Fractional Distillation notes

Reminder: These notes are meant to supplement, not replace the laboratory manual.
Fractional Distillation notes
History and Application:
Fractional distillation is one of the most widely used separation techniques for the purification of volatile liquids. It is routinely used in research labs and ubiquitously used throughout industrial chemical processes. Distillation is one of the first processes crude oil encounters while being transformed into gasoline, kerosene and other high value products. Large distillation columns can often be seen while driving past chemical plants. Commercial distillation columns are typically tall and narrow structures. There are several in the image of a plant below.
Safety considerations for this experiment:
A sand bath, especially the top portion, can get very hot during the experiment, and you won’t be able to tell by looking. Handle the sand bath with care.
Also, a sand bath is an electrical apparatus; notify your instructor if its wires are frayed or expos ed, and don’t splash water on it.
You’ll be using a thermometer. These are fragile--handle them carefully, especially when you’re installing them in the top of the distillation apparatus.
Ethyl Acetate and Butyl Acetate are both highly flammable. Our lab equipment and safety regulations are selected to avoid flames. Also, avoid direct contact with these compounds. Work in the hood if sufficient space is available. This will reduce any inhalation hazards and isolate any fires which may occur.
When distilling an organic liquid it is important not to boil all of the liquid out of this flask. This is called distilling to dryness. Peroxides can form, concentrate and detonate if this is done.
Use a boiling stone whenever heating a liquid. The boiling stone provides a nucleation site onto which vapors can start to form bubbles. Boiling stones
allow smooth boiling to occur. If a boiling stone is not present, the vapors can coalesce all at once and shoot out of the flask. This is called bumping.
It often sprays boiling hot reagents out of the vessel hitting equipment, bench tops and exposed body parts. If the liquid contacts a spark, it can ignite. Always have a boiling stone or other nucleation center present when heating liquids.
Terminology.
A theoretical plate is one cycle of evaporation and condensation in a distillation.
The more theoretical plates the better the separation. Fractional distillations have many theoretical plates; true simple distillations have only one theoretical plate.
In reflux, liquids boil in the lower part of an apparatus, condense in the upper part, and drip down to the lower portions again. This is a continuing repeating process of vaporization and condensation. In a stable reflux
(without separation by distillation) the composition and the temperature remain constant.
Distillation. A process whereby the vapor above a heated liquid is collected, condensed and separated away from the bulk liquid. A successful distillation will result in pure components being separated from a mixture.
Boiling point. The temperature at which the vapor pressure of a liquid is equal to the atmospheric pressure on that liquid. It is the temperature at which a liquid is in a steady state equilibrium with the gas. The higher the vapor pressure of a liquid, the lower the boiling point, and vice versa.
Azeotrope: A mixture of liquids that distills at a constant temperature without changing composition. The best-known azeotrope is 95.5% ethanol and
4.5% water.
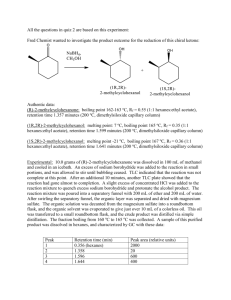
1. First, study the notes for the Gas Chromatography and Refractive Index experiment. You are responsible for that material as well as that given below. Be sure you remember how to calculate composition of mixtures from pure RI values of components. Review RI notes if unsure how to perform this calculation.
2. Typical atmospheric pressure distillations are carried out on volatile organic compounds. These materials have boiling points of 225 o
C or less. Boiling points decrease as pressure is reduced. If a material has low volatility (low vapor pressure, a boiling point over 200 o
C) or if the material decomposes at increased temperature, a vacuum distillation may be required. Special equipment and precautions are needed for these distillations.
3. The critical principal that allows distillations to succeed is the fact that the composition of vapor over a liquid composed of two or more materials will be higher in the material with the lower boiling point. A mixture of two or more liquids will boil at given temperature, but the composition of the vapor will be different than the composition of the liquid. This is illustrated by the ethyl acetate : butyl
acetate distillation composition diagrams below i
, ii
.
In both of these diagrams, the composition of the mixture is on the x-axis and the temperature is on the y-axis, To determine the temperature at which a liquid mixture of 33% Ethyl acetate and 66% butyl acetate will boil, start from that composition on the x-axis and draw a vertical line up to the bubble point (boiling point) curve. The temperature corresponding to this point is approximately
105 o
C. This is the temperature which a 33 :66 mixture will boil. To find the composition of the vapor over that liquid, make a line horizontally from that point over to the dew point (condensation curve), extend this line straight down to the x-axis. Where this line intersects the x axis will be the composition of the vapor over that liquid. In this case it is 45% ethyl acetate and 55 % butyl acetate. The vapor has increased in concentration of the lower boiling point material, from
33 % to 45%. If this vapor was then condensed (as 45% ethyl acetate and 55% butyl aceteate) and then vaporized again, the vapor composition would be even higher in ethyl acetate (58% ethyl acetate, 42% butyl acetate). This is shown in the chart on the right. The more condensation-vaporoization cycles that are carried out the higher purity samples may be obtained.
4. Each vaporization and condensation cycles is one theoretical plate. The more theoretical plates in a distillation set up the better the separation and the more pure the fractions. In practice, simple distillation is carried out without any column packing and can have as little as one theoretical plate. Fractional distillation has many theoretical plates. Column packing such as the copper sponge is often added to a column to increase the surface area for condensation and increase the number of theoretical plates. The more theoretical plates, the more efficient the separation.
Simple distillation is useful for separating materials with large differences in boiling points (50 o
C or more). Typically simple distillation is used to separate volatile materials from nonvolatile materials. For columns with a high number of theoretical plates, fractional distillation can be used to separate materials whose boiling points differ by as few as 1 or 2 degrees Celsius. More typically fractional distillation is used to separate materials whose boiling points are at least 10 o
C apart.
5. The purpose of a distillation is to separate a mixture of volatile liquids into pure components .The more successful the distillation the higher purity of the
components. In a “perfect” distillation, our 33% ethyl acetate, 66% butyl acetate mixture will be separated into one fraction of 100 % ethyl acetate 0% butyl acetate and another fraction of 0% ethyl acetate 100% butyl acetate. The flask residue (the liquid left in the flask when the distillation is complete) should be composed entirely of the higher boiling point material. How close the actual composition of the fractions come to these ‘perfect’ values determines the success or failure of the distillation.
6. Here is a rough drawing, not to scale, of the apparatus used in this experiment for fractional distillation. Note the placement of the thermometer. The top of the bulb is even with the bottom of the side arm of the distillation head.
7. The temperature of each drop falling into the receiver should be recorded. A graph will be prepared. (Remember you graphing skills learned in CHEM 1011L).
A ‘perfect’ distillation will result in a graph similar to the one on the left. A representative result from this distillation may result in something closer to the graph on the right.
130
120
110
100
90
80
70
0 20 40
Drops
60 80
120
110
100
90
80
70
60
0 20 40
Drops
60 80
8. Here are some problems that can occur while distilling
If you place the thermometer bulb too high, the vapors won’t reach it before they go into the sidearm to be collected, and your observed boiling point will be lower than it should be. If you place the thermometer bulb too low, an artificially high reading for the boiling point range will result.
Superheating most often happens when you leave out the boiling stones. Boiling of a superheated sample can be irregular, complete and violent. The very hot liquid and gas may rapidly shoot out of the flask; this is called bumping. If you forget to add a boiling stone, remove flask from heat source, allow flask to cool, add boiling stone then resume heating. Do not add a boiling stone to a hot liquid. If this is done bumping may occur.
Many organic compounds react with oxygen in the air over time to give low concentrations of peroxides. Distillation of such samples to dryness may cause them to explode.
If you try to distil in a closed system (this most often happens when you use a connector to attach your receiver), the pressure of the heated gases inside the apparatus may cause a rupture. Most often you’ll launch your thermometer.
9. The chemicals used in this experiment are identical to the materials used in the
Refractive Index and Gas Chromatography experiment. They are ethyl acetate and butyl acetate.
Compound ethyl acetate butyl acetate
Literature Boiling Point
77
126 o o
C
C
Literature Index of
Refraction
1.3723
1.3941
Notes for topics that may be included in quizzes given after the experiment:
10. Here are factors that chemists consider when deciding what type of distillation to use:
Simple distillation is primarily used to separate compounds that have very large differences in boiling point, usually more than 100° C. Example: acetone
(b. p. 57° C) can be separated from dodecane (C
12
H
26
, b.p. 216° C) using simple distillation.
Fractional distillation is used to separate compounds that have boiling points that are closer to each other. The compounds separated in this experiment are an example.
Vacuum distillation is used to separate and purify compounds that have very high boiling points, as well as those that decompose or react with air at temperatures near their boiling points.
References i
Boiling point binary diagrams, W. R. Salzman, http://www.chem.arizona.edu/~salzmanr/480a/480ants/vpdiag&/vpdiag&.html
( October 27. 2012) ii
Williamson, Macroscopic and Microscopic Organic Experiments, Brooks/Cole, 2011, p88
Revised December 15, 2014 S.L. Weaver