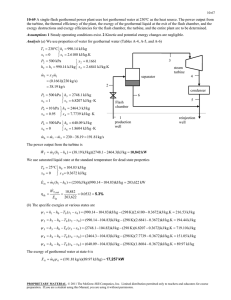
is to be determined. 3 R
advertisement
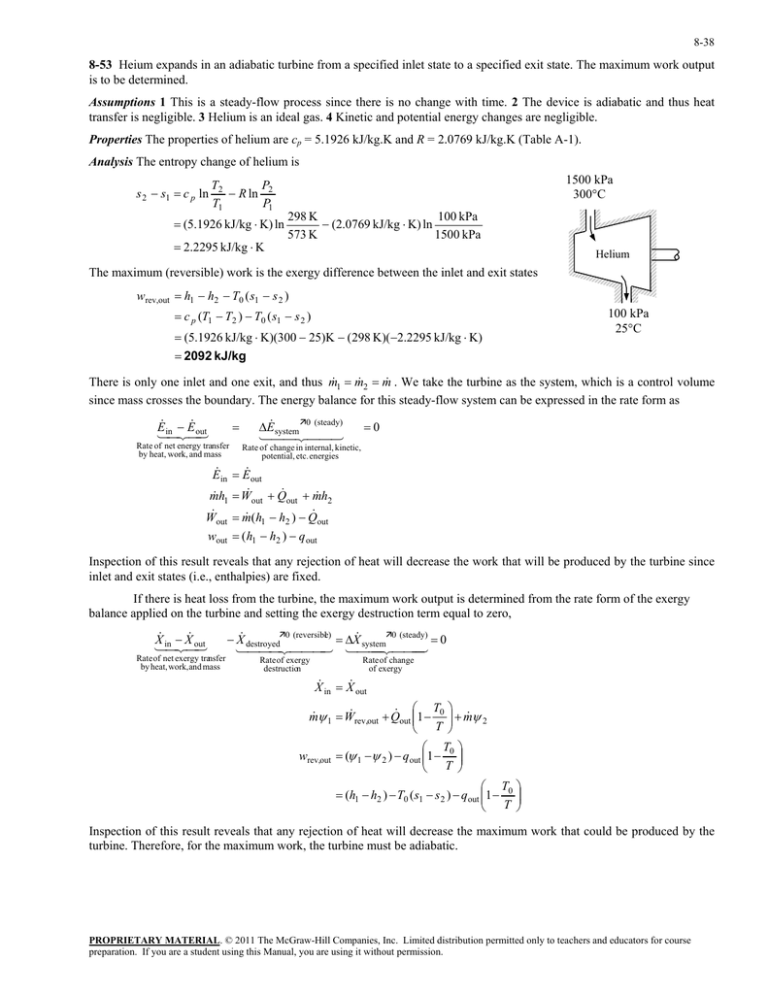
8-38 8-53 Heium expands in an adiabatic turbine from a specified inlet state to a specified exit state. The maximum work output is to be determined. Assumptions 1 This is a steady-flow process since there is no change with time. 2 The device is adiabatic and thus heat transfer is negligible. 3 Helium is an ideal gas. 4 Kinetic and potential energy changes are negligible. Properties The properties of helium are cp = 5.1926 kJ/kg.K and R = 2.0769 kJ/kg.K (Table A-1). Analysis The entropy change of helium is s 2 s1 c p ln T2 P R ln 2 T1 P1 (5.1926 kJ/kg K) ln 2.2295 kJ/kg K 1500 kPa 300°C 298 K 100 kPa (2.0769 kJ/kg K) ln 573 K 1500 kPa Helium The maximum (reversible) work is the exergy difference between the inlet and exit states wrev,out h1 h2 T0 ( s1 s 2 ) c p (T1 T2 ) T0 ( s1 s 2 ) 100 kPa 25°C (5.1926 kJ/kg K)(300 25)K (298 K)(2.2295 kJ/kg K) 2092 kJ/kg There is only one inlet and one exit, and thus m 1 m 2 m . We take the turbine as the system, which is a control volume since mass crosses the boundary. The energy balance for this steady-flow system can be expressed in the rate form as E E inout Rate of net energy transfer by heat, work, and mass E system0 (steady) 0 Rate of change in internal, kinetic, potential, etc. energies E in E out m h1 W out Q out m h2 W m (h h ) Q out 1 2 out wout (h1 h2 ) q out Inspection of this result reveals that any rejection of heat will decrease the work that will be produced by the turbine since inlet and exit states (i.e., enthalpies) are fixed. If there is heat loss from the turbine, the maximum work output is determined from the rate form of the exergy balance applied on the turbine and setting the exergy destruction term equal to zero, X X inout Rate of net exergy transfer by heat, work,and mass X destroyed0 (reversible) X system0 (steady) 0 Rate of exergy destruction Rate of change of exergy X in X out T m 1 W rev,out Q out 1 0 m 2 T T wrev,out ( 1 2 ) qout 1 0 T T (h1 h2 ) T0 (s1 s 2 ) qout 1 0 T Inspection of this result reveals that any rejection of heat will decrease the maximum work that could be produced by the turbine. Therefore, for the maximum work, the turbine must be adiabatic. PROPRIETARY MATERIAL. © 2011 The McGraw-Hill Companies, Inc. Limited distribution permitted only to teachers and educators for course preparation. If you are a student using this Manual, you are using it without permission.