer cells while upregulating expression of MHC class I antigens and promoting the differentiation of CD4+ T cells to type 1 helper T cells. L 132 IMMUNOLOGY 2005 The vaccine also was effective in a therapeutic setting. It markedly suppressed growth and dissemination of established pulmonary metastases of both D2F2 breast and D121 non–small cell lung carcinomas. The primary mediators of this protective immunity were CD8+ T cells, which secreted IFN-γ, a proinflammatory cytokine associated with type 1 helper T cells, and were activated in both lymphoid and nonlymphoid tissues, especially in tumor tissues of lung and liver. Furthermore, our finding that a single vaccination can upregulate CD4+ T cells releasing IL-2 in Peyer’s patches supports the contention that the mechanism of tumor protection depends on CD4 + T cells and is mediated by effector functions of helper T cells. We also showed that the vaccine induced the establishment and long-term maintenance of immunologic memory CD8 + T cells in successfully vaccinated mice. When passively transferred to mice with severe combined immunodeficiency disease, such memory cells maintained sufficient memory in the absence of tumor antigen to markedly suppress dissemination and growth of a lethal challenge of D2F2 breast tumor cells. Importantly, after vaccination, CD8 + T cells in the tumor microenvironment also migrated to nonlymphoid tissues such as lungs, where some of the T cells were found as long-lived, dormant memory T cells ready to respond to reencounter with the same tumor antigen. Strikingly, such lymphocytes released more IFN-γ and contained a higher percentage of CD8 + cytotoxic T lymphocytes and memory CD8+ T cells than did their splenic counterparts, suggesting that these CD8+ T cells can play a key role in the cellular response against tumor metastases. In fact, these results point to the existence of a population of extralymphoid effector memory T cells poised for an immediate antitumor immune response. On the basis of data in the literature that T-cell proliferation induced by IL-18 depends on IFN-γ, we examined the effect of IL-18 on T-cell turnover. We found that expression of secretory IL-18 in our vaccine induced the rapid turnover of CD8+ T memory cells in mice within 24 hours after immunization and that such cells can be maintained in lymphoid tissues as well as locally in lung tumor tissues. Interestingly, CD8+ T cells obtained from the lungs of our vaccinated mice proliferated and released IFN-γ after exposure to antigen in vitro. Consequently, we conclude that persistently activated T cells and memory CD8+ T cells in the lung can play a key role in the cellular immune response against tumor metastases. We also demonstrated in our tumor models that a specific memory T-cell response is induced and mainPublished by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. tained in the absence of tumor antigen. We showed that CD8 + T cells adoptively transferred from successfully vaccinated mice to syngeneic mice with severe combined immunodeficiency and parked there for 7 or 30 days could maintain an effective and long-lived memory in the absence of both tumor antigen and naive T cells. In fact, 6 of 8 of these successfully reimmunized mice lived 3 times longer than control mice did in the absence of any detectable tumor growth up to 56 days after challenge with tumor cells. Taken together, our data indicate that an oral DNA vaccine encoding ubiquitinated Fra-1 and IL-18 can protect mice against a lethal challenge of murine breast cancer cells and of non–small cell lung carcinoma cells. Moreover, this vaccine can break T-cell tolerance to the Fra-1 self-antigen and generate a long-lived memory T-cell immune response against recurring breast cancer, which can be maintained constantly in both lymphoid and nonlymphoid organs in mice with severe combined immunodeficiency in the absence of tumor antigen. PUBLICATIONS King, D.M., Albertini, M.R., Schalch, H., Hank, J.A., Gan, J., Surfus, J., Mahvi, D., Schiller, J.H., Warner, T., Kim, K.M., Eickhoff, J., Kendra, K., Reisfeld, R.A., Gillies, S.D., Sondel, P. Phase I clinical trial of the immunocytokine EMD 273063 in melanoma patients. J. Clin. Oncol. 22:4463, 2004. Lo, J.F., Zhou, H., Fearns, C., Reisfeld, R.A., Yang, Y., Lee, J.D. Tid1 is required for T cell transition from double-negative 3 to double-positive stages. J. Immunol. 174:6105, 2005. Loeffler, M., Kruger, J.A., Reisfeld, R.A. Immunostimulatory effects of low-dose cyclophosphamide are controlled by inducible nitric oxide synthase. Cancer Res. 65:5027, 2005. Luo, Y., Zhou, H., Mizutani, M., Mizutani, N., Liu, C., Xiang, R., Reisfeld, R.A. A DNA vaccine targeting Fos-related antigen 1 enhanced by IL-18 Induces long-lived T-cell memory against tumor recurrence. Cancer Res. 65:3419, 2005. Mizutani, N., Luo, Y., Mizutani, M., Reisfeld, R.A., Xiang, R. DNA vaccines suppress angiogenesis and protect against growth of breast cancer metastases. Breast Dis. 20:81, 2004. Niethammer, A.G., Wodrich, H., Loeffler, M., Lode, H.N., Emmerich, K., Abdollahi, A., Krempien, R., Debus, J., Huber, P.E., Reisfeld, R.A. Multidrug resistance-1 (MDR-1): a new target for T cell-based immunotherapy. FASEB J. 19:158, 2005. Xiang, R., Mizutani, N., Luo, Y., Chiodoni, C., Zhou, H., Mizutani, M., Ba, Y., Becker, J.C., Reisfeld, R.A. A DNA vaccine targeting survivin combines apoptosis with suppression of angiogenesis in lung tumor eradication. Cancer Res. 65:553, 2005. Zhou, H., Luo, Y., Lo, J.F., Kaplan, C.D., Mizutani, M., Mizutani, N., Lee, J.D., Primus, F.J., Becker, J.C., Xiang, R., Reisfeld, R.A. DNA-based vaccines activate innate and adaptive antitumor immunity by engaging the NKG2D receptor. Proc. Natl. Acad. Sci. U. S. A. 102:10846, 2005. Zhou, H., Luo, Y., Mizutani, M., Mizutani, N., Reisfeld, R.A., Xiang, R. T cellmediated suppression of angiogenesis results in tumor protective immunity. Blood, in press. IMMUNOLOGY 2005 133 Protective Protease-Activated Receptor 1 Signaling by the Protein C Pathway M. Riewald, C. Feistritzer, R.A. Schuepbach, R. Lenta hrombin and activated protein C (APC) are major regulators of the blood coagulation system. Thrombin not only is the key procoagulant enzyme but also activates the anticoagulant protein C pathway on the surface of endothelial cells. The generated APC inhibits blood coagulation by downregulating prothrombin activation in a negative feedback loop. Results from animal models and clinical trials indicate that APC has potent protective effects in systemic inflammation that are independent of its anticoagulant function, and recently recombinant APC was approved to treat patients with severe sepsis. The molecular basis for the anti-inflammatory effects of APC is incompletely understood. Previously, we showed that APC signaling in endothelial cells requires binding to endothelial protein C receptor and activation of protease-activated receptor 1 (PAR-1), the thrombin receptor. Thrombin–PAR-1 signaling has well established proinflammatory effects, including disruption of endothelial barrier function, raising the question of how the same receptor can also mediate protective effects of APC. Incubation of an endothelial monolayer with APC and low concentrations of thrombin potently enhanced barrier integrity through PAR-1–dependent transactivation of the barrier protective sphingosine 1-phosphate (S1P) signaling pathway (Fig. 1). These results revealed an unexpected role for cross-communication between the prototypical barrier protective S1P and barrier-disruptive PAR-1 pathways and suggest that S1P signaling may mediate protective effects of APC in sepsis. In addition, largescale gene expression profiling indicated that thrombin and APC can have distinct PAR-1–dependent effects in inflammatory cytokine-perturbed endothelial cells. APC–PAR-1, but not thrombin–PAR-1, downregulated transcript levels of several proapoptotic proteins, including p53 and thrombospondin-1. Taken together, these results indicate that the same receptor, PAR-1, can mediate different biological effects depending on the rate of receptor activation, and they suggest that APC elicits powerful protective responses precisely because it is a relatively poor PAR-1 activator compared with thrombin. We are elucidating how T Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. F i g . 1 . PAR-1 can mediate opposite effects on endothelial bar- rier integrity. Inflammatory disorders such as sepsis are associated with increased permeability of the endothelial cell monolayer at the blood-tissue interface. Proinflammatory signaling by thrombin through PAR-1 can disrupt endothelial barrier integrity. APC enhanced endothelial barrier integrity dependent on binding to endothelial protein C receptor (EPCR) and activation of PAR-1, cellular sphingosine kinase-1 (SK1), and S1P receptor-1 (S1P1). Thus, barrier protection by APC proceeds via cross talk between the barrier-disruptive PAR-1 and barrier-protective S1P pathways. Incubation of cells with low concentrations (~40 pM) of thrombin had an equally potent barrier-enhancing effect dependent on the S1P pathway. differences in the level of receptor activation translate into activation of different cellular signaling pathways. To dissect the contributions of PAR-1 activation by APC and thrombin, we designed PAR-1 variants that are efficiently activated by APC but not by thrombin. Transgenic mice expressing these variants in endothelial cells have been generated and will be analyzed in sepsis models to define the in vivo roles of PAR-1 signaling in systemic inflammation. PUBLICATIONS Feistritzer, C., Mosheimer, B.A., Kaneider, N.C., Riewald, M., Patsch, J.R., Wiedermann, C.J. Thrombin affects eosinophil migration via protease-activated receptor-1. Int. Arch. Allergy Immunol. 135:12, 2004. Feistritzer, C., Riewald, M. Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine 1-phosphate receptor-1 crossactivation. Blood 105:3178, 2005. Riewald, M., Ruf, W. Protease-activated receptor-1 signaling by activated protein C in cytokine-perturbed endothelial cells is distinct from thrombin signaling. J. Biol. Chem. 280:19808, 2005. 134 IMMUNOLOGY 2005 Chemical and Genetic Approaches to Adaptive and Innate Immunity H. Rosen, G. Sanna, C. Alfonso, E. Jo, P. Gonzalez-Cabrera, A. Don, M. Peterson, Y. Gon ymphocytes develop in the thymus (T cells) and bone marrow (B cells) and upon maturation leave their sites of development to enter the bloodstream. Because the numbers of lymphocytes with specific receptors for antigen are limited, the probability of random productive collision of specific lymphocyte, antigen, and antigen-presenting cell in a permissive environment for an efficient immune response is low. In the immune system, this probability is enhanced by rapid recirculation of lymphocytes through secondary lymphoid organs, so that each lymphocyte has many opportunities to respond to its specific antigen. A sufficient number of blood lymphocytes are therefore essential for the development of efficient immune responses, and this number is maintained by the recirculation of lymphocytes through the secondary lymphoid organs. Using small synthetic druglike organic molecules, we elucidated specific molecular gatekeepers that control the numbers of recirculating lymphocytes. These compounds alter lymphocyte trafficking and induce clinically useful immunosuppression by activating a single sphingosine 1-phosphate (S1P) receptor subtype, S1P1. L F i g . 1 . Postulated contributions of lymphocytes and endothelial cells to mediation of lymphocyte trafficking by the S1P-S1P1 system. A, Stromal gate control: Lymphocytes pass through open endothelial junctions (left). S1P or synthetic agonists ligate S1P1 receptors on the surfaces of endothelial cells, stimulating Rac GTPase-dependent endothelial junctional tightening. Lymphocytes cannot pass through the closed gate of sinus-lining endothelial cells (right) and accumulate in the lymph node. B, S1P1 intrinsic lymphocyte control: S1P normally stimulates migration of T cells from the lymph node into the sinus with concentration-dependent responses (left). High concentrations of S1P may downregulate S1P1 expression on T cells, and S1P1 antagonists may block S1P chemotactic signaling of T cells to promote the retention of T cells in the lymph node (right). Both mechanisms could contribute simultaneously to the control of T-cell trafficking. M O L E C U L A R C O N T R O L O F LY M P H O C Y T E M I G R AT I O N Molecular control of the migration of lymphocyte subsets within the recirculation pathway is a fundamental issue of therapeutic importance. Although transplantation involves the sensitization of an immunologically naive host, most autoimmune diseases require intervention in a sensitized host that already has autoreactive effector T cells in the periphery. We approached this problem by examining the role of the S1P system in the control of lymphocyte egress from lymph nodes and thymus, and we have delineated 2 potentially synergistic mechanisms that alter lymphocyte migration (Fig. 1). CHEMICAL PROBES OF RECEPTOR INTERACTIONS, A C T I VAT I O N , A N D FAT E One of our goals has been to define the rules for chemical tractability of therapeutic targets in signaling Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. lipids. Using high-throughput screening of commercial chemical libraries, we identified potent selective agonists of the S1P1 receptor. These agonists produced lymphopenia in blood by sequestering lymphocytes in lymph nodes, but not in the spleen. The minimal signals required for lymphocyte sequestration are being defined by using selective S1P1-specific agonists that generate prolonged signals upon ligand stimulation and induce receptor internalization but rapid recycling to the cell surface. Receptor docking and mutagenesis studies with A. Parrill, University of Memphis, Memphis, Tennessee, and G. Tigyi, University of Tennessee, Memphis, Tennessee, indicated that these ligands overlap the binding site for the natural lipid mediation S1P, and that key requirements for headgroup interactions for S1P (Fig. 2) can be replaced by ion- IMMUNOLOGY 2005 135 cells, disrupts tight junctions between epithelial cells but not capillary endothelium and results in breakdown of the lung barrier. We also have evidence for a synergistic interaction between the S1P-S1P3 axis and exposure to tumor necrosis factor. Whereas neither tumor necrosis factor nor S1P induces pathologic changes in the lungs when given alone in subthreshold doses, the 2 molecules produce severe breakdown of lung barriers with lethal pulmonary leakage when administered together. These data have led us to a model in which S1P controls physiology in different systems through the use of discrete receptor subtypes that have different cellular and spatial distributions and through downstream signal coupling (Fig. 3). F i g . 2 . A space-filling model of the S1P 1 -selective ligand SEW2871 docked into the receptor shows the critical headgroup mimetic interactions. Image courtesy of A. Parrill, University of Memphis, Memphis, Tennessee. dipole interactions in this synthetic tetra-aromatic chemical series. These studies suggest that continuous agonism is a requirement for sequestration. ROLE OF SIGNALING LIPIDS IN THE CONTROL OF LUNG INTEGRITY Pulmonary abnormalities, including acute respiratory distress syndrome, are characterized by disruption of pulmonary integrity and edema that compromise respiratory function. S1P is a lipid mediator synthesized and/or stored in mast cells, platelets, and epithelial cells, and its production is upregulated by the proinflammatory cytokines IL-1 and tumor necrosis factor. We suspected that S1P could be an independent regulator of lung barrier function and therefore a contributor to lung injury. In collaboration with J. Chun, Department of Molecular Biology, and M. Woods and B. Kiosses, Core Microscopy Facility, we used a combination of chemical and genetic approaches in mice lacking genes for S1P receptor subtypes to examine lung barrier integrity. We found that barrier integrity is regulated through S1P3 activation, whereas lymphocyte recirculation is controlled by S1P1. It is now apparent that different S1P receptor subtypes regulate lung barrier function in spatially distinct and functionally opposite ways. S1P1, found on lung capillaries, tightens capillary junctions and protects from leakage, whereas S1P3, found on lung epithelial Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. Spatially and mechanistically distinct S1P receptor subtypes have opposing effects on pulmonary epithelial and endothelial barriers. S1P modulates epithelial and endothelial barrier function. Fig. 3. S1P-induced S1P 3 activation in alveolar epithelium results in increased permeability via opening of tight junctions and loss of zonula occludens protein, most likely through Rho activation. In contrast, activation of S1P1 receptors on endothelial cells activates Rac1 GTPase, inducing downstream assembly and stabilization of cell-cell junctions with reorganization of the actin cytoskeleton and VE-cadherin. G indicates G protein; filled circles indicate cellcell junctions. S T R AT E G I C O U T L O O K The S1P system thus regulates adaptive immunity in at least 3 discrete ways: egress of naive cells from lymph nodes, sequestration of effector T cells in lymph nodes, and egress of mature medullary T cells from the thymus. The system can therefore affect both the peripheral diversity of lymphocytic responses and the efficiency of T-cell activation by misdirecting T cells to the wrong lymph nodes and by inhibiting the egress of antigen-specific effector T cells from lymph nodes after antigen activation and clonal proliferation. 136 IMMUNOLOGY 2005 These effects can alter adaptive immune responses and the expression of tissue damage while providing potentially important advantages to patients by sparing innate host defenses to bacteria and pathogenic fungi. The fine molecular control of this system and its effect on immune responses as a fundamental approach to organization of the immune system and potential therapeutic agents will remain our primary focus. The recent discovery of a critical role for chemically tractable S1P receptors in the innate immune system, where the S1P system regulates lung epithelial barrier function, is a new focus in molecular pathogenesis of inflammatory lung disease that is of long-term interest to us. Regulation of Protease Signaling Pathways W. Ruf, J. Ahamed, M. Belting,* A. Dorfleutner, E. Hintermann,** M. Kerver, B.M. Mueller,*** F. Niessen, T. Kurokawa, Y. Kurokawa, Y. Lee, M. Majumdar, H. Peterson, Y. Takada,**** H.H. Versteeg * University of Lund, Lund, Sweden ** Department of Cell Biology, Scripps Research *** La Jolla Institute for Molecular Medicine, San Diego, California **** University of California, Davis, California THERAPEUTIC INTERVENTION WITH TISSUE PUBLICATIONS Goetzl, E.J., Rosen, H. Regulation of immunity by lysosphingolipids and their G protein-coupled receptors. J. Clin. Invest. 114:1531, 2004. Gon, Y., Wood, M.R., Kiosses, W.B., Jo, E., Sanna, M.G., Chun, J., Rosen, H. S1P3 receptor-induced reorganization of epithelial tight junctions compromises lung barrier integrity and is potentiated by TNF. Proc. Natl. Acad. Sci. U. S. A. 102:9270, 2005. Hale, J.J., Doherty, G., Toth, L., Li, Z., Mills, S.G., Hajdu, R., Keohane, C.A., Rosenbach, M., Milligan, J., Shei, G.J., Chrebet, G., Bergstrom, J., Card, D., Rosen, H., Mandala, S. The discovery of 3-(N-alkyl)aminopropylphosphonic acids as potent S1P receptor agonists. Bioorg. Med. Chem. Lett. 14:3495, 2004. Hale, J.J., Doherty, G., Toth, L., Mills, S.G., Hajdu, R., Keohane, C.A., Rosenbach, M., Milligan, J., Shei, G.J., Chrebet, G., Bergstrom, J., Card, D., Forrest, M., Sun, S.Y., West, S., Xie, H., Nomura, N., Rosen, H., Mandala, S. Selecting against S1P3 enhances the acute cardiovascular tolerability of 3-(N-benzyl)aminopropylphosphonic acid S1P receptor agonists. Bioorg. Med. Chem. Lett. 14:3501, 2004. Hale, J.J., Lynch, C.L., Neway, W., Mills, S.G., Hajdu, R., Keohane, C.A., Rosenbach, M.J., Milligan, J.A., Shei, G.J., Parent, S.A., Chrebet, G., Bergstrom, J., Card, D., Ferrer, M., Hodder, P., Strulovici, B., Rosen, H., Mandala, S. A rational utilization of high-throughput screening affords selective, orally bioavailable 1-benzyl-3-carboxyazetidine sphingosine-1-phosphate-1 receptor agonists. J. Med. Chem. 47:6662, 2004. Hale, J.J., Neway, W., Mills, S.G., Hajdu, R., Keohane, C.A., Rosenbach, M., Milligan, J., Shei, G.J., Chrebet, G., Bergstrom, J., Card, D., Koo, G.C., Koprak, S.L., Jackson, J.J., Rosen, H., Mandala, S. Potent S1P receptor agonists replicate the pharmacologic actions of the novel immune modulator FTY720. Bioorg. Med. Chem. Lett. 14:3351, 2004. Hale, J.J., Yan, L., Neway, W.E., Hajdu, R., Bergstrom, J.D., Milligan, J.A., Shei, G.J., Chrebet, G.L., Thornton, R.A., Card, D., Rosenbach, M., Rosen, H., Mandala, S. Synthesis, stereochemical determination and biochemical characterization of the enantiomeric phosphate esters of the novel immunosuppressive agent FTY720. Bioorg. Med. Chem. 12:4803, 2004. Jo, E., Sanna, M.G., Gonzalez-Cabrera, P.J., Thangada, S., Tigyi, G., Osborne, D.A., Hla, T., Parrill, A.L., Rosen, H. S1P1-selective in vivo-active agonist from high-throughput screening: off-the-shelf chemical probes of receptor interactions, signaling and fate. Chem. Biol. 12:703, 2005. Rosen, H. Chemical approaches to the lysophospholipid receptors. Prostaglandins Other Lipid Mediat. 77:179, 2005. Yan, L., Hale, J.J., Lynch, C.L., Budhu, R., Gentry, A., Mills, S.G., Hajdu, R., Keohane, C.A., Rosenbach, M.J., Milligan, J.A., Shei, G.J., Chrebet, G., Bergstrom, J., Card, D., Rosen, H., Mandala, S.M. Design and synthesis of conformationally constrained 3-(N-alkylamino)propylphosphonic acids as potent agonists of sphingosine-1-phosphate (S1P) receptors. Bioorg. Med. Chem. Lett. 14:4861, 2004. Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. FA C T O R – S I G N A L I N G PAT H WAY S e are interested in protease systems that regulate inflammation, cancer, and angiogenesis. Activation of the coagulation pathway by tissue factor (TF) triggers cell signaling events that contribute to lethality in sepsis. Therapy with activated protein C, the natural counterbalance of TF-initiated coagulation, markedly improves survival in severe sepsis. We recently showed that activated protein C elicits a unique cellular response in inflamed endothelial cells through protease-activated receptor 1 (PAR-1) signaling. The established gene profile is consistent with antiapoptotic and anti-inflammatory protection of the endothelium. Importantly, the pattern of gene induction is distinct from the profile of the proinflammatory protease thrombin, which signals through the same G protein–coupled receptor PAR-1. Thus, signaling specificity appears to be determined by accessory protease-binding receptors, and protease-specific effects appear to be crucial for therapeutic benefit. How to appropriately target direct TF-initiated cell signaling pathways remains an active area of research. Inhibition of TF can improve survival in animal models of lethal hemorrhagic fever, similar to the results of earlier studies of bacterial septicemia. However, clinical trials with a recombinant form of TF pathway inhibitor, the physiologic inhibitor of TF, have been disappointing. We established that the recombinant inhibitor is a poor inhibitor of signaling of the TF initiation complex at doses that are highly effective in blocking activation of coagulation. Because bleeding complications are the major concern with TF-directed inhibitors, the ratio of anticoagulant efficiency to antisignaling potency of TF-targeted inhibitors is a consideration in using such drugs to treat inflammatory disorders or cancer. W IMMUNOLOGY We are identifying downstream signaling responses that predict inhibitory potency of TF-directed strategies. We are also continuing basic mechanistic studies on the specificity of the TF signaling pathway; our goal is to identify inhibitors with highly selective antisignaling activities. We identified a candidate that specifically blocks signaling by the complex consisting of TF and coagulation factor VIIa without impairing the coagulant response. Such strategies may allow intervention in TF signaling pathways while reducing the risk of impairments in hemostasis. 2005 137 stream of the TF cytoplasmic domain and the cross talk of TF with PARs and integrins. PUBLICATIONS Ahamed, J., Belting, M., Ruf, W. Regulation of tissue factor-induced signaling by endogenous and recombinant tissue factor pathway inhibitor 1. Blood 105:2384, 2005. Belting, M., Ahamed, J., Ruf, W. Signaling of the tissue factor coagulation pathway in angiogenesis and cancer. Arterioscler. Thromb. Vasc. Biol. 25:1545, 2005. Dorfleutner, A., Hintermann, E., Tarui, T., Takada, Y., Ruf, W. Cross-talk of integrin α3β1 and tissue factor in cell migration. Mol. Biol. Cell 15:4416, 2004. Majumdar, M., Tarui, T., Shi, B., Akakura, N., Ruf, W., Takada, Y. Plasmininduced migration requires signaling through protease-activated receptor 1 and integrin α9β1. J. Biol. Chem. 279:37528, 2004. R E G U L AT I O N O F I N T E G R I N F U N C T I O N B Y T F CYTOPLASMIC DOMAIN SIGNALING We discovered an important regulatory role of the TF cytoplasmic domain in tumor and developmental angiogenesis. In diabetic eye diseases, we showed that the cytoplasmic domain of TF is phosphorylated specifically in neovasculature associated with pathologic changes but not in normal endothelial cells or in the vessel wall. PAR-2, the target for TF-VIIa signaling, was also expressed in neovasculature. These data suggest that the TF-VIIa complex is an important regulator of vascular cell signaling in angiogenesis. Results of recent in vitro studies lend further support to the concept that the TF–PAR-2 pathway regulates cell migration in angiogenesis. The extracellular domain of TF is involved in an interaction with integrins, but we found no evidence that the TF-integrin interaction results in competition or inhibition of binding to the extracellular matrix by these adhesive receptors. Rather, TF specifically suppressed cell migration on laminin 5 that depends on activation of integrin α3β1. Inhibition of promigratory α3β1 depended on the TF cytoplasmic domain. Mutagenesis indicated that integrin function is suppressed when the TF cytoplasmic domain is not phosphorylated. However, phosphorylation of the TF cytoplasmic domain by TF-VIIa–mediated PAR-2 signaling is sufficient to release integrin inhibition. Thus, protease-driven signaling pathways of TF regulate cell migration by targeting the cross talk between the cytoplasmic domain of TF and integrins. Laminin 5 is a component of basement membranes, including the subendothelial matrix. In addition, α3β1 is targeted by antiangiogenic molecules. We are using genetic approaches to define components of the TF–VIIa– PAR-2 signaling pathway and the relationship between the pathway and integrins during angiogenesis in vivo. In vitro, we are mapping the signaling pathways downPublished by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. Peerschke, E.I.B., Petrovan, R.J., Ghebrehiwet, B., Ruf, W. Tissue factor pathway inhibitor-2 (TFPI-2) recognizes the complement and kininogen binding protein gCIqR/p33 (gCIqR): implications for vascular inflammation. Thromb. Haemost. 92:811, 2004. Riewald, M., Ruf, W. Protease-activated receptor-1 signaling by activated protein C in cytokine-perturbed endothelial cells is distinct from thrombin signaling. J. Biol. Chem. 280:19808, 2005. Ruf, W. Emerging roles of tissue factor in viral hemorrhagic fever. Trends Immunol. 25:461, 2004. Shi, X., Gangadharan, B., Brass, L.F., Ruf, W., Mueller, B.M. Protease-activated receptors (PAR1 and PAR2) contribute to tumor cell motility and metastasis. Mol. Cancer Res. 2:395, 2004. Structural Analysis of the Host-Pathogen Interface E. Ollmann Saphire, M.L. Havert, D.M. Abelson, C.R. Kimberlin, J.E. Lee e are crystallizing proteins that play key roles in the pathogenesis and lethality of viruses that cause hemorrhagic fever. The resulting crystal structures will provide (1) information for design of vaccines and inhibitors against the viruses as the microbes exist naturally and (2) structural templates that will enable us to anticipate and rapidly respond to newly emerging and synthetic versions of the virus and viral proteins. W EBOLA AND MARBURG VIRUSES At least 10 recognized outbreaks of infection with Ebola virus in humans have occurred; in each outbreak, 50%–90% of those infected died. Six outbreaks of infection by the closely related Marburg virus have also occurred. In outbreaks of Marburg virus, typically 25%–40% of those infected die; however, in a recent outbreak in Angola, mortality was 90%. To date no vaccines or treatments are available for infections caused by either virus. 138 IMMUNOLOGY 2005 With these 2 viruses, death usually occurs 7–12 days after infection. Events early in infection and innate immune responses are critical for survival in those infected. However, filoviruses have evolved mechanisms by which the host immune system is suppressed. For example, the viral protein VP35 is a required component of the Ebola and Marburg viral capsids and transcription complexes. VP35 also blocks activation of immunomodulatory genes by type I interferon and may play a significant role in viral suppression of the host immune system. Hence, structural analysis of the VP35 protein will provide insights into viral replication and type I interferon suppression and will provide the structural basis for the design of antiviral compounds and attenuated viral strains. An additional, unusual feature of the genome of Ebola virus is its ability to encode 2 different glycoproteins, sGP and GP, from the same gene. These 2 glycoproteins share 295 amino acids of N-terminal sequence, but a transcriptional editing event causes them to have different C-terminal sequences that result in unique patterns of disulfide bonding, structures, and roles in pathogenesis. Comparative structural analysis of sGP and GP should explain how 2 structures arise from the same sequence and should provide templates for the design of vaccines that elicit antibodies that target the virus rather than the secreted proteins. In contrast, Marburg virus expresses only the membrane-embedded GP. Although Ebola and Marburg viruses are closely related, antibodies to Ebola GP do not cross-react with Marburg GP. Comparative structural analysis of Ebola and Marburg GP should illustrate the fusion machinery required for infection and the structural mechanisms by which the viruses escape from immune surveillance. Additional crystal structures of these proteins in complex with rare human antibodies derived from survivors of infection will assist in vaccine design. DENGUE VIRUS Dengue virus is a mosquito-borne flavivirus that causes up to 100 million infections each year. Infection with dengue virus results in either dengue fever or the much more severe disease dengue hemorrhagic fever. Dengue hemorrhagic fever usually occurs upon secondary infection with a different viral subtype or in infants born to dengue virus–immune mothers. This potential antibody-mediated enhancement of infection is a major concern in the testing and use of vaccines against dengue virus because antibodies elicited by the vacPublished by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. cines could trigger severe disease. To aid in vaccine design, we are determining crystal structures of envelope proteins of contemporary field isolates of dengue virus, alone and in complex with antibodies, to determine structural features of epitopes associated with neutralization and enhancement. Autoimmune Mechanisms and Compensatory Responses N. Sarvetnick, M. Cleary, S. Dabernat, D. Dietz, C. Fine, N. Hill, H. Hua, A. Ilic, H.-B. Jie, V. Judkowski, A. Kayali, C. King, M. Kritzik, X. Li, G. Liu, A. Maday, A. Marleau, E. Rodriguez, P. Secrest, M. Solomon, L. Sterling, A. Stotland, W. Wu, D. Yadav, Y.Q. Zhang nfection with coxsackievirus is associated with the development of autoimmunity in humans, and infection with the CB4 strain of this virus is strongly linked to the development of type 1 diabetes mellitus. However, the development of autoimmunity in general depends on the availability of autoimmune T cells in the periphery. Normally, autoreactive T cells are deleted during negative selection in the thymus, and few T cells with high affinity for self-antigens gain access to the body. We tested the hypothesis that CB4 infection inhibits negative selection in the thymus, allowing the maturation of self-reactive T cells and migration of the cells into the periphery. We found that novel central tolerance mechanisms are responsible for coxsackievirus-induced autoimmunity. We have now determined the role of coxsackievirus in the generation of an autoimmune T-cell repertoire. Recently, we found that CB4 infects the thymus. We extended this observation to show that productive infection leads to dramatic changes in the developmental processes that occur during T-cell differentiation in the thymus. In investigating this novel aspect of virusinduced autoimmunity, we determined that CB4 infection inhibits the negative selection of self-reactive T cells in the thymus. On the basis of our recent data, we selected several interesting mechanisms for further study. We are using a diversified molecular approach to gain information that will bring a novel mechanism linking virus infection with autoimmunity into focus, fueling new directions in therapy for virus-induced autoimmunity. The cytokines IFN-γ and TNF-α play major roles in the destruction of pancreatic islets during the develop- I IMMUNOLOGY ment of diabetes and in the acute rejection of islet tissue allografts. The protein termed suppressor of cytokine signaling-1 (SOCS-1) negatively regulates interferon signaling by inhibiting activation of the proteins Janus kinase and signal transducer and activator of transcription. We investigated whether modulation of interferon signaling by SOCS-1 could prevent the destruction of pancreatic islet tissue allografts in mice. We found that islets expressing SOCS-1 that were transplanted beneath the kidney capsule of MHC-mismatched recipient mice had delayed allograft rejection and reversed streptozotocin-induced diabetes for at least 2 weeks longer than did normal islets. Surprisingly, although SOCS-1 negatively regulates interferon signaling, the islets expressing SOCS-1 responded to stimulation with IFN-γ and upregulated class I MHC selfantigens, suggesting that this negative regulation was not a factor in improved islet allograft survival. Islets expressing SOCS-1 were significantly more resistant than normal islets to cytokine-induced cell death after treatment with TNF-α alone or with TNF-α plus IFN-γ. Protection against cytokine-induced cytotoxic effects correlated with degradation of the IκB inhibitor of the upstream transcription factor NF-κB and inhibition of the transcription factor interferon regulatory factor-1, reflecting enhanced NF-κB–regulated cell survival signals. Our findings indicate that intragraft expression of SOCS-1 makes islets insensitive to the deleterious effects of cytokines and will be important in the development of therapies to prevent acute allograft rejection. Activins regulate the growth and differentiation of a variety of cells. During the development of islets in the pancreas, activins are required for the specialization of pancreatic precursors from the gut endoderm during midgestation. We probed the role of activin signaling during the development and regeneration of pancreatic islet cells. We found that both activins and activin receptors are upregulated in duct epithelial cells during islet differentiation. Interestingly, the expression of endogenous cellular inhibitors of activin signaling, follistatin and Cripto, were also augmented. Inhibition of activins significantly enhanced survival and expansion of pancreatic epithelial cells but decreased the numbers of differentiated cells. Our results suggest that the homeostasis of growth and terminal differentiation requires a precise context-dependent regulation of activin signaling. Follistatin participates in this process by promoting proliferation of precursor cells during pancreas growth. Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. 2005 139 PUBLICATIONS Jie, H.-B., Sarvetnick, N. The role of NK cells and NK cell receptors in autoimmune disease. Autoimmunity 37:147, 2004. Judkowski, V., Rodriguez, E., Pinilla, C., Masteller, E., Bluestone, J.A., Sarvetnick, N., Wilson, D.B. Peptide specific amelioration of T cell mediated pathogenesis in murine type 1 diabetes. Clin. Immunol. 113:29, 2004. Judkowski, V., Allicotti, G.M., Sarvetnick, N., Pinilla, C. Peptides from common viral and bacterial pathogens can efficiently activate diabetogenic T-cells. Diabetes 53:2301, 2004. Yadav, D., Judkowski, V., Flodstrom-Tullberg, M., Sterling, L., Redmond, W.L., Sherman, L., Sarvetnick, N. B7-2 (CD86) controls the priming of autoreactive CD4 T cell responses against pancreatic islets. J. Immunol. 173:3631, 2004. Zhang, Y.-Q, Cleary, M.M., Si, Y., Liu, G.. Eto, Y., Kritzik, M., Dabernat, S., Kayali, A.G., Sarvetnick, N. Inhibition of activin signaling induces pancreatic epithelial cell expansion and diminishes terminal differentiation of pancreatic beta-cells. Diabetes 53:2024, 2004. Promotion of Cell Migration and Invasion by Tyrosine Kinase Signaling D.D. Schlaepfer, J.A. Bernard-Trifilo, X.L. Chen, A. Chi, D.A. Hanson, S. Hou, S.T. Lim, Y.M. Lim, S.K. Mitra, J. Molina, S. Uryu e wish to understand how intracellular signaling networks coordinate a complex biological response such as cell motility. In order for a cell to correctly process different environmental stimuli, these networks must contain critical intracellular signaling proteins that act as signal “integrators.” These proteins should be activated by various extracellular inputs and act to regulate multiple downstream signaling pathways. One such integrator is focal adhesion kinase (FAK), an intracellular protein tyrosine kinase that is associated with sites of binding of integrin receptors to matrix proteins such as fibronectin. FAK catalytic activity is enhanced by integrin binding to fibronectin, and FAK tyrosine phosphorylation is increased in a receptor-proximal fashion by transmembrane growth factor and G protein–linked receptors. In many cells, increased FAK phosphorylation promotes binding of the protein tyrosine kinase c-Src to FAK, thereby generating a dual FAK-Src protein tyrosine kinase signaling complex. Because genetic inactivation of FAK or Src results in the inhibition of cell migration, our research strategy is to perform rescue and/or gainof-function assays in cells that lack FAK expression. We are elucidating the importance of FAK catalytic activity, FAK-Src signaling connections, novel interactions W 140 IMMUNOLOGY 2005 between FAK and its binding partners, and the molecular basis for FAK relocalization to distinct intracellular sites after treatment with motility-promoting stimuli. Additionally, we are investigating the effects of the upregulated expression of the FAK-related proline-rich tyrosine kinase 2 in cells that lack FAK. During cancer progression, tumor cells can become highly motile and invasive. These properties contribute to tumor spread and metastasis. Elevated FAK expression has been correlated with increased tumor growth and metastasis. However, the molecular signaling connections that link FAK to tumorigenesis remained undefined. The possibility that FAK-mediated signaling may promote increased tumor growth has generated much interest in determining the molecular mechanisms of FAK function in both normal cells and tumor cells. We found that FAK-Src signaling promotes an invasive cell phenotype in a manner that is distinct from the signaling role of FAK in promoting cell motility. In these studies, we are using live-cell imaging, recombinant viral vectors, RNA interference, cell culture–based signaling, and in vivo tumor growth and experimental metastasis assays to elucidate the molecular signaling connections of FAK in tumorigenesis. PUBLICATIONS Mitra, S.K., Hanson, D.H., Schlaepfer, D.D. Focal adhesion kinase: in command and control of cell motility. Nat. Rev. Mol. Cell. Biol. 6:56, 2005. The Consequences of T-Cell Recognition of Self-Antigens and Tumor Antigens L.A. Sherman, H.T.C. Kreuwel, W.L. Redmond, M.A. Lyman, C.H. Wei, J.A. Biggs, K.L. Marquardt, R.L. Trenney, J. Martinez, B. Marincek he consequence of antigen recognition by naive CD8 + T cells can be either tolerance or immunity, depending on the activation status of the antigen-presenting dendritic cells. If a CD8+ cell recognizes antigen on a quiescent dendritic cell that expresses relatively low levels of costimulatory molecules, then activation of the T cell results in deletion and tolerance. Inflammatory signals, such as those due to the presence of foreign pathogens and activated lymphocytes, activate dendritic cells to express cellsurface costimulatory molecules and cytokines. If T Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. CD8 + T cells recognize antigen on activated dendritic cells, the costimulatory molecules and cytokines prevent deletion and promote the clonal expansion of the CD8 + cells and the development of effector functions. Understanding the signals that result in either T-cell deletion or immunity is of importance in preventing autoimmunity, which represents a failure to control self-destructive T lymphocytes. This understanding is also important in promoting tumor immunity, in which the goal is to promote the autoimmune destruction of tumor cells. We are comparing the consequence of the interaction of naive CD8+ T lymphocytes with a transgenic self-antigen (the influenza virus hemagglutinin) expressed by the insulin-producing beta cells in the pancreatic islets in 3 different types of mice: normal mice (Ins-HA mice), diabetes-prone nonobese diabetic mice (NOD-InsHA mice) and mice in which the beta cells express an oncogene that promotes spontaneous transformation and production of tumors (RIP-Tag2-InsHA mice). In all 3 types of mice, the interaction between antigen and naive CD8 + T lymphocytes specific for hemagglutinin first occurs in the pancreatic lymph nodes. There, antigen is recognized on dendritic cells that obtain it from beta cells in the islets and cross-present it to naive T cells in the lymph nodes. In normal mice, this interaction results in an abortive activation of the T cells and subsequent deletion of the potentially autoreactive T cells specific for hemagglutinin. DELETION OF NAIVE CD8+ T CELLS Using the transgenic animals that were our source of naive CD8 + T cells specific for hemagglutinin, we examined the requirements for peripheral deletion in vivo. We found that independent of the amount of antigen used for stimulation, a single dose of antigen did not result in complete clonal deletion. Instead, further antigenic exposure was required to completely eliminate all of the activated T cells. Consecutive stimulations with low doses of antigen were highly effective in promoting deletion. In contrast, although stimulation with high doses of antigen initially led to the programmed cell death of many of the activated T cells, it induced hyporesponsiveness in part of the responding cells, thereby sparing the cells from further activation and deletion. These data explain why some conditions promote tolerance through clonal deletion whereas others promote anergy. Furthermore, the data provide a framework for devising protocols for effective deletion of potentially autoreactive T cells. IMMUNOLOGY R E S T O R AT I O N O F C D 8 + T - C E L L T O L E R A N C E I N NOD MICE The development of autoimmune diseases such as type 1 diabetes is mediated by multiple genetic and environmental factors. Although genes that may control type 1 diabetes can now be identified, defining the resulting cellular events mediated by each locus is a major challenge. In congenic NOD mice, the genetic regions that control diabetes, designated as insulindependent diabetes (Idd) loci, have been replaced by resistant alleles obtained from nondiabetic strains of mice. We hypothesize that critical genetic susceptibility loci regulate the maintenance of self-specific CD8+ T cells. We compared the fate of islet-reactive CD8+ and CD4 + T cells in diabetes-susceptible NOD mice with the fate of the same kinds of cells in diabetes-resistant NOD congenic mice with protective alleles at Idd3, Idd5.1, and Idd5.2 (Idd3/5 strain) or at Idd9.1, Idd9.2, and Idd9.3 (Idd9 strain). We found that protection from diabetes in each instance is correlated with functional tolerance of islet-specific CD8+ T cells; however, this tolerance is achieved in different ways. In Idd3/5 mice, tolerance occurs during the initial activation of islet-specific CD8+ and CD4+ T cells in the pancreatic lymph nodes, where the presence of CD25 + regulatory T cells prevents accumulation of the CD8+ and CD4 + T cells. In contrast, resistance alleles in Idd9 mice do not prevent the accumulation of islet-specific CD8+ and CD4+ T cells in the pancreatic lymph nodes, suggesting that tolerance occurs at a later checkpoint. These results underscore the variety of ways that autoimmunity can be prevented and indicate the elimination of islet-specific CD8+ T cells as a common indicator of high-level protection. FAT E O F L O W - A F F I N I T Y T U M O R - S P E C I F I C C D 8 + T CELLS IN TUMOR-BEARING MICE An important issue in tumor immunology is how to best activate and mobilize the low-avidity self-specific and tumor-specific T cells that remain in the T-cell repertoire after the development of central and peripheral tolerance. We generated transgenic mice that express a low-avidity T-cell receptor (clone 1 mice) specific for hemagglutinin, a model self-antigen. When CD8+ T cells from clone 1 mice were transferred into InsHA mice, little proliferation occurred in response to low amounts of cross-presented hemagglutinin, indicating that the low-avidity clone 1 CD8+ T cells ignore the cross-presented hemagglutinin self-antigen. In contrast, the Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. 2005 141 expression of hemagglutinin as a tumor-associated antigen on spontaneous hemagglutinin-expressing beta cell tumors in RIP-Tag2-InsHA mice led to high levels of cross-presented antigen that could activate the lowaffinity clone 1 T cells. However, because of the absence of inflammatory signals, this activation resulted in deletion of the clone 1 cells. This model should be useful in optimizing protocols for immunotherapy of solid tumors with low-affinity tumor-specific T cells. PUBLICATIONS Kuball, J., Schmitz, F.W., Voss, R.H., Ferreira, E.A., Engel, R., Guillaume, P., Strand, S., Romero, P., Huber, C., Sherman, L.A., Theobald, M. Cooperation of human tumor-reactive CD4+ and CD8+ T cells after redirection of their specificity by a high-affinity p53A2.1-specific TCR. Immunity 22:117, 2005. Lyman, M.A., Nugent, T.C., Marquardt, K.L., Biggs, J.A., Pamer, E.G., Sherman, L.A. The fate of low affinity tumor specific CD8+ T cells in tumor-bearing mice. J. Immunol. 174:2563, 2005. Redmond, W.L., Marincek, B.C., Sherman, L.A. Distinct requirements for deletion versus anergy during CD8 T cell peripheral tolerance in vivo. J. Immunol. 174:2046, 2005. Redmond, W.L., Sherman, L.A. Peripheral tolerance of CD8 T lymphocytes. Immunity 22:275, 2005. Yadav, D., Judkowski, V., Flodstrom-Tullberg, M., Sterling, L., Redmond, W.L., Sherman, L., Sarvetnick, N. B7-2 (CD86) controls the priming of autoreactive CD4 T cell response against pancreatic islets. J. Immunol. 173:3631, 2004. T-Cell Specificity and the Thymus J. Sprent, O. Boyman, J.-H. Cho, N. Ishimaru, M. Kovar, M.P. Rubinstein ature T cells arise in the thymus through a process of positive and negative selection directed to MHC molecules complexed with self-peptides. In the secondary lymphoid tissues, T cells survive for prolonged periods through covert signaling elicited by interaction with self-peptide–MHC complexes and various cytokines. Contact with complexes composed of foreign peptide and MHC molecules causes T cells to proliferate and differentiate into effector cells, with subsequent production of small numbers of memory cells. M S T I M U L AT I O N O F N A I V E C D 8 + T C E L L S B Y FRAGMENTS OF ANTIGEN-PRESENTING CELLS Typical immune responses of naive CD8+ T cells are stimulated by foreign peptide–MHC complexes on viable antigen-presenting cells (APCs) such as dendritic cells and take place in the T-dependent areas of the lymphoid tissues. Under certain conditions, however, 142 IMMUNOLOGY 2005 CD8 + cells can respond to small membrane vesicles (exosomes) secreted by APCs. In addition, CD8 + cells can be stimulated by fragments of plasma membranes derived from sonicated APCs. These membrane fragments can be directly immunogenic for purified naive CD8 + T cells in the absence of APCs, but only when the fragments express specific peptide-MHC complexes and also coexpress both B7 and intracellular adhesion molecule 1 (the ligands for CD28 and lymphocyte function–associated antigen 1, respectively). Membrane fragments from APCs are also immunogenic in vivo and can be used to prime mice for tumor rejection. CYTOKINE CONTROL OF CD8+ T MEMORY CELLS CD8+ T memory cells are kept alive and divide intermittently through contact with cytokines, especially IL-15. Another cytokine, IL-2, can have a negative effect on CD8 + memory cells, as indicated by the finding that the number of CD8+ memory cells are greatly increased in mice that lack IL-2 or components of the receptor for IL-2 such as CD25 (IL-2Rα) and CD122 (IL-2/IL15Rβ). Unexpectedly, we found massive proliferation of normal CD8 + memory cells after the cells were transferred to mice that lack the gene for IL-2 or the gene for the IL-2 receptor. Why CD8+ memory cells proliferate under these conditions is unclear. I N F L U E N C E O F N F - κB O N T - C E L L P R O L I F E R AT I O N Stimulation of T cells and other lymphoid cells is controlled by heterodimers of NF-κB1 (p50) and NF-κB2 (p52) bound to RelA and RelB subunits, respectively. In the case of NF-κB2, activation and nuclear translocation are under the control of NF-κB–inducing kinase, which is mutated in aly/aly mice. Confirming the results of others, we found that unseparated aly/aly CD4 + T cells have poor proliferative responses, implying an important role for NF-κB2 in proliferation. Surprisingly, however, quite different results occur when CD4+ T cells are separated into purified subsets of naive (CD44lo) and memory (CD44 hi) phenotype cells. Thus, CD44lo cells have enhanced proliferative responses and IL-2 synthesis, whereas CD44lo cells have reduced responses. Significantly, inhibition occurs when CD44hi cells are added to CD44 lo cells. These findings indicate that primary responses of T cells can be negatively influenced by NF-κB2. PUBLICATIONS Ganesh, K.A., Thomas, S., Thompson, L., Sprent, J., Murali-Krishna, K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J. Exp. Med., in press. Goodnow, C.C., Sprent, J., de St. Groth, B.F., Vinuesa, C.G. Cellular and genetic mechanisms of self tolerance and autoimmunity. Nature. 435:570, 2005. Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. Sprent, J. Direct stimulation of naive T cells by antigen-presenting cell vesicles. Blood Cells Mol. Dis. 35:17, 2005. Sprent, J. Proving negative selection in the thymus. J. Immunol. 174:3841, 2005. Sprent, J. Swapping molecules during cell-cell interactions. Sci. STKE pe8, 2005, March 1 issue. Surh, C.D., Sprent, J. Regulation of mature T cell homeostasis. Semin. Immunol. 17:183, 2005. Regulation of Homeostasis of Mature T Cells C.D. Surh, J. Tan, C. Ramsey, J. Purton, J.Y. Lee, W.C. Kieper, J.T. Burghardt, D. Kim, C. Ahn* * Seoul National University Hospital, Seoul, Korea he pool of mature T cells is constantly regulated by homeostatic mechanisms to remain at a steady size and maintain predictable proportions of naive and memory T cells. Homeostatic signals are received as a result of interactions of T cells with self-MHC molecules and the cytokines IL-7 and IL-15. Thus, contact with self-MHC molecules and IL-7 is required for survival of naive T cells, and a combination of IL-7 and IL-15 is required for survival of memory T cells. A hallmark of homeostatic regulation is the ability of both naive and memory T cells to undergo spontaneous homeostatic proliferation in response to severe T-cell depletion. A slower rate of homeostatic proliferation is also evident for memory T cells under normal T cell–sufficient conditions, but not for most naive T cells. Because the requirements for homeostatic proliferation closely mirror the requirements for cell survival, most likely the survival signals delivered by low levels of IL-7 and IL-15 become mitogenic signals when the concentrations of these cytokines are elevated. Homeostatic proliferation of naive T cells is driven by signals induced by contact of the cells with low-affinity ligands composed of self-antigens, MHC molecules, and peptides that have been intensified by elevated levels of IL-7. Thus, polyclonal naive T cells transferred into syngeneic wild-type mice treated to be lymphopenic undergo mostly homeostatic proliferation as indicated by the cell division rate of 1 division every 24–36 hours. In congenitally T cell–deficient syngeneic hosts, however, a fraction of polyclonal naive T cells undergoes a prodigious rate of proliferation and outcompetes the rest of the naive cells that are undergoing slow homeostatic proliferation. Such rapid T-cell proliferation resembles proliferation driven by high-affinity foreign antigens, T IMMUNOLOGY because the resultant T cells have characteristics of effector cells. The proliferation also occurs largely independent of homeostatic factors, that is, in the absence of IL-7 and in T cell–sufficient hosts that are nonetheless devoid of functional T-cell immunity. Consistent with the idea that foreign antigens induce such a rapid form of proliferation, congenitally immunodeficient mice raised under germ-free conditions had only slow homeostatic proliferation, not the rapid T-cell proliferation that occurred in conventionally raised immunodeficient mice. Thus, polyclonal proliferation of naive T cells in T cell–deficient hosts can be driven predominantly by either self-antigens or foreign antigens, depending on the host’s previous state of T-cell immunocompetency. The finding in germ-free mice also indicates that much of the homeostatic proliferation of naive T cells occurs in the absence of any foreign antigens. Among the various subsets of T cells, memory CD4 cells are probably the least well understood in terms of their homeostatic requirements. Recent research indicates that survival of antigen-specific memory CD4 cells is supported by IL-7, whereas spontaneously generated polyclonal memory phenotype cells can also use signals from contact with self-MHC-peptide ligands. One potential problem with studying memory phenotype cells in normal mice is that these cells appear to be heterogeneous in terms of their homeostatic requirements. Thus, whereas antigen-specific memory CD4 cells are acutely dependent on IL-7 for survival, a population of polyclonal memory phenotype cells is independent of IL-7 (and IL-15) for survival and homeostatic proliferation. The origin and the homeostatic requirements of these IL-7/IL-15–independent memory phenotype CD4 cells are unclear, but compared with antigen-specific memory CD4 cells, these cells appear to be at a higher state of activation, because they undergo a faster rate of homeostatic proliferation. The factors that support the generation and maintenance of various populations of memory phenotype T cells are currently unknown. Nonetheless, these cells may play a significant role in influencing the homeostatic behavior of other subsets of T cells through competition for homeostatic factors. PUBLICATIONS Baccala, R., Witherden, D., Gonzalez-Quintial, R., Dummer, W., Surh, C.D., Havran, W.L., Theofilopoulos, A.N. γδ T cell homeostasis is controlled by IL-7 and IL-15 together with subset-specific factors. J. Immunol. 174:4606, 2005. 2005 143 Surh, C.D., Sprent, J. Regulation of mature T cell homeostasis. Semin. Immunol. 17:183, 2005. Structure-Function Studies of Innate and Adaptive Immunity L. Teyton, B. Atteberry, K. Bennett, H. Burt, C. Cantu, S. Chabot, S.Y. Chang, W. Cheung, S. Freigang, H. Issafras, C. Li, N. Schrantz, J. Sim, R. Stefanko, C. Wang, K. Yoshida A C T I VAT I O N O F T - C E L L R E C E P T O R S ur goal is to understand the molecular switches that lead to activation of T cells. Assembly of functional complexes of T-cell receptors (TCRs) on artificial bilayers with recombinant forms of TCRαβ, CD3δε, CD3γε, and CD8αβ is in progress. We use a combination of single-molecule, multicolor imaging by total internal reflection fluorescence microscopy, in collaboration with K. Fish, Molecular and Integrative Neurosciences Department, and electron microscopy to examine the dynamics and membrane relationships of each subunit within the complex. Similar observations are carried out in the presence of MHC ligands displayed in solution or at the surface of polystyrene beads and liposomes. Interactions of MHC and TCR molecules with their respective membranes could provide simple switches essential to T-cell activation. This hypothesis is supported by our structure determination, in collaboration A.K. Mitra, University of Auckland, Auckland, New Zealand, of the structure of an MHC molecule attached to a phospholipid bilayer that shows parallel orientation of the long axis of the molecule with the lipid leaflet. In collaboration with I.A. Wilson, Department of Molecular Biology, we are determining 3-dimensional structures of CD3, TCR complexes and CD8αβ. O AUTOIMMUNE DIABETES We are using MHC multimers to detect antigenspecific T-cell populations in nonobese diabetic mice. Pathogenic T cells are characterized by analyzing secretion of cytokines and use of TCRs by single cells. We are also trying to treat insulin-dependent diabetes by depleting antigen-specific T cells in vivo during the preclinical phase of the disease. For this therapy, we are using MHC molecules to deliver doxorubicin liposomes to autoreactive T cells. The specificity of the intervention will limit the side effects and complications of general immunosuppression. L I N K B E T W E E N I N N AT E A N D A D A P T I V E I M M U N I T Y Kieper, W.C., Troy, A., Burghardt, J.T., Ramsey, C., Lee, J.Y., Jiang, H.-Q., Dummer, W., Shen, H., Cebra, J.J., Surh, C.D. Recent immune status determines the source of antigens that drive homeostatic T cell expansion. J. Immunol. 174:3158, 2005. Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. We are studying lipid binding to CD1 to determine the factors that govern the presentation of the lipids to 144 IMMUNOLOGY 2005 T cells. A family of lipid transfer proteins known as saposins, which are involved in the catabolism of lipids, are critical for the loading of natural glycolipids onto CD1 and the selection of natural killer T cells. Other lipid transfer proteins most likely account for the loading of other exogenous ligands. In collaboration with A. Bendelac, University of Chicago, we are using RNA interference, genetic techniques, and recombinant biochemistry to study CD1 within the context of lipid metabolism. I N N AT E I M M U N E R E C E P T O R S Recognition of unique features of the prokaryotic world is embedded in a series of receptors of the innate immune system called pattern recognition molecules. Each of these receptors can sense the presence of a family of unique prokaryotic compounds such as glycolipids, proteoglycans, DNA, or RNA and allow activation of macrophages, dendritic cells, and neutrophils. We are collaborating with R. Ulevitch and P. Tobias, Department of Immunology, to decipher the structural basis of this mode of recognition. We expressed recombinant forms of receptor family members from Drosophila, mice, and humans to compare the biophysical and structural characteristics of the receptors and to delineate new activation pathways. PUBLICATIONS Goff, R.D., Gao, Y., Mattner, J., Zhou, D., Yin, N., Cantu, C. III, Teyton, L., Bendelac, A., Savage, P.B. Effects of lipid chain lengths in α-galactosylceramides on cytokine release by natural killer T cells. J. Am. Chem. Soc. 126:13602, 2004. Kelker, M.S., Foss, T.R., Peti, W., Teyton, L., Kelly, J.W., Wuthrich, K., Wilson, I.A. Crystal structure of human triggering receptor expressed on myeloid cells 1 (TREM-1) at 1.47 Å. J. Mol. Biol. 342:1237, 2004. Malherbe, L., Hausl, C., Teyton, L., McHeyzer-Williams, M.G. Clonal selection of helper T cells is determined by an affinity threshold with no further skewing of TCR binding properties. Immunity 21:669, 2004. Mattner, J., Debord, K.L., Ismail, N., Goff, R.D., Cantu, C. III, Zhou, D., SaintMezard, P., Wang, V., Gao, Y., Yin, N., Hoebe, K., Schneewind, O., Walker, D., Beutler, B., Teyton, L., Savage, P.B., Bendelac, A. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 434:525, 2005. Ranheim, E.A., Tarbell, K.V., Krogsgaard, M., Mallet-Designe, V., Teyton, L., McDevitt, H.O., Weissman, I.L. Selection of aberrant class II restricted CD8+ T cells in NOD mice expressing a glutamic acid decarboxylase (GAD)65-specific T cell receptor transgene. Autoimmunity 37:555, 2004. Reiser, J.B., Teyton, L., Wilson, I.A. Crystal structure of the Drosophila peptidoglycan recognition protein (PGRP)-SA at 1.56 Å resolution. J. Mol. Biol. 340:909, 2004. Robey, I.F., Peterson, M., Horwitz, M.S., Kono, D.H., Stratmann, T., Theofilopoulos, A.N., Sarvetnick, N., Teyton, L., Feeney, A.J. Terminal deoxynucleotidyltransferase deficiency decreases autoimmune disease in diabetes-prone nonobese diabetic mice and lupus-prone MRL-Faslpr mice. J. Immunol. 172:4624, 2004. Zhou, D., Mattner, J., Cantu, C. III, Schrantz, N., Yin, N., Gao, Y., Sagiv, Y., Hudspeth, K., Wu, Y.P., Yamashita, T., Teneberg, S., Wang, D., Proia, R.L., Levery, S.B., Savage, P.B., Teyton, L., Bendelac, A. Lysosomal glycosphingolipid recognition by NKT cells. Science 306:1786, 2004. Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. Genes and Genetics of Systemic Autoimmunity and T-Cell Homeostasis in Autoimmunity and Cancer A.N. Theofilopoulos, D.H. Kono, R. Baccala, R. Chintalapati, R. Gonzalez-Quintial, M.K. Haraldsson, C.A. Louis-Dit-Sully, K.M. Pollard,* J. Schettini * Department of Molecular and Experimental Medicine, Scripps Research ur main interests are identifying predisposing loci and genes in murine models of systemic autoimmunity, clarifying the role of type I interferons in systemic lupus erythematosus (SLE), determining why activated/memory phenotype T cells accumulate in SLE and why cyclin-dependent kinase inhibitors are increased in these cells, and characterizing factors that influence acute homeostatic proliferation of T-cell subsets and the relevance of this process in autoimmunity and cancer. O GENETIC BASIS OF SYSTEMIC AUTOIMMUNITY Susceptibility to SLE is in large part determined by genetic predisposition. Thus, defining the specific genes and how certain alterations lead to autoimmunity should yield new insights into the pathogenesis of SLE and facilitate the development of innovative approaches to disease management. Because of the complexity of defining susceptibility genes in humans, we use both spontaneous and induced models of SLE in well-characterized inbred mouse strains. Previously, we identified loci that predispose mice to spontaneous manifestations of SLE in NZB, NZW, BXSB, MRL-Faslpr, and C57BL/6Faslpr strains and a DBA/2 locus associated with resistance to mercury-induced autoimmunity. Currently, we are identifying the underlying genes and their specific roles in autoimmunity for 4 loci: Lbw2, Lbw5, Lmb3, and Hmr1. Lbw2 is a locus on chromosome 4 in NZB mice that promotes spontaneous activation of B cells, production of autoantibodies, glomerulonephritis, and autoimmune hemolytic anemia. The Lbw2 locus appears to contain at least 3 subloci that affect different component phenotypes mapped to this interval. Lbw5 is a recessive locus on chromosome 7 in NZW mice that enhances production of IgG autoantibodies, glomerulonephritis, and autoimmune hemolytic anemia. Further mapping of Lbw5 suggests that this interval contains as least 2 subloci. IMMUNOLOGY The dominant MRL Lmb3 is also a locus on chromosome 7, but it occurs at a different, more distal location than does Lbw5. Lmb3 congenic MRL-Faslpr mice containing an introgressed chromosome 7 fragment of C57BL/6 have marked reductions in lymphoproliferation, production of autoantibodies, glomerulonephritis, and early mortality. This locus was recently mapped to a 0.8 Mb-sized interval, and a likely candidate gene with a functional mutation has been identified. The Hmr1 locus on chromosome 1 does not confer resistance to deposition of glomerular immune complexes in congenic NZB and SJL mice that have the DBA/2 Hmr1 interval, suggesting that epistatic interactions with other DBA/2 resistance genes are required. In support of this notion, the reciprocal congenic DBA/2 mice with the NZB Hmr1 region are susceptible to mercury-induced autoimmunity. We are mapping the number and location of the various subloci and are identifying and characterizing possible genes in the reduced intervals. TYPE I INTERFERONS IN SLE Type I interferons (IFN-α/β) are highly pleiotropic cytokines that affect both innate and adaptive immune responses. Long-standing observations indicate the central role of these cytokines in the pathogenesis of SLE in humans and in animal models. In our studies of SLE-prone NZB mice that lack the common receptor for IFN-α/β, we clearly delineated the pathogenic role of these effector molecules. Compared with mice that had the receptor, mice that lacked the receptor had significant decreases in humoral, cellular, and histologic characteristics of SLE and increases in survival. Several questions remain unanswered, however, particularly about the mechanisms associated with endogenous stimuli for production of IFN-α/β. We are determining the efficacy of a nonviral vector that encodes the IFN-α/β–binding IFNAR2 chain fused to the Fc fragment of IgG1 in inhibiting disease when applied either at the early (prophylactic) stage or the late (therapeutic) stage of the disease in various models of spontaneous SLE. The efficacy of this approach will provide the basis for translating these results to similar contemplated efforts for treatment of SLE in humans. We are also interested in differentiating the effects of IFN-α from those of IFN-β and in defining the postulated central role of plasmacytoid dendritic cells as the major producers of IFN-α/β in this disease. Most importantly, in collaboration with B. Beutler and K. Hoebe, Department of Immunology, we are creating congenic Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. 2005 145 SLE mice that lack adaptor molecules involved in the production of IFN-α/β mediated by the Toll-like receptors. We are also concentrating on identifying the exact nature of the endogenous (self) factors that stimulate production of IFN-α/β, particularly the roles of apoptotic materials and immune complexes composed of autoantibodies and particles containing DNA and/or RNA. CYCLIN-DEPENDENT KINASE INHIBITORS IN SYSTEMIC AUTOIMMUNITY In recent studies, we focused on the role of the cell-cycle inhibitor p21 in normal immune responses and autoimmunity. The cell cycle, which plays a critical role in determining both the fate and the differentiation of cells, is highly regulated by complexes of proteins, including cyclin, cyclin-dependent kinases, and cyclin-dependent kinase inhibitors, that are themselves controlled by external stimuli via a number of signaling pathways. Previously, in SLE-prone BXSB mice, we found that high levels of certain cyclin-dependent kinase inhibitors, such as p21, p18, and p27, are present in activated/memory (CD44 hi) phenotype CD4+ T cells, a population commonly increased in SLE. Therefore, we hypothesized that repeated stimulation of T cells reactive to self-antigens might lead to a state similar to “replicative senescence,” in which T cells are no longer cycling but are resistant to apoptosis, accumulate, and transcribe autoimmune-promoting proinflammatory cytokines. In support of this notion, we found that male BXSB mice lacking p21 had a marked reduction in SLE-like disease associated with both enhanced apoptosis of T and B lymphocytes and significant decreases in the number of activated/memory CD4 + T cells. Recently, we created diabetes-susceptible nonobese diabetic mice that lacked the gene for p21. In sharp contrast to the situation in SLE-prone mice, p21 deficiency had no effect on the development and severity of diabetes, indicating that p21 plays different roles in systemic and organ-specific diseases. Currently, we are addressing the role of p21 in other SLE-prone strains, immune responses to foreign antigens and viral infection, and the observed reduction in Fas-mediated apoptosis. H O M E O S TAT I C T - C E L L P R O L I F E R AT I O N I N AUTOIMMUNITY AND CANCER Homeostasis is defined as the ability of a biological system to maintain its internal equilibrium by adjusting critical physiologic properties. Recent studies have largely defined the factors that control homeostasis of naive and memory T cells under states in which the 146 IMMUNOLOGY 2005 number of lymphocytes is sufficient or is markedly reduced (lymphopenia). Of particular relevance to autoimmunity is the phenomenon termed “acute homeostatic T-cell proliferation,” which signifies proliferation of the remaining T cells after a lymphopenia-inducing event (e.g., treatment with cytotoxic drugs, viral infection) to reestablish a pool with normal numbers of lymphocytes. Efficient acute homeostatic proliferation appears to be based on recognition of self-peptide–MHC complexes and signaling by trophic cytokines, such as IL-7 and IL-15. We recently hypothesized that such lymphopeniamediated T-cell proliferation may be a contributing factor to autoimmunity, and we have discussed several examples in the literature in which lymphopenia was paradoxically associated with autoimmune phenomena. Our recent experiments in SLE-prone mice that lack the gene for the α-chain of the T-cell receptor and thus lack T cells, provided evidence that homeostatic proliferation of syngeneic cells in this empty environment can recapitulate an SLE-like disease. Others have shown that increased proliferation but inefficient survival of T cells can lead to lymphopenia and can be a contributing factor in the organ-specific autoimmune disease of nonobese diabetic mice. Thus, the perplexing association of lymphopenia with autoimmunity might be explained on the basis of compensatory self-mediated homeostatic proliferation of T cells. Overall, we postulate that in normal mice, the rare occurrence of lymphopenia and physiologic proliferation of a polyclonal T-cell population containing few (if any) autoreactive cells will be a physiologic process without pathologic consequences. Similarly, in animals that have more autoreactive T cells, a rare occurrence of homeostatic proliferation of T cells most likely will be innocuous. In contrast, in animals predisposed to autoimmunity, lymphopenia might contribute to the initiation and/or progression of recurrent or chronic disease (Fig. 1). T cells that express γδ T-cell receptors constitute a considerable fraction of lymphocytes in secondary lymphoid organs and blood and predominate in the mucosa and epithelia of various tissues. Considerable evidence indicates that γδ T cells have important immunologic functions, including antitumor activities, and may contribute to the pathogenesis of autoimmune diseases. Among subsets of T cells, γδ T cells uniquely have a tissue distribution based on their antigen receptors, but what defines the preferential homing and homeostasis of these cells is unknown. To address this question, we Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. F i g . 1 . Postulated mechanisms for recurrent lymphopenia-induced expansion of autoreactive T cells and autoimmunity. In animals with a normal genetic background, the rare occurrence of lymphopenia leads to homeostatic proliferation (HP) and survival of diverse T cells (white areas, nonautoreactive; black areas, autoreactive) without predominance of the few potentially autoreactive clones in the periphery. Similarly, in animals with a genetic predisposition to autoimmunity (autoimmune background), which have a higher frequency of potentially autoreactive T cells, the rare occurrence of homeostatic proliferation might not lead to autoimmunity because the frequency of the autoreactive cells remains low and other requirements are absent. By contrast, in animals with a genetic predisposition to autoimmunity in which lymphopenia occurs recurrently or chronically, proliferation and selection of autoreactive T cells together with other factors such as adequate antigen presentation and costimulation might lead to autoimmunity. studied the resources that control the homeostasis of γδ T cells in secondary lymphoid organs. We found that γδ and αβ T cells are controlled by partially overlapping resources, because lymphopeniainduced acute homeostatic proliferation of γδ T cells was inhibited by an intact αβ T-cell compartment and both γδ and αβ T cells were dependent on IL-7 and IL-15. Significantly, acute homeostatic proliferation of γδ T cells also required depletion of γδ cells. Thus, homeostasis of γδ T cells is maintained by trophic cytokines commonly used by other types of lymphoid cells and by additional, as yet unidentified, γδ-specific ligands. Efforts to develop effective antitumor immunotherapies are hampered by the difficulty of overcoming tolerance against tumor antigens, which in most instances are normal gene products that are overexpressed, preferentially expressed, or reexpressed in cancer cells. Because lymphopenia-induced homeostatic proliferation of T cells is mediated by recognition of self-peptide–MHC complexes and because the expanded cells acquire some effector functions, we hypothesized that lymphopenia- IMMUNOLOGY induced homeostatic proliferation could be used to break tolerance against tumor antigens. Our earlier studies with W. Dummer, Genentech, Inc., South San Francisco, California, A.G. Niethammer and R. Reisfeld, Department of Immunology, in mouse models of melanoma and colon carcinoma indicated that availability of tumor antigens during homeostatic proliferation of T cells indeed leads to effective antitumor autoimmunity with specificity and memory. We hypothesize that this effect is mediated by a reduction in the activation threshold of low-affinity tumorspecific T cells, leading to preferential engagement and proliferation of the cells in the presence of a high concentration of tumor antigens. We are further defining the parameters of this approach, particularly its efficacy in the treatment of primary and metastatic tumors with different immunologic and histologic characteristics. Our emphasis is on mechanistic issues, such as the exact process by which tolerance is broken, modes of antigen presentation (direct vs indirect), the efficacy of refined T-cell subsets, and the potentiating effects of appropriate vaccines and cytokines. Overall, we think that because of its simplicity, this approach will have considerable application in the treatment of malignant neoplasms in humans because it relies on conventional lymphopeniainducing cancer therapies, tumor-specific vaccination at the early phases of lymphopenia, and, optimally, infusion of autologous lymphocytes. PUBLICATIONS Baccala, R., Gonzalez-Quintial, R., Dummer, W., Theofilopoulos, A.N. Tumor immunity via homeostatic T cell proliferation: mechanistic aspects and clinical perspectives. Springer Semin. Immunopathol. 27:75, 2005 Baccala, R., Kono, D.H., Theofilopoulos, A.N. Interferons as pathogenic effectors in autoimmunity. Immunol. Rev. 204:9, 2005. Baccala, R., Theofilopoulos, A.N. The new paradigm of T-cell homeostatic proliferation-induced autoimmunity. Trends Immunol. 26:5, 2005. Baccala, R., Witherden, D., Gonzalez-Quintial, R., Dummer, W., Surh, C.D., Havran, W.L., Theofilopoulos, A.N. γδ T cell homeostasis is controlled by IL-7 and IL-15 together with subset-specific factors. J. Immunol. 174:4606, 2005. Haraldsson, M.K., dela Paz, N.G., Kuan, J.G., Gilkeson, G.S., Theofilopoulos, A.N., Kono, D.H. Autoimmune alterations induced by the New Zealand Black Lbw2 locus in BWF1 mice. J Immunol. 174:5065, 2005. Kono, D.H., Theofilopoulos, A.N. Genetics of autoantibody production in mouse models of lupus. In: Autoantibodies and Autoimmunity. Pollard, K.M. (Ed.). WileyVCH, New York, in press. Pollard, K.M., Arnush, M., Hultman, P., Kono, D.H. Costimulation requirements of induced murine systemic autoimmune disease. J. Immunol. 173:5880, 2004. Pollard, K.M., Hultman, P., Arnush, M., Hildebrand, J.A., Kono, D.H. Immunology and genetics of xenobiotic-induced autoimmunity. In: From Animal Models to Human Genetics: Research on the Induction and Pathogenicity of Autoantibodies. Conrad, K., et al. (Eds.). Pabst Science Publishers, Lengerich, Germany, 2004, p. 130. Theofilopoulos, A.N., Baccala, R., Beutler, B., Kono, D.H. Type I interferons (α/β) in immunity and autoimmunity. Annu. Rev. Immunol. 23:307, 2005. Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. 2005 147 Initiation of Inflammation by the Innate Immune System P.S. Tobias, H.-K. Lee, L.K. Curtiss,* P. Dawson,** T. Kirkland,*** D. Liebler**** * Department of Immunology, Scripps Research ** Department of Cell Biology, Scripps Research *** University of California, San Diego, California **** Vanderbilt University, Nashville, Tennessee e focus on understanding the mechanisms by which cells use the innate immune system to initiate defensive inflammatory responses. First, we seek to understand the structural features of the Toll-like receptors (TLRs) and their allied proteins lipopolysaccharide-binding protein, CD14, MD-2, and CD36, which enable the receptors to bind their ligands. Second, we seek to understand the structural changes by which binding of a microbial ligand to the extracellular domain of the receptor leads to signal transduction across the cell membrane and initiation of intracellular signaling cascades. Third, we seek to understand the involvement of endogenous and exogenous inflammatory stimuli in atherosclerosis. Ten TLRs are known. For most of these, ligands derived from microorganisms are known; binding to the ligands initiates signaling, leading to expression of inflammatory mediators and other defensive responses. In addition, some of the TLRs that may be involved in sterile inflammatory conditions such as arthritis or atherosclerosis may have endogenous ligands. However, these ligands are not yet clearly identified. To understand the structural features of ligand-receptor binding, we use 2 approaches. In the traditional mutation approach, amino acid residues in the proteins are mutated, and the proteins are then studied for functional changes. In the second approach, we use crosslinking agents to create covalent attachments of the ligands to the proteins. The proteins are then degraded chemically to determine the site of attachment. Binding of ligands to TLRs starts an intracellular signaling cascade that results in activation of a number of cellular responses. Prominent hypothesized mechanisms by which ligand binding to TLRs incurs transmembrane signaling are (1) the ligand induces dimerization of receptors and (2) binding of the ligand induces conformational changes in the receptor. Our studies indicate that pairs of TLRs are associated even in the absence W 148 IMMUNOLOGY 2005 of ligand and that the pairs undergo a conformational change upon ligand binding. We are using a variety of approaches to understand the structural basis for associations among the TLRs and their associated intracellular signaling partners. Atherosclerosis is an inflammatory disease of the large arteries. Evidence suggests that inflammatory components derived from microbes can induce progression of atherosclerosis. However, most of the development of atherosclerotic lesions is due to endogenous inflammatory factors. Because the TLR system is so intimately involved with inflammation, we are determining whether the TLRs are involved in atherosclerosis. Our initial data clearly indicate that TLR2, whether activated by endogenous ligands or by exogenous ligands, drives progression of atherosclerosis. For these experiments, we are using mouse models of the disease and mice deficient in individual TLRs. PUBLICATIONS Dunzendorfer, S., Lee, H.-K., Soldau, K., Tobias, P.S. TLR4 is the signaling but not the lipopolysaccharide uptake receptor. J. Immunol. 173:1166, 2004. Dunzendorfer, S., Lee, H.-K., Tobias, P.S. Flow-dependent regulation of endothelial Tolllike receptor 2 expression through inhibition of SP1 activity. Circ. Res. 95:684, 2004. Tobias, P., Curtiss, L.K. Paying the price for pathogen protection: Toll receptors in atherogenesis. J. Lipid Res. 46:404, 2005. Molecular Mechanisms of Host-Pathogen Interactions R.J. Ulevitch, V.V. Kravchenko, C. Fearns, T.-H. Chuang, J.C. Mathison, Q. Pan, J. da Silva Correia, K. Iwata, K.D. Janda, G. Kaufmann, M. Meijler nfection by microbial pathogens often sets in motion chains of events that cause severe injury to the host, and nowhere is this phenomenon illustrated more dramatically than in the response by humans to infection by gram-negative bacteria. In his book Lives of a Cell, Lewis Thomas characterized the host response to the endotoxin, or lipopolysaccharide, of gram-negative bacteria as being “read by our tissues as the very worst of bad news. . . . There is nothing intrinsically poisonous about endotoxin, but it must look awful, or feel awful, when sensed by cells. Cells believe that it signifies the presence of gram-negative bacteria, and they will stop at nothing to avoid this threat.” In other words, the innate immune response to infection has caused a serious disease in humans. I Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. Clearly, much human suffering could be eased if such overzealous host responses could be tempered. However, such responses, when not overzealous, are a normal part of the host’s homeostatic mechanisms, designed to respond to the threat of infection by gramnegative bacteria. Accordingly, we are attempting to (1) define the mechanisms of innate immunity and (2) learn how to control these responses without compromising host defenses against pathogens. Most recently, we contributed to the understanding of innate immunity through our studies of Toll-like receptors (TLRs) and of effector mechanisms that mediate host responses to infection. It is now well appreciated that the innate immune system is positioned at the intersection of multiple host pathways, including those for microbial and viral recognition, enhancement of adaptive immune responses, and, possibly, cancer immunosurveillance. Each pathway depends on ligand recognition by specific cellular receptors that are either membrane bound (plasma membrane as well as endosomal compartments) or cytosolic. The most important class of membrane-bound receptors are the TLRs. Among cytosolic receptors, an important family known as the Nod/Caterpillar family has been identified. Within this family, 2 proteins, Nod1 and Nod2, are involved in recognition of bacterial ligands distinct from the ligands for TLRs. Activation of TLR and Nod signaling pathways leads to production of multiple cytokines with proinflammatory and antiinflammatory activities. Such responses are central to host responses to infection. However, when a breakdown occurs in the normal regulatory mechanisms that control these pathways, disease may result. Perhaps the most well-understood link between innate immunity and human disease is in the host response to infection. When dysregulation of innate immune responses occurs, clinical abnormalities such as septic shock and acute respiratory distress syndrome occur. Dysregulation of innate immune responses may also play a role in human diseases in which chronic inflammation is responsible for disease progression, including autoimmune and autoinflammatory diseases. Genetic studies in humans have revealed strong associations among various members of the Nod family of proteins and human diseases. During the past year, we made considerable progress in several different areas. First we established mice that lack both copies of the gene for Triad3A, an E3 ubiquitin ligase that controls the expression of some TLRs. We are breeding these mice to begin studies of IMMUNOLOGY the cellular phenotype that occurs when the innate immune system is activated. At the same time, we are continuing our studies of the interactions between Triad3A and potential endogenous substrates. Although it is too early to know the exact consequences of the deletion of Triad3A, our initial observations suggest that homozygous Triad 3A–/– mice have lower body weight and size during the first 8 weeks of life than do their wild-type littermates. We continue to study the biological functions of Nod1 and Nod2. We discovered a signaling pathway in MCF-7 cells in which Nod1 negatively regulates the response of this human breast cancer cell line to estrogen and also influences the level of expression of the estrogen receptor. We found that MCF-7 cells containing Nod1 had greater proliferative responses in tissue culture than did cells lacking Nod1. Most importantly, in a xenograft model of tumor growth in mice with severe combined immunodeficiency, growth of MCF-7 cells containing Nod1 was greater than growth of cells lacking Nod1. We are working out the molecular details of the pathway leading from Nod1 to the estrogen receptor. We are also continuing our studies on the biological function of Nod2, with a specific emphasis on its role in the assembly of a macromolecular protein complex that induces release of the cytokine IL-1 from cells after activation with the Nod2 ligand. Using cells genetically deficient in specific components associated with IL-1 release, we showed that the Nod2 pathway requires the kinase receptor interacting protein-2 and an adaptor protein known as ASC. Currently, we are using cells deficient in other proteins that may also be implicated in the release of IL-1. Our long-term goal is to identify the nature of this activating complex so that strategies can be devised to block IL-1 release in chronic inflammatory diseases. Finally, in collaboration with K.D. Janda, Department of Chemistry, we are studying a class of bacterial products known as quorum-sensing factors. These factors play a crucial role in the adaptation of bacteria to the host and serve as a means for bacteria to communicate with one another. Quorum-sensing factors are involved in the induction of virulence factors and the establishment of biofilms. Thus, this class of bacterial molecules clearly plays a role in pathogenesis of infectious processes. Now we are investigating the role in quorum sensing of members of the homoserine lactone family, which induce inflammation and cell death via their effects on host cells. Inflammation and cell death induced by homoserine lactones may be important processes in diseases Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. 2005 149 such as cystic fibrosis in which the local concentrations of the quorum-sensing factor can be quite high in the lung and in which inflammation and cell remodeling and/or death are unchecked. By understanding how the quorum-sensing molecules activate host cells, we may be able to devise new therapeutic strategies for treatment of diseases in which these molecules are involved. PUBLICATIONS Fort, M.M., Mozaffarian, A., Stover, A.G., da Silva Correia, J., Johnson, D.A., Crane, R.T., Ulevitch, R.J., Persing, D.H., Bielefeldt-Ohmann, H., Probst, P., Jeffery, E., Fling, S.P., Hershberg, R.M. A synthetic TLR4 antagonist has anti-inflammatory effects in two murine models of inflammatory bowel disease. J. Immunol. 174:6416, 2005. Ulevitch, R.J. Therapeutics targeting the innate immune system. Nat. Rev. Immunol. 4:512, 2004. Ulevitch, R.J., Mathison, J.C., da Silva Correia, J. Innate immune responses during infection. Vaccine 22(Suppl. 1):S25, 2004. Regulation of CD4+ T-Cell Responses by Accessory Molecules S. Webb, N.S. Kim, J. Kovarova, L.H. Lopez, J.G. Melton, R.R. Mendoza ctivation of CD4+ T cells leads to the development of a heterogeneous collection of functionally distinct effector cells. For several years, we have focused on how the activities of these various effector cells are regulated. Both cytokines and interactions between T-cell receptors and peptide-MHC molecules make important contributions to the effector activity of CD4+ T cells. We have shown that the particular array of accessory molecule receptors engaged during priming of naive CD4+ cells also critically influences the subsequent activities and survival of developing effector cells. In previous studies with Drosophila cell lines transfected with selected murine MHC molecules and accessory molecule ligands, we discovered an important role for interactions between lymphocyte function–associated antigen 1 (LFA-1) and intracellular adhesion molecule 1 (ICAM-1) in the regulation of cytokine production. In these studies, engaging LFA-1 during priming suppressed synthesis of IL-4 and IL-10 and enhanced secretion of IFN-γ and IL-2. Two broad possibilities might explain how interactions between LFA-1 and ICAM-1 mediate these effects. One possibility is that LFA-1 regulates cytokine production by promoting cell adhesion and thus strengthening the relative degree of signaling via T-cell receptors and costimulatory receptors. Alternatively, or additionally, ligation of LFA-1 triggers signaling events A 150 IMMUNOLOGY 2005 that lead to altered activation of transcription factors and, ultimately, expression of cytokine genes. To distinguish between these possibilities, we are generating a panel of LFA-1 constructs with selected mutations within the cytoplasmic domain of the LFA-1 β-chain to interfere selectively with either adhesion or putative signal transduction pathways. These constructs will be expressed in LFA-1–deficient CD4+ T cells during primary peptide-dependent stimulation. By monitoring the effects of these mutations on the subsequent capacity of LFA-1 to alter cytokine production, we will begin to define how LFA-1 alters the function of CD4+ T cells. In other studies, we are using Drosophila cell lines cotransfected with murine MHC molecules, inducible costimulator (ICOS) ligand, programmed cell death 1 (PD-1) ligands, and/or B7.1 to examine the costimulatory activity of members of the CD28 family. These Drosophila cells are used to present peptide antigen to T-cell receptor transgenic CD4+ cells. We tested a currently popular hypothesis that ICOS, in contrast to CD28, preferentially costimulates secondary responses. To our surprise, cells expressing ICOS ligand did not effectively costimulate proliferative responses and/or cytokine production by either naive or primed CD4+ T cells in the absence of B7 expression. These results raise the possibility that the primary function of ICOS is to amplify CD28-initiated responses by increasing the effective level of engagement of costimulatory receptors after initial proliferation of the T cells. This possibility will be tested in future experiments. PD-1, like cytotoxic T lymphocyte antigen 4, has been implicated in the negative regulation of T-cell responses. To study the mechanisms by which PD-1 engagement suppresses the function of CD4+ cells, we expressed the PD-1 ligands, PD-L1 and PD-L2, in Drosophila cells expressing class II MHC molecules with or without B7 and/or ICAM-1. Expression of PD-1 ligands on the Drosophila cells during peptide-mediated stimulation of naive CD4 + cells did not significantly inhibit either proliferative responses of or cytokine production by CD4 + cells. In preliminary experiments, Drosophila antigen-presenting cells expressing PD-1 ligands did induce higher and/or more sustained production of the inhibitory cytokine IL-10 than did antigenpresenting cells lacking PD-1 ligand expression. This finding raises the possibility that the negative regulation of T-cell responses by PD-1 may function indirectly via IL-10 rather than directly via PD-1–mediated signaling. Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. We are also studying the influence of selected accessory molecules in generating CD4+ effector cells that provide help for CD8 + cells in an in vivo model for type 1 diabetes. These experiments are a collaboration with L.A. Sherman, Department of Immunology. Prion Diseases: Insights Into the Biology of an Infectious Protein L. Solforosi, M. Schaller, A. Bellon, G. Moroncini, E. Ollmann Saphire, G. Abalos, J. Cruite, E. Wiseman, R.A. Williamson he prion diseases, or transmissible spongiform encephalopathies, are diseases of protein conformation that cause profound neurodegeneration and death. They include bovine spongiform encephalopathy, also known as mad cow disease; scrapie in sheep; and chronic wasting disease, which is spreading rapidly in deer and elk within the United States. Human consumption of foodstuffs contaminated with the prions that cause bovine spongiform encephalopathy led to the emergence of a variant of CreutzfeldtJakob disease (vCJD). To date, more than 180 cases of vCJD have occurred, primarily in the United Kingdom. More recently, transmission of vCJD via blood products obtained from apparently healthy donors in whom vCJD later developed has been documented. These events have reignited concern about the widespread dissemination of prion diseases in humans. Because no diagnostic test for early-stage prion infections exists, the risks to public health cannot be accurately quantified. Uniquely, the infectious agent in transmissible spongiform encephalopathies, the prion, is thought to be composed largely of PrP Sc, an abnormally shaped version of the cellular prion protein PrPC, a molecule of unknown function that is found in all healthy individuals. Once established within an infected host, prions replicate by converting the normal PrPC form of the protein into additional molecules of the diseaseassociated form, PrP Sc , through a templating-type mechanism that is poorly understood. Over time, PrPSc accumulates in the CNS, and its appearance is closely associated with profound neuropathologic changes. We have developed antibody reagents that specifically recognize different components of either the normal PrPC or the abnormal PrPSc conformers of the prion T IMMUNOLOGY protein. We have used these immunologic reagents to gain insights into several aspects of prion biology that remain poorly understood. For example, the mechanisms through which the accumulation of PrPSc within the CNS leads to the destruction of brain tissues are undetermined, although the presence of PrP Sc in and of itself appears to be insufficient to promote damage in the absence of PrPC. We hypothesized that PrPC may contribute directly to the prion-induced neurodegenerative cascade, perhaps through an unknown signaling pathway. To test this possibility experimentally, we introduced recombinant monoclonal IgG antibodies that recognize PrPC into the brain in mice. Upon binding to and effectively cross-linking PrP C on the surface of neuronal cells, the antibodies rapidly triggered extensive neuronal death by apoptosis. These findings indicate that PrPC may be co-opted twice in prion diseases, once as a substrate for conformational conversion into nascent PrPSc molecules and additionally as a signaling vehicle that promotes neuronal injury and death, perhaps after cross-linking by oligomeric forms of PrPSc. In additional experiments, we are using PrPSc-specific antibodies to map the association between PrPC and PrP Sc , a key event in the formation of the prion replicative complex. Elucidating how these different PrP conformers interact will enhance the prospect of efficiently inhibiting their association and thereby halting prion replication and disease. Finally, our comprehension of prion disease would be greatly enhanced if a detailed molecular structure of PrP Sc were solved. We are using the PrPSc-reactive antibodies in an effort to cocrystalize the disease-associated form of PrP. In the longer term, lessons learned in the study of prion disease most likely will increase our understanding of other more common neurodegenerative conditions that are intimately linked to abnormally folded proteins, such as Alzheimer’s and Parkinson’s diseases. PUBLICATIONS Deleault, N.R., Geoghegan, J.C., Nishina, K., Kascsak, R., Williamson, R.A., Supattapone, S. Protease-resistant prion protein amplification reconstituted with partially purified substrates and synthetic polyanions. J. Biol. Chem. 280:26873, 2005. Yadavalli, R., Guttmann, R.P., Seward, T., Centers, A.P., Williamson, R.A., Telling, G.C. Calpain-dependent endoproteolytic cleavage of PrPSc modulates scrapie prion propagation. J. Biol. Chem. 279:21948, 2004. Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. 2005 151 Legumain as a Target for a Genetic Vaccine Against Breast Cancer R. Xiang, C. Dolman, C. Liu, D. Markowitz, Y. Luo n our efforts to develop cancer immunotherapies, we are using legumain as a target to induce a strong, cell-mediated immunity against breast cancer cells. Legumain, an asparaginyl endopeptidase, is a newly discovered stress protein that is an almost ideal target for our DNA-based vaccines. First, positional gene expression profiling of tumor tissues in a search for genes that are upregulated in tumors and tumor vasculature indicated that legumain is highly expressed in many murine breast tumor tissues. In contrast, expression of legumain is very low or absent in all normal tissues from which breast tumors arise. In addition, the protein is overexpressed by endothelial cells in the breast tumor vasculature and by tumor-associated macrophages in the breast tumor microenvironment. It is well known that tumor-associated macrophages are recruited by chemokine gradients established by tumor cells into the tumor stroma, where the macrophages mediate immunosuppression by making T cells ineffective and by promoting angiogenesis, leading to increased tumor cell growth and metastases. Importantly, cell-surface expression of legumain occurs under stress, such as tumor growth in vivo, but not in tissue culture. We obtained the following evidence in our initial experiments. First, we found that legumain was overexpressed in vivo by most of the solid tumors we tested, especially on neoplastic cells, neovasculature, and tumor-associated macrophages, but not by the corresponding cultured tumor cell lines in tissue culture. Most normal tissues had either no or essentially undetectable levels of legumain expression. Second, we found that legumain is a stress-responsive protein induced under certain conditions such as heat shock, drug treatment, and hypoxia and is associated with tumor invasion, dissemination of metastases, and tumor angiogenesis. Third, in a prophylactic setting, the legumain-based DNA vaccine induced suppression of both growth of 4T1 primary breast tumors and dissemination of spontaneous pulmonary metastases. Finally, this antitumor effect could be achieved by suppression of angiogenesis in the tumor vasculature combined with tumor-cell killing mediated by cytotoxic T lym- I 152 IMMUNOLOGY 2005 phocytes and/or antibodies. Taken together, the results indicate that legumain can be used as an effective target for a DNA vaccine against breast cancer. This approach may lead to the rational design of such vaccines for future clinical application. Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved.