Nuclear Physics Chapter 29 Answers to Even Numbered Conceptual Questions
advertisement
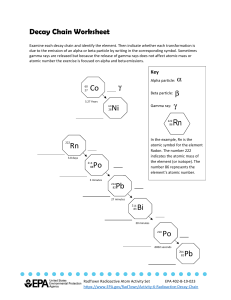
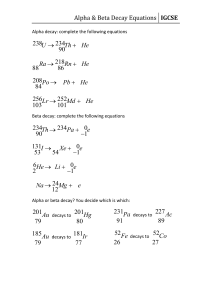
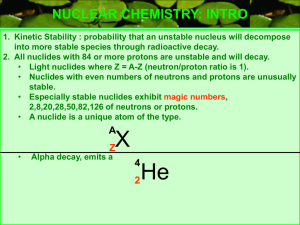
Chapter 29 Nuclear Physics Answers to Even Numbered Conceptual Questions 4. An alpha particle is a doubly positive charged helium nucleus, is very massive and does not penetrate very well. A beta particle is a singly negative charged electron and is very light and only slightly more difficult to shield from. A gamma ray is a high energy photon, or high frequency electromagnetic wave, and has high penetrating ability. 6. Beta particles have greater penetrating ability than do alpha particles. 8. The much larger mass of the alpha particle as compared to that of the beta particle ensures that it will not deflect as much as does the beta, which has a mass about 7000 times smaller. Problem Solutions 29.8 13 Using r = rA , with r0 = 1.2× 10−15 m = 1.2 fm , gives: 0 (a) For 24 H e,A = 4 , and (b) For 29.12 238 92 r = ( 1.2 fm H e,A = 238 , and r = ( 1.2 fm 13 )( 4) = 1.9 fm = 1.9× 10−15 m 13 )( 238) = 7.4 fm = 7.4× 10−15 m ∆m = Z m H + ( A − Z ) m n − m and Eb A = ∆m ( 931.5 M eV u ) A 55 25 ∆m ( in u ) ( in M eV ) (A – Z) 25 30 54.938 048 0.517 527 8.765 Fe 26 30 55.934 940 0.528 460 8.786 Co 27 32 58.933 198 0.555 357 8.768 Mn 56 26 59 27 ( in u ) Z Nucleus m Eb A Therefore, 56 26 Fe has a greater binding energy per nucleon than its neighbors. This gives us finer detail than is shown in Figure 29.4. 457 458 CHAPTER 29 29.15 The decay constant is λ = ln 2 , so the activity is T1 2 3.0 × 1016 ) ln 2 ( N ln 2 R = λN = = = 1.7 × 1010 decays s 4 T1 2 ( 14 d ) ( 8.64× 10 s d ) ⎛ ⎞ 1 Ci R = ( 1.7 × 1010 decays s) ⎜ ⎟ = 0.46 C i 10 ⎝ 3.7 × 10 decays s⎠ or 29.20 Recall that the activity of a radioactive sample is directly proportional to the number of radioactive nuclei present and hence, to the mass of the radioactive material present. R N m = = R0 N 0 m 0 and R = R 0e− λt becom es The decay constant is λ= ln 2 ln 2 = = 0.181 d −1 T1 2 3.83 d Thus, m = m 0e− λt If m 0 = 3.00 g and the elapsed time is t= 1.50 d , the mass of radioactive material remaining is ( ) − 0.181 d −1 ( 1.50 d ) m = m 0e− λt = ( 3.00 g ) e 29.22 Using R = R 0 e− λt , with R R 0 = 0.125 , gives λt= − ln ( R R 0 ) t= − or 29.25 = 2.29 g 212 83 95 36 Bi → Kr → 144 60 ln ( R R 0 ) λ ⎡ ln ( R R 0 ) ⎤ ⎡ ln ( 0.125) ⎤ 4 = − T1 2 ⎢ ⎥ = − ( 5730 yr) ⎢ ⎥ = 1.72× 10 yr l n 2 l n 2 ⎣ ⎦ ⎣⎢ ⎦⎥ 208 81 Tl + 24H e 95 37 Rb + −01e N d → 24H e + 140 58 Ce Nuclear Physics 29.30 The energy released in the decay 238 92 U → 24H e + Th is 234 90 Q = ( ∆m ) c2 = ⎡⎣ m 238 U − ( m 4 H e + m 234 Th ) ⎤⎦ c2 = ⎡⎣ 238.050784 u − ( 4.002602 u + 234.043 583 u ) ⎤⎦ ( 931.5 M eV u ) = 4.28 M eV 29.53 From R = R 0e− λt , the elapsed time is t= − ln ( R R 0 ) λ = − T1 2 ln ( R R 0 ) ln 2 = − ( 14.0 d ) ln ( 20.0 m C i200 m C i) ln 2 = 46.5 d 459