cooling rate of the high-temperature evaporator, the power required by... 11-61
advertisement

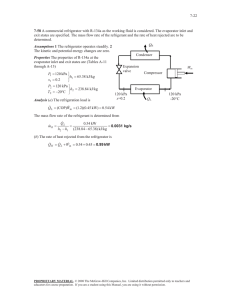
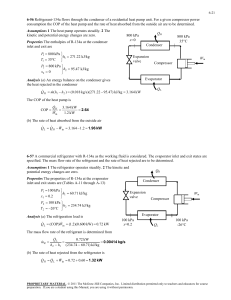
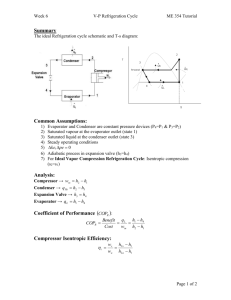
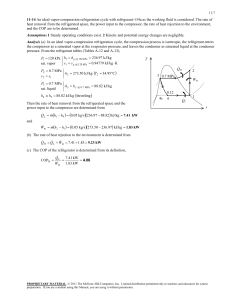
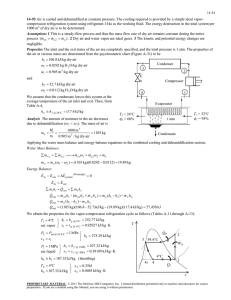
11-47 11-61 A two-evaporator compression refrigeration cycle with refrigerant-134a as the working fluid is considered. The cooling rate of the high-temperature evaporator, the power required by the compressor, and the COP of the system are to be determined. Assumptions 1 Steady operating conditions exist. 2 Kinetic and potential energy changes are negligible. Condenser 2 T 3 · QH Compressor 1 Expansion valve Expansion valve 3 800 kPa 4 · Win 5 -26.4C Expansion valve 7 0C 4 Evaporator 1 5 2 6 Evaporator 2 · 7 1 QL s 6 Analysis From the refrigerant tables (Tables A-11, A-12, and A-13), P3 800 kPa h3 h f sat. liquid @ 800 kPa 95.47 kJ/kg h4 h6 h3 95.47 kJ/kg ( throttling) T5 0C h h g @ 0C 250.45 kJ/kg sat. vapor 5 T7 26.4C h7 h g @ 26.4C 234.44 kJ/kg sat. vapor The mass flow rate through the low-temperature evaporator is found by Q L 8 kJ/s Q L m 2 (h7 h6 ) m 2 0.05757 kg/s h7 h6 (234.44 95.47) kJ/kg The mass flow rate through the warmer evaporator is then m 1 m m 2 0.1 0.05757 0.04243 kg/s Applying an energy balance to the point in the system where the two evaporator streams are recombined gives m h m 2 h7 (0.04243)(250.45) (0.05757)(234.44) m 1 h5 m 2 h7 m h1 h1 1 5 241.23 kJ/kg m 0.1 Then, P1 Psat @ 26.4C 100 kPa s1 0.9789 kJ/kg K h1 241.23 kJ/kg P2 800 kPa h2 286.26 kJ/kg s 2 s1 The cooling rate of the high-temperature evaporator is Q m (h h ) (0.04243 kg/s)(250.45 95.47) kJ/kg 6.58 kW L 1 5 4 The power input to the compressor is W m (h h ) (0.1 kg/s)(286.26 241.23) kJ/kg 4.50 kW in 2 1 The COP of this refrigeration system is determined from its definition, Q (8 6.58) kW COPR L 3.24 4.50 kW Win PROPRIETARY MATERIAL. © 2011 The McGraw-Hill Companies, Inc. Limited distribution permitted only to teachers and educators for course preparation. If you are a student using this Manual, you are using it without permission.