Practice Quiz 2 - Chemistry Courses: About
advertisement
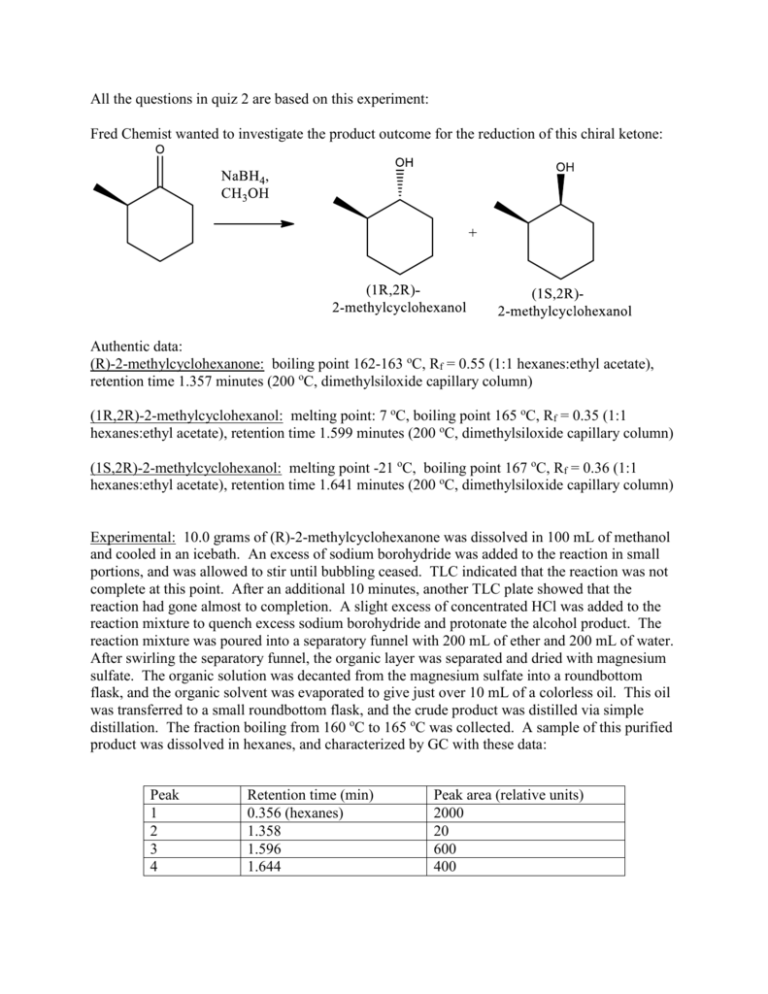
All the questions in quiz 2 are based on this experiment: Fred Chemist wanted to investigate the product outcome for the reduction of this chiral ketone: Authentic data: (R)-2-methylcyclohexanone: boiling point 162-163 oC, Rf = 0.55 (1:1 hexanes:ethyl acetate), retention time 1.357 minutes (200 oC, dimethylsiloxide capillary column) (1R,2R)-2-methylcyclohexanol: melting point: 7 oC, boiling point 165 oC, Rf = 0.35 (1:1 hexanes:ethyl acetate), retention time 1.599 minutes (200 oC, dimethylsiloxide capillary column) (1S,2R)-2-methylcyclohexanol: melting point -21 oC, boiling point 167 oC, Rf = 0.36 (1:1 hexanes:ethyl acetate), retention time 1.641 minutes (200 oC, dimethylsiloxide capillary column) Experimental: 10.0 grams of (R)-2-methylcyclohexanone was dissolved in 100 mL of methanol and cooled in an icebath. An excess of sodium borohydride was added to the reaction in small portions, and was allowed to stir until bubbling ceased. TLC indicated that the reaction was not complete at this point. After an additional 10 minutes, another TLC plate showed that the reaction had gone almost to completion. A slight excess of concentrated HCl was added to the reaction mixture to quench excess sodium borohydride and protonate the alcohol product. The reaction mixture was poured into a separatory funnel with 200 mL of ether and 200 mL of water. After swirling the separatory funnel, the organic layer was separated and dried with magnesium sulfate. The organic solution was decanted from the magnesium sulfate into a roundbottom flask, and the organic solvent was evaporated to give just over 10 mL of a colorless oil. This oil was transferred to a small roundbottom flask, and the crude product was distilled via simple distillation. The fraction boiling from 160 oC to 165 oC was collected. A sample of this purified product was dissolved in hexanes, and characterized by GC with these data: Peak 1 2 3 4 Retention time (min) 0.356 (hexanes) 1.358 1.596 1.644 Peak area (relative units) 2000 20 600 400 S342 Quiz 2 9/25/14 Name_____________________________________ AI or Section_______________________________ All the questions in this quiz are based on the experiment on the separate page. Only answers on this page will be corrected. If what you write contains right and wrong information, the answer is wrong. 1. These two TLC plates were obtained during the reaction using 1:1 hexane:ethyl acetate. A. (1pts) Fred Chemist did not label the times each of these plates were taken in the reaction. Which one was taken first? B. (1pts) What does Plate A tell you about the product distribution between the (1R,2R) and (1S,2R) alcohols? C. (1pt) Would you expect a TLC plate developed in pure ethyl acetate to give more or less useful data? 2. (1pts) Distillation was used rather than recrystallization to separate the cyclohexanol mixture. Given the significant difference in melting points, why was recrystallization not performed? 3. (1pts) Distillation was used rather than column chromatography for the purification of this reaction even though it didn’t work completely. A. What data shows that the isolated material contains more than a mix of cyclohexanol products? B. Column chromatography might have given greater final product purity, but distillation was chosen in this situation. Why? 4. Answer these questions concerning the extraction: A. (2pts) Why was ether added in the extraction step? Why was magnesium sulfate used? B. (3pts) Would you expect this extraction to be useful or not useful for removing each of these impurities: 6. (6pts) List three pieces of data that suggest that your final isolated product was predominately a mixture of the (1R,2R) and (1S,2R) alcohols. Be specific. A. Data 1: B. Data 2: C. Data 3: 7. (3pts) What was the final product distribution of (1R,2R)-2-methylcyclohexanol and (1S,2R)-2-methylcyclohexanol? Show your calculations: