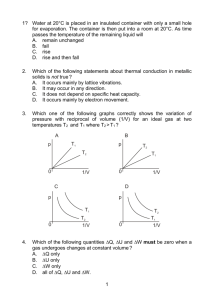
kT v m K
advertisement
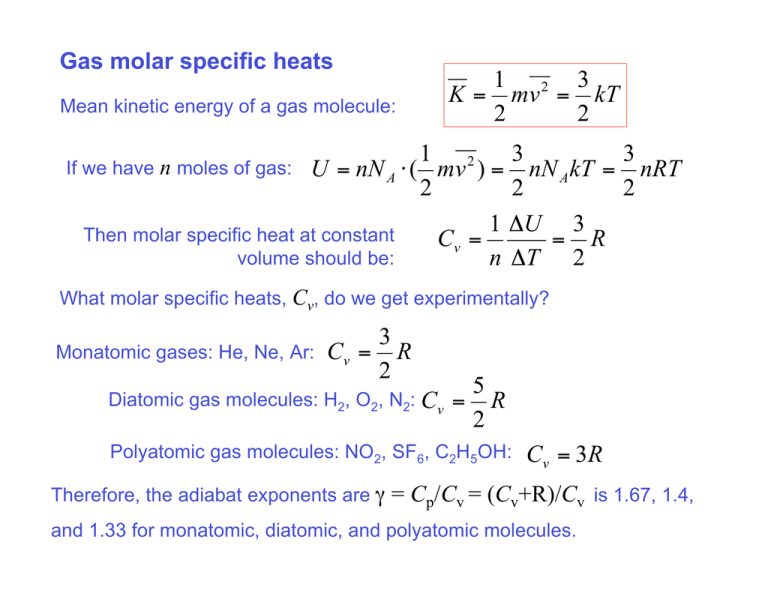
Gas molar specific heats Mean kinetic energy of a gas molecule: 1 2 3 K = mv = kT 2 2 1 2 3 3 If we have n moles of gas: U = nN A ! ( mv ) = nN A kT = nRT 2 2 2 1 !U 3 Then molar specific heat at constant Cv = = R volume should be: n !T 2 What molar specific heats, Cv, do we get experimentally? Monatomic gases: He, Ne, Ar: 3 Cv = R 2 Diatomic gas molecules: H2, O2, N2: Cv 5 = R 2 Polyatomic gas molecules: NO2, SF6, C2H5OH: Therefore, the adiabat exponents are γ Cv = 3 R = Cp/Cv = (Cv+R)/Cv is 1.67, 1.4, and 1.33 for monatomic, diatomic, and polyatomic molecules. Gas molar specific heats Equipartition theorem: When a system is in thermodynamic equilibrium the average energy per molecule is ½·kT per each degree of freedom. It means that the molar specific heat is ½·R per degree of freedom. Monatomic molecules only have 3 translational degrees of freedom. Diatomic molecules have 3 translational plus 2 rotational – a total of 5. Polyatomic molecules have 3 translational and 3 rotational – a total of 6. Is this the entire story? Not really!!! It takes a finite temperature to “activate” rotational degrees of freedom. For H2, the 2 rotational degrees of freedom get activated at ~100 K +kT in molar specific heat at const. volume. Below that temperature, H2 behaves as a monatomic gas At still higher temperatures, you activate further degrees of freedom, which are due to oscillations of the atoms along the axis connecting the dumbbell: an addition of 2 degrees of freedom and another kT in Cv at ~1000 K. Reversibility. Where do we find reversible processes? In mechanics – • elastic collisions; • oscillations with no friction; http://www.myphysicslab.com/pendulum1.html • rotation of planets… No mechanical energy is dissipated into heat-internal energy! You can run the movie back and it will still be a plausible process. Irreversibility. Where do we find irreversible processes?... Pretty much everywhere, @#%$@!.. And we are not getting any younger either!.. You can’t possibly run that movie back… Losing, breaking, destroying, saying stupid things…. Seriously. Three common scenarios of irreversibility in thermodynamics. 1) Mixing and loosing structural order in general. Two molecularly mixed fluids never “unmix”. http://mutuslab.cs.uwindsor.ca/schurko/animations/irreversibility/happy.htm A broken vase never repairs itself… 2) Conversion of mechanical energy into internal energy (dissipation into heat). Ordered motion of an object is converted into disordered motion of its molecules. Never coming back… http://mutuslab.cs.uwindsor.ca/schurko/animations/secondlaw/bounce.htm 3) Heat transfer from a hotter to a cooler object – never goes in the opposite direction. Entropy – the story of lost opportunities... Gas expands without doing any mechanical work vs. There was an opportunity for a spontaneous process – heat flow from Th to Tc. Heat transfer between a hot and cold object without mechanical work done. What do these two processes have in common? Spontaneous (NOT quasi-static) expansion of a gas and heat transfer between two objects with different temperatures are both irreversible processes – lost opportunities. What kind of simple reversible processes do we have in stock? T = const isothermal Thermal reservoir with constant temperature No heat transfer at all. !U = 0 W =Q Q=0 W = " !U adiabatic How do we convert internal energy or heat into work? We build a heat engine. "U = Q ! W PV = nRT W = ! PdV Isothermal engine !U = 0 W =Q 100% of the heat transferred to the system is converted to work…. & V2 # W = nRT ln$$ !! % V1 " In principle one can get an unlimited amount of work… BUT it will require an infinitely large expansion! What are we going to do after the gas expands? Run it back? Isothermal engine W = ! PdV & V2 # W = nRT ln$$ !! % V1 " As the system expands all the heat transferred to the system is converted to work…. W<0 W>0 As the system contracts back, though, the same amount of work is done by the surroundings and all the energy is returned to the thermal reservoir. Adiabatic engine W = ! PdV Q=0 W = "!U The positive work is now limited by the internal energy of the insulated system. But again, no net work is done if you go back and forth along the same adiabat. W<0 W>0 We need an engine working in cycles and converting heat supplied from the outside into mechanical work, possibly, with a high efficiency… How efficient can it be? The isothermal engine could convert 100% heat into work, but did not work cyclically. Can we match this performance with an engine operating in cycles? Any fundamental law prohibiting it? The second law of thermodynamics (Kelvin-Plank statement): It is impossible to construct a heat engine operating in a cycle that extracts heat from a reservoir and delivers and equal amount of work. It is impossible to construct a heat engine operating in a cycle that extracts heat from a reservoir and delivers and equal amount of work That would be an ideal heat engine… What is a real heat engine doing? • Works between two temperatures, a hot reservoir and a cold reservoir. (Hot side and cold side). • Gets some heat Qh (obtained from, say, burning a fuel) from the hot side • Rejects some heat Qc to the cold side. • Works in a cycle, so that the internal energy does not change, ΔU=0. • Does work W = Qh - Qc • Has an efficiency e = W/Qh W Qh ! Qc e= = Qh Qh Carnot cycle http://www.ntu.edu.sg/home2000/S7231633I/ A-B Carnot cycle !U AB = 0 WAB = Qh > 0 A B-C Q = 0 WBC = " !U BC > 0 B C-D D C !U CD = 0 WCD = !Qc < 0 D-A What is the total work by the gas? Q = 0 WDA = " !U DA < 0 !U AB + !U BC + !U CD + !U DA = 0 " !U BC + !U DA = 0 WBC + WDA = "( !U BC + !U DA ) = 0 Wtotal = WAB + WCD = Qh ! Qc