Atomistic Simulations of Radiation Damage in 1MASSACHUSETTS
advertisement

Atomistic Simulations of Radiation Damage in
Amorphous Metal Alloys
by
Richard E. Baumer
B.S.E., LeTourneau University (2008)
Submitted to the Department of Materials Science and Engineering
in partial fulfillment of the requirements for the degree of
AACHNE0"
1MASSACHUSETTS
Doctor of Philosophy
MAY 14 2014
at the
-LIBRARIES
_
MASSACHUSETTS INSTITUTE OF TECHNOLOGY
September 2013
0 Massachusetts Institute of Technology 2013. All rights reserved.
Author..........................
Certified by ......................
.............
.
Department of Materials Science and Engineering
August 6, 2013
.....
Michael J. Demkowicz
Assistant Professor of Materials Science and Engineering
Thesis Supervisor
Accepted by ...................
...........
..
_._.
erbrand Ceder
Chair, Department Committee on Graduate Students
1
IdWME,
OF TECHNOLOGY
2
Atomistic Simulations of Radiation Damage in
Amorphous Metal Alloys
by
Richard E. Baumer
Submitted to the Department of Materials Science and Engineering
on August 6, 2013, in partial fulfillment of the requirements
for the degree of Doctor of Philosophy in
Materials Science and Engineering
Abstract
While numerous fundamental studies have characterized the atomic-level radiation
response mechanisms in irradiated crystalline alloys, comparatively little is known regarding the
mechanisms of radiation damage in amorphous alloys. Knowledge of collision cascade dynamics
is lacking, both with respect to the possibility of sub-cascade formation and concerning the types
of damage created in individual cascades. This Thesis resolves these knowledge gaps through a
systematic simulation study of the radiation response of amorphous metal alloys.
Using a molecular dynamics simulation of /2 MeV ion irradiation in a realistic 2 billion-
atom molecular dynamics simulation in amorphous Cu 5oNb 5o, I show that radiation creates
isolated nanometer-scale zones with rapidly quenching liquids. Quenched liquids reach large
pressures and emit stress pulses that trigger polarized plastic deformation in adjacent material.
In order to identify liquid zones in irradiated amorphous Cu 5oNb 5 o, I use molecular
dynamics simulations to characterize the properties and glass transition temperature of uniform
liquid Cu-Nb alloys. I show that radiation-induced liquid zones rapidly quench to material with
the same properties as a uniform liquid quenched at an equivalent quench rate approaching 1014
K/s. These "super-quenched zones" (SQZs) are approximately 10 nm in diameter and provide a
mechanistic explanation for radiation-induced swelling and ductilization in metallic glasses.
The identification of plasticity adjacent to SQZs is an unexpected damage mechanism
that could prove a limiting factor for the application of amorphous alloys in radiation
environments. To aid selection of amorphous alloys with resistance to collision-induced
plasticity, I formulate a micro-mechanical model for collision-induced plasticity in irradiated
metallic glasses. The analytical model successfully ranks the damage-resistance of irradiated CuNb alloys and should enable selection of amorphous alloys with optimized radiation tolerance.
Finally, through characterization of quenched Cu5 oNb 5o, I reveal that glass transition in
Cu5 oNb 5 o occurs by gelation due to formation of a mechanically stiff, percolating network of
atoms with icosahedral local packing at the interfaces between compositionally enriched regions.
These features of glass transition are similar to gelation processes in polymeric and colloidal gels
and suggest new approaches for understanding glass transition in bulk metallic glasses.
Thesis Supervisor: Michael J. Demkowicz
Title: Assistant Professor of Materials Science and Engineering
3
4
Acknowledgments
First, I wish to acknowledge and thank my PhD Thesis advisor, Michael Demkowicz, for
his patient, thoughtful guidance as I have grown from a welding engineer to a computational
materials scientist. It has been a slow, at times painful, journey, fraught with more wrong turns
than right, and I am forever grateful for his clear direction and encouragement.
A variety of individuals have contributed to this research. I thank my Thesis
Committee-Jeff Grossman, Chris Schuh, and Frans Spaepen-for their guidance and support
throughout my Thesis research. I thank Vasily Bulatov and Tomas Oppelstrup for welcoming me
during the Summer of 2010 at Lawrence Livermore National Laboratory. I am grateful to both
Vasily and Tomas for useful discussions and assistance with the grant application for computing
resources on uBGL. Tomas, thank you for getting me up and running on uBGL and for
assistance with parallelizing my analysis code. I thank Professor Van Vliet for useful comments
on my characterization of the glass transition in Cu 5oNb 5o and Felice Frenkel for discussions on
visualization. I thank J. Ziegler for providing a version of SRIM modified to exclude electronic
stopping. It was critical to designing the radiation damage simulations.
The material in this Thesis is based primarily upon financial support through a National
Science Foundation Graduate Research Fellowship under primary Grant No. 1122374. I also
gratefully acknowledge two Departmental Fellowships-the Salapatas Fellowship and John F.
Elliott Fellowship-for support during my first year at MIT. Thank you, Mrs. Elliott, for your
generosity and encouragement. I acknowledge support through a DuPont/MIT Alliance
Fellowship, as well as funding support by the DoE Office of Nuclear Energy, Nuclear Energy
Enabling Technologies, Reactor Materials program, contract DE-NE0000533. The computations
were performed at Lawrence Livermore National Laboratory through a LLNL 5th Institutional
Unclassified Grand Challenge Computing Allocation.
I thank my undergraduate research mentors who guided me towards graduate school and
the study of computational materials science. I thank my undergraduate advisor, Professor
Warke, for introducing me to computational materials science and for giving me my first
research project in materials modeling. I thank Professor Adonyi for giving me welding research
experience. I thank Professor Ayers and Professor Gonzalez for giving me the opportunity to
work on the LEGS Project. Finally, I thank Professor Daraio for giving me the chance to work in
her lab during the summer of 2007. That research experience confirmed my decision to apply to
graduate school.
On a more personal level, I am grateful for the support and feedback of all the members of
the Demkowicz Group. In particular, I thank Kedar Kolluri and Abishek Kashinath. Kedar, thank
you for many conversations, both technical and personal. Your mentorship helped me to develop
into a productive researcher, and your friendship was a bright spot during my first two, often
difficult, years at MIT and continues to be a valued source of encouragement to this day.
Abishek, thank you for many discussions at the whiteboard. When I was stumped, you often
were my sounding board that kept me on the right track.
The courses and qualifying exams at MIT were challenging and took me to the breaking
point. I made it this far only through the support and help of numerous individuals. I am grateful
for the friendship and support of my first-year study group-Ahmed Al-Obeidi, Satoru Emori,
and James Paramore. I would never have passed the core classes or our written quals without
your friendship, camaraderie, and help. I am indebted to my first-year TAs, particularly Charles
Moore, Jeremy Mason, and Gilbert Nessim. You each listened patiently when I was confused
5
and clarified concepts difficult for me to grasp. Many thanks to all my classmates who helped me
prepare for the oral qualifying exam-Tracey Brommer, Matt Connors, Abishek Kashinath,
Heather Murdoch, and Alexis Turjman-and the older students who practiced with me-Eric
Homer and Tim Rupert.
Toiling away in the basement at MIT can make for a lonely existence, but a few people
brought levity on a nearly daily basis. To my basement compatriots-Uwe Bauer, Satoru Emori,
and Liz Rapoport-and by extension-Ahmed Al-Obeidi and Charles Sing-thank you for
making me smile and convincing me to open the office window blinds!
My MIT experience was greatly enriched through a few courses at Sloan. I am grateful to a
few specific MBA students who warmly welcomed me into their world-Ari Oxman, Jonathan
Bloom, Shanshan Gong, and Dameng Yue. Ari, I am particularly grateful for the opportunity to
work with you on the MIT $1 00k Entrepreneurship Competition.
I thank Professor Gibson for giving me the opportunity to work as her TA in 3.032.
Thank you for accommodating my busy travel schedule and providing an inspiring example in
the classroom. I learned much and enjoyed working with you. To my fellow TA, Alan Lai, thank
you for your teamwork throughout the semester. It was a pleasure working with you.
I thank my wonderful friends at Christ the King Presbyterian Church in Cambridge who
welcomed my wife and me to Boston and made us feel at home. So many have encouraged,
counseled, and prayed for us throughout this process. I thank those fellow PhD students-Sean
O'Hern, Anthony Wong, and Rachel Liao-for perspective. I thank those who already obtained
their PhDs-Ryan Shenvi, Derek Chang, and Erik Baldwin-for encouragement. I thank
Ambrose and Gi Huang for welcoming us into their lives. Ambrose, thanks for all the long runs
together. I thank John and Rachel Churchill for their friendship. Churchills, we should vacation
together again! Laura and Aaron Winn, I am already missing our super club adventures. Sean
and Nicole O'Hern, thank you for your friendship and encouragement. I miss our weekly
community group. Anthony and Emily Wong, thank you for your support. Anthony, I am
grateful for our weekly coffee outings.
I thank my wonderful family for their support. Mom and Dad, thanks for encouraging
for me, and listening to me throughout my undergraduate and graduate studies. It
praying
me,
has been a long journey and you have been there every step of the way. Thanks to my sisters for
always thinking what I was doing was "amazing" even when I didn't feel that way. I thank my
mother and father-in-law for their support, for their prayers, and for trying to understand what I
have spent the last five years studying. Mrs. Callaway, I am forever grateful that you spent the
past two months with us so I could focus on finishing my PhD. This could not have happened
without your help.
Finally, I thank my wonderful wife, Jordan, for her unwavering support. When I got
sidetracked, you pointed me in the right direction. When I was discouraged and depressed, you
listened, encouraged me, and told me to go for a run. Thanks for making time, in the midst of
pursuing your own career, to support me. Any success that I have achieved is a direct result of
your sacrifices. Your courage and perseverance as you have cared for our son has inspired me in
the final push. I love you.
6
Table of Contents
S Introduction
2
1.1
t.............................................................................................................................
Fundamental radiation response mechanisms in crystalline metals .............................
19
19
1.2
Knowledge gaps-the radiation response of metallic glasses ......................................
21
1.3
Thesis outline ...................................................................................................................
22
Review of m etallic glasses and their radiation response ........................................................
2.1 Introduction to glasses .................................................................................................
2.1.1
2.2
25
Synthesis...................................................................................................................
25
2.2.2
Structure....................................................................................................................
26
2.2.3
M echanical properties...........................................................................................
28
Radiation response of metallic glasses: Experim ents ..................................................
29
2.3.1
Radiation-induced sw elling ...................................................................................
29
2.3.2
Radiation-enhanced ductility .................................................................................
30
2.3.3
Radiation-enhanced diffusion...............................................................................
31
2.3.4
Ion-induced plasticity ..........................................................................................
31
2.3.5
Radiation-induced crystallization..........................................................................
32
2.3.6
Summ ary...................................................................................................................
33
Radiation response of m etallic glasses: Simulations ....................................................
33
2.4
2.4.1
Increased free volum e...........................................................................................
33
2.4.2
Reduced short-range topological order..................................................................
34
2.4.3
Enhanced plasticity ...............................................................................................
34
2.4.4
Sum m ary...................................................................................................................
35
Open research questions ...............................................................................................
35
2.5
4
Overview of m etallic glasses ...........................................................................................
23
2.2.1
2.3
3
G lass transition ......................................................................................................
23
23
A tom istic sim ulation m ethods ............................................................................................
3.1 M olecular dynam ics......................................................................................................
37
37
3.2
M olecular statics ..............................................................................................................
37
3.3
A tom ic structure analysis.............................................................................................
38
3.3.1
Analysis of average structural order with the pair correlation function-g(r)........ 38
3.3.2
Classification of bond topology with common neighbor analysis (CNA) ...........
Parallelized atom istic data analysis .....................................................................................
7
39
41
4.1
5
42
4.1.1
Density and potential energy .................................................................................
42
4.1.2
Tem perature..............................................................................................................
42
4.1.3
Diffusivity.................................................................................................................
42
4.1.4
Stress tensor..............................................................................................................
42
4.1.5
Strain tensor..............................................................................................................
43
Atom istic m odeling of m etallic glasses ...............................................................................
5.1 Modeling of amorphous Cu-Nb alloys with molecular dynamics...............................
45
45
45
5.1.1
Cu-Nb as a m odel am orphous alloy system ........................................................
5.1.2
Construction of amorphous Cu-Nb alloy configurations with molecular dynamics 46
5.2
Glass transition by gelation in Cu5 oNb 5O.......................................................................
54
5.2.1
Introduction ..............................................................................................................
54
5.2.2
M ethods ....................................................................................................................
55
5.2.3
Result -Glass transition temperature is 1500 K ...................................................
56
5.2.4
Result - Glass transition m echanism is gelation ..................................................
60
5.2.5
Discussion - Glass transition by gelation.............................................................
65
5.3
Glass transition temperatures in Cu 2 5Nb 75, Cu5oNb 5o, and Cu 75Nb 25 .............
. .. . . . . . . . . . . .
66
5.3.1
Length-scale of com positional order ....................................................................
67
5.3.2
Flow stress ..........................................................................................................
69
5.3.3
Icosahedral short-range order ...............................................................................
69
5.3.4
Therm al expansion ...............................................................................................
69
5.3.5
Heat capacity ........................................................................................................
70
5.4
Properties of amorphous Cu 25Nb 75, Cu5 oNb5 o, and Cu75Nb 25 ...................
... .............. . .
71
5.4.1
Elastic constants ...................................................................................................
72
5.4.2
Yield stress ...............................................................................................................
73
5.5
6
Voxel field calculations ...............................................................................................
Synthesis of
/2billion
atom amorphous alloy configurations...................
Atom istic simulations of irradiated m etallic glasses ..........................................................
6.1 Design of /2MeV molecular dynamics collision cascade studies ...............................
73
77
77
6.1.1
Prim ary knock-on atom energy selection .................................................................
78
6.1.2
Selection of simulation cell size ...........................................................................
80
6.2
Radiation response mechanisms in metallic glasses: Isolated super-quenched zones and
polarized plasticity ....................................................................................................................
8
80
6.2.1
Simulation setup ...................................................................................................
80
6.2.2
Result 1 - Simulation output is reliable...............................................................
81
6.2.3
Result 2 - PKA produces isolated thermal spikes without ion tracks ...................
84
6.2.4
Result 3 - Thermal spikes are liquids that quench to "Super-quenched zones" ...... 87
6.2.5
Result 4 - Thermal spikes produce stress pulses that trigger polarized plasticity ... 93
6.2 .6
D iscussion .................................................................................................................
6.3
7
Role of composition and free volume in radiation response of metallic glasses........... 100
6 .3.1
Introduction ............................................................................................................
100
6.3.2
Therm al spike size ..................................................................................................
100
6.3.3
C ollision-induced plasticity ....................................................................................
101
Micro-mechanical model for collision-induced plasticity ....................................................
7 .1 Introdu ction ....................................................................................................................
105
105
7.2
105
M icro-m echanical model ...............................................................................................
7.2.1
Transient analytical solution to pressurized spherical cavity .................................
7.2.2
Model-based predictions of transient stress adjacent to thermal spikes................. 108
7.2.3
Model-based predictions of maximum stress adjacent to thermal spikes .............. 111
7.3
Modeling onset of collision-induced plasticity..............................................................
105
112
7.3.1
Maximum von Mises stress adjacent to thermal spikes .........................................
112
7.3.2
Maximum pressure inside thermal spikes ..............................................................
113
7.3.3
Collision-induced plasticity susceptibility parameter X.......
7.4
....... 115
Validation of micro-mechanical model with irradiated Cu-Nb alloys...........................
116
Testing damage resistance parameter X with simulation data ................................
116
7.4.1
8
9
99
C onclu sion s...........................................................................................................................
Referen ces.............................................................................................................................
9
119
12 1
10
List of Figures
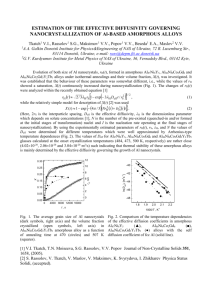
Fig. 1.1: Molecular dynamics simulation of 1 keV self-ion irradiation of 32,000 atom FCC
copper configuration at 300 K (interatomic potential is copper Voter EAM potential splined to
ZBL at short-distances [8]). Atoms are colored by the number of nearest neighbors. Perfectly
coordinated (N = 12) FCC atoms are removed for clarity. (a) Ballistic stage of the cascade
results in numerous displacements. (b) The primary damage state includes vacancies (e.g.
indicated by under-coordinated atoms) and interstitials (e.g. indicated by over-coordinated
0
ato ms)............................................................................................................................................2
Fig. 2.1: Variation of volume (or enthalpy) with temperature in a quenched liquid. Sufficiently
slow cooling causes crystallization at the melting temperature (TM). Fast cooling causes
undercooling below TM, suppressing crystallization and leading to formation of glass "a." Faster
cooling leads to glass "b." Reprinted by permission from Macmillan Publishers Ltd: Nature 410:
24
259-267, C 200 1. ..........................................................................................................................
Fig. 2.2: Metallic glasses are disordered, but not random, at the atomic scale. (a) Atomic
configuration of an amorphous metal alloy-Cu 5oNb 5o-produced with atomistic modeling (See
Chapter 5 for details). Nb atoms colored in dark gray; Cu atoms colored with light gray. (b) Total
pair correlation function computed for the visualized structure shown in (a). ......................... 26
Fig. 3.1: Calculation of the pair correlation function. (a) g(r) computed as the number of atoms
within a spherical shell of width Ar at a distance r from a given atom, relative to bulk density. (b)
Pair-correlation function computed in liquid Cu5 oNb 5o at 4000 K. The dashed line indicates the
38
value for the norm alized bulk density......................................................................................
Fig. 3.2: Application of common neighbor analysis (CNA). (a) Bonded atoms are identified on
the basis of a cutoff distance (here, 3.5 A). The charcoal and red colored atoms are the nearest
neighbors to the light gray atoms. (b) The nearest neighbors common to the two light gray atoms
are highlighted in red and correspond to a 5-5-5 CNA index for the root pair bond................ 39
Fig. 5.1: Molecular dynamics "virtual quenching" procedure for synthesis of amorphous metal
structures. (a) Simulation temperature versus time, with inset showing the stepwise cooling
procedure. Simulation pressure, potential energy, and volume (b, d, e, respectively) are plotted
against the simulation temperature during the quench. The initial crystalline and final amorphous
configuration (c and f, respectively). Cu atoms are shown in light gray; Nb atoms in dark gray. 48
Fig. 5.2: Topological and chemical ordering in a-Cu5 oNb5 o quenched at 10"3 K/s. (a) Pair-pair
correlation functions for Cu-Cu, Cu-Nb, and Nb-Nb interactions. (b) Total structure factor,
computed from the total g(r), computed for r values out to r=4.5 nm. (c) Partial structure factors
Sa (q) computed for each of the individual g(r),p curves in (a), but with radial distances out to
r=4.5 nm. (d) Composition-composition structure factor factor Sec(q), computed from the partial
50
structure factors (shown at low q-values in the inset)...............................................................
Fig. 5.3: Cu5oNb 5o via molecular dynamics quenching. (a) Quenching at 1010 K/s yields a phase
separated structure with crystallization in the Nb phase. Quenching at 1011 K/s and 10" K/s
11
yields phase separated amorphous structures, (b) and (c) respectively. Quenching at 1013 K/s and
1014 K/s amorphous structures with interpenetrating networks of compositionally enriched
material, (d) and (e) respectively. All structures shown at 300 K, after quenching from 4000 K
liquid under P = 0 GPa at the indicated quench rate. Atomic structure visualizations performed
w ith O V IT O [87]..........................................................................................................................
51
Fig. 5.4: Critical quench rate for crystallization in rapidly quenched CuMoNb 5 o. (a) Paircorrelation functions-g(r)-at quench rates of 10" K/s (top) and 1010 K/s (bottom). (b)
Potential energy versus temperature in Cu5 oNb 5 o quenched from 4000 K liquid to 300 K solid at
rates of 1011 K/s (dashed line) and 1010 K/s (solid line). .........................................................
52
Fig. 5.5: Length-scale of compositional medium range order (CMRO). Variation of local
composition as a function of distance along a 2 nm diameter cylinder in 300 K amorphous
53
Cu 55Nb 45 produced by MD quenching. Compare with Fig. 2(d) in Ref. [36]. .........................
Fig. 5.6: Visualizations of Cu5 oNb 5 o at 1400 K (left) and 1600 K (right) after 20 ns of annealing.
(a and b) A 1 nm thick slice is shown with Cu-rich regions colored light gray and Nb-rich
regions colored black. (c and d) Atoms at the CMRO interface, 40 < < 60% Cu, in the slice of
the top panel are visualized. Atoms participating in ISRO packing are colored red and
emphasized with a 20% larger radius. Bonds between ISRO nearest neighbor atoms are colored
red. (e and f) Probability pfl""(r) of finding an atom in the icosahedra network at distance r
from C M RO interfaces .................................................................................................................
57
Fig. 5.7: Properties in annealed Cu 5oNb5 o. Temperature dependence of (a) CMRO wavelength
Ac ; (b) percent of atoms in full icosahedra f""""ll and size of largest ISRO cluster divided by
simulation cell edge length L> = Lo /L 0; (c) flow stress o-F ; and (d) diffusion exponent n . The
vertical lines at 1500 K correspond to the glass transition temperature. All quantities computed
after 20 ns annealing at indicated temperature. .........................................................................
58
Fig. 5.8: Variation of potential energy as a function of local composition in 300 K amorphous
Cu 5 oNb 5o. Open data points correspond to the average local composition and potential energy in
the Nb-rich (triangle), interface (squre), and Cu-rich region (diamond). Dashed line is the
interpolated value of potential energy versus local concentration based on the values of the Nbrich and Cu-rich data points. Filled star corresponds to the average local composition and
potential energy for ISRO atoms. Uncertainty on the mean values reported of order of the size of
the sym bols and therefore not shown........................................................................................
59
Fig. 5.9: Harmonic elastic response of icosahedra network. Change in potential energy for the
icosahedra network and non-icosahedral atoms (closed and open symbols, respectively) as a
function of applied strain ezz below the elastic limit.............................................................
61
Fig. 5.10: Variation in the fraction of icosahedra fo""" in Cu 5oNb 5o deformed at 1400K. (a)
f
" at applied strain EZZtof"o"" at EZZ = 0. (b) Ratio of far." between two zerostrain configurations, Cl and C2. Cl is the initial, zero strain configuration. C2 is the final
Ratio
12
configuration after Cl has been loaded to a total strain of EAPP at ta = 2 x 109 s-'and unloaded
to zero strain at the same rate. Error bars represent the uncertainty on the mean value, determined
by averaging over 5 (a) and 30 (b) independent simulations....................................................
63
Fig. 5.11: Reversible deformation. (a) Average displacement magnitude IAr I and (b) average
difference in potential energy APE between two zero-strain configurations, C1 and C2, as a
function of applied strain EAPP (see text for details). The vertical lines indicate the global yield
strain. Error bars represent the uncertainty on the mean value, determined by averaging over 30
63
independent simulations................................................................................................................
Fig. 5.12: Diffusion behavior of annealed CusoNb5 o. Variation of mean-squared displacement
with temperature for 9,826 atom Cu 5oNb 5o annealed at temperatures between 500 K and 2500 K
(100 K increments). The cage breaking interval is shaded in blue; the fitting window is shaded in
cyan; and the phase separation interval is shaded in red. .........................................................
65
Fig. 5.13: CMRO versus temperature and composition. Visualization of alloys (Cu 2 5 Nb 75 ,
Cu5 oNb 5 o, and Cu75 Nb 25 at top, middle, and bottom, respectively) following 20 ns annealing. A 1
nm thick slice is shown with Cu-rich regions colored light gray and Nb-rich regions colored
b lack ..............................................................................................................................................
67
Fig. 5.14: Variation of properties with composition and annealing temperature. (a) Variation of
the length-scale of compositional medium range order (AC) with temperature. (b) Variation of the
flow stress with temperature. (c) Fraction of atoms in full icosahedra as a function of
temperature. (d) Glass transition temperature as a function of composition. All values computed
after annealing for 20 ns at the indicated temperature...............................................................
68
Fig. 5.15: Variation of properties with composition and temperature. (a) Variation of thermal
expansion with respect to temperature for three alloy systems. (b) Temperature dependence of
the second derivatives of the curves in (a). The inflection point is where the second derivative
equals zero. (c) Variation of heat capacity with respect to temperature for three alloy systems. (d)
Temperature dependence of the second derivatives of the curves in (c). The inflection point is
where the second derivative equals zero....................................................................................
70
Fig. 5.16: Atomistic calculation of mechanical properties of of a-Cu5 oNb5 o. (a) Stress versus
strain computed under quasi-static uniaxial tension. Solid lines indicate linear fits. (b) Von Mises
stress versus work equivalent strain under volume conserving deformation at 300 K with a strain
rate of = 2 - 10' 1/s. Solid line indicates 0.2% strain offset..................................................
73
Fig. 5.17: Comparison of thermodynamic output between 50k and 474M atom configurations of
quenched Cu 5 oNb 5o. (a) Potential energy versus simulation time. (b) Volume per atom versus
sim ulation tim e..............................................................................................................................
74
Fig. 6.1: (a) The nuclear and electronic stopping powers (Sn and Se, respectively) as a function
of PKA energy for Nb and Cu (black and red, respectively), as computed in an a-Cu oNbso
5
system of density p=8.193 g/cm with standard SRIM [125]. (b) Ratio of Sn and Se as a function
of PKA energy, for Cu (red) and Nb (black) PKAs.................................................................
78
13
Fig. 6.2: (a) Distributions of final positions of 475 keV Nb PKAs in an a-Cu5oNb5 o system of
density p= 8 .193 g/cm computed with SRIM. The solid line and filled circles are for no electronic
stopping (modified SRIM [126]) while the dashed line and open circles include electronic
stopping (standard SRIM [125]). (b) Histogram of primary recoil energies due to 475 keV Nb
ions, averaged over 1,000 Nb PKAs, computed using SRIM without and with electronic stopping
79
(solid and dashed lines, respectively). .......................................................................................
Fig. 6.3: Energy is conserved in MD simulation of 475 keV Nb ion irradiation of a Cu5 oNb5 o. (a)
Variation of simulation timestep size with total simulation time. (b) PKA energy and change in
81
total system energy as a function of simulation time...............................................................
Fig. 6.4: Trajectory of 475 keV Nb ion in a-Cu5 oNb5o, computed with NVE molecular dynamics.
(a) Visualization of PKA trajectory. The simulation cell boundaries are indicated. The dashed
line indicates the specified PKA direction. (b) PKA position as a function of simulation time.
82
The simulation cell has an edge length of 196 nm....................................................................
Fig. 6.5: Change in the simulation center of mass position as a function of simulation time...... 83
Fig. 6.6: Quantifying PKA collision events. (a) PKA energy versus integral trajectory distance.
(b) Histogram of number of recoils created at a given energy, computed using SRIM without
[126] and with [125] electronic stopping (solid and dashed lines, respectively), as previously
shown in Fig. 6.2. Blue symbols with dashed line correspond to MD data, computed as the
85
histogram of PKA energy drops [from part (a)]. .......................................................................
Fig. 6.7: Displacement zones and thermal spikes in irradiated a-Cu5 oNb 5o. (a) Displaced atom
trajectories in a-Cu5oNb5 o: 475keV Nb PKA plotted in red, knock-on atoms acquiring at least 1
keV in black; atoms displaced between 0.5-1 nm in blue. (b) Temperature fields due to internuclear collisions. Red voxels have a maximum temperature greater than TG = 1500 K [39]. The
blue contour is for Tmax = 350 K after a total simulation time of 12 ps. (c) Isolated thermal
spikes identified on the basis of nearest-neighbor cluster analysis. (d) Energy flux into a single
representative therm al spike, boxed in (c). ................................................................................
86
Fig. 6.8: Thermal spike volume versus deposited energy. The straight line corresponds to the
87
linear fit: VTs = (15.1 ± 0.6 nm 3 /keV) ETS-( 8 .9 + 11.2 nm 3 ).................................................
Fig. 6.9: Time-dependent properties of a single voxel inside thermal spike shown in Fig. 6.7 (d).
(a) Voxel temperature and density versus time. (b) Voxel mean-squared displacement (MSD)
and derivative diffusivity versus time. (c) Voxel density and (d) diffusivity versus voxel
temperature (open symbols), compared with values from uniform CuMoNbMo liquid quenched at
89
6 - 1 0 K / s...................................................................................................................................
Fig. 6.10: Mapping of thermal spike properties to rapidly quenched, uniform liquid. Diffusivity
(a) and density (b) at t=5 ps plotted versus temperature for voxels with Tmax > TG (open symbols:
voxel data; blue line: binned average). The values for Cu 5 oNb5 o liquid quenched at 6- 1013 K/s are
90
shown for com parison (black line). ...........................................................................................
14
Fig. 6.11: Changes in voxel potential energy (a) and density (b) between the initial and postirradiation SQZs are plotted versus voxel quench rate. Property changes for a-Cu5 oNb5 o
quenched at various rates, with respect to 1 - 10" K/s are shown for comparison (black line).. 91
Fig. 6.12: Schematic representation of radiation-induced SQZ formation, responsible for
radiation-induced swelling and ductilization .............................................................................
92
Fig. 6.13: Confined melting leads to pressurization of thermal spike and initiation of a stress
pulse. (a) Close-up view of thermal spike. (b) Pressure, (c) temperature, and (d) density plotted
versus simulation time for thermal spike (black line) and adjacent material within 4 nm of
thermal spike surface (gray line). Shaded band indicates uncertainty of the mean.................. 93
Fig. 6.14: Liquid thermal spikes emit stress pules. (a) Pressure as a function of distance from the
surface of the thermal spike visualized in Fig. 6.13 (a). Inset plot is position of the peak of the
pressure pulse as a function of time. (b) Stress pulse front after 5 ps......................................
94
Fig. 6.15: Material response in a radiation damage zone. (a) Close-up view of thermal spike
boxed in Fig. 1(a). A cylindrical coordinate system is defined along the major (z) axis of the
thermal spike. (b) Average temperature in the thermal spike and in adjacent voxels versus time.
(c) Variation of diagonal components of plastic strain in cylindrical coordinates with location
along the thermal spike major axis, with uncertainty indicated by shaded bands. (d) Von Mises
stress and tensile work equivalent plastic strain versus time, averaged over all adjacent voxels
w ithin 4 nm of the therm al spike. ..............................................................................................
95
Fig. 6.16: Voxel average plastic strain components in material adjacent to the seven largest
thermal spikes as a function of the thermal spike energy. Arrow indicates the thermal spike
analyzed in F ig. 6.15.....................................................................................................................96
Fig. 6.17: (a) Distribution of maximum pressure inside thermal spikes; (b) Distribution of
maximum average von Mises equivalent stress (Uvm) in material within 4 nm of thermal spikes.
.......................................................................................................................................................
97
Fig. 6.18: Summary of radiation damage in irradiated Cu-Nb alloys. Left column, PKA
trajectories shown with red lines; KA (ke>1keV) shown with black lines. Center column, red
cubes correspond to regions with Tmax > TG; blue contour corresponds Tmax = 350 K. Right
column, voxels adjacent to liquid zones ( Tmx > 350 K ) with plastic strains
102
EV (t-12 ps) > 0.01 are shown as black cubes..........................................................................
Fig. 6.19: Number of clusters, sorted largest to smallest, comprising 80% of the total thermal
spike volume, versus glass transition temperature of irradiated alloys (Cu 2 5Nb 7 5, TG=1400 K;
Cu5 oNbso, TG=1500 K ; Cu 7 5Nb 2 5, TG=1600 K). .........................................................................
104
Fig. 6.20: Variation of collision-induced plasticity with material glass transition temperature and
annealing. Open symbols correspond to as-quenched state and filled symbols indicate relaxed
state .............................................................................................................................................
10 4
15
Fig. 7.1: Schematic of the micro-mechanical model for the onset of collision-induced plasticity.
(a) Spherical cavity of radius a loaded with an internal pressure P at time t = 0. An analytical
solution describes the transient stress response at a material point r. (b) Schematic of the
106
assumed step-function loading pressurization of the spherical cavity........................................
Fig. 7.2: Comparison of thermal spike data from molecular dynamics simulation of 475 keV Nb
irradiation of Cu5oNb 5 o and transient linear elastic model. (a) Thermal spike zone, with red cubes
indicating voxels with Tmax>1500 K and black cubes indicating EP>0.01. (b) Spherical cavity
approximation of thermal spike in (a) with a radius r=4 nm. .....................................................
109
Fig. 7.3: Application of transient elastic model to model stress response of material adjacent to
thermal spikes. The pressure input is modeled with a single step function (a), two step functions
(b), and multiple step functions (c). The dashed blue line (a-c) is the pressure measured in the
thermal spike shown in Fig. 7.2 (a). The approximation for P(t) is shown in the solid black line
(a-c). Using the P(t) approximation shown in (a-c), the stress response of a material point at r = 7
nm is plotted with a solid black line. The actual stress data measured at this material point
110
adjacent to the thermal spike is plotted in the dashed black line................................................
Fig. 7.4: Model-based prediction of maximum von Mises stress (OvM) as a function of distance
111
from the surface of the therm al spike. ........................................................................................
Fig. 7.5: (a) Variation of thermal spike pressure with material. (b) variation of X with material
116
type, evaluated using pressure measured in thermal spikes........................................................
Fig. 7.6: Collision-induced susceptibility parameter X computed from material properties versus
X evaluated directly from thermal spike properties. Open symbols correspond to as-quenched
117
systems while filled symbols indicate relaxed systems..............................................................
16
List of Tables
Table 3.1: Common neighbor analysis (CNA) indices for different atomic structures [38, 86].. 40
Table 5.1: Variable quench rate synthesis procedure for 50k atom model glasses and resulting
prop erties.......................................................................................................................................
53
Table 5.2: Synthesis procedure for 50k atom model glasses and resulting properties. ............ 72
Table 5.3 Synthesis procedure for % billion atom model glasses and resulting properties..... 75
Table 6.1: Plasticity in irradiated Cu-Nb alloys. Cascade direction determined by the major axis
of the best-fit ellipsoid to voxels withTmax > 350 K; eigenvalues computed from the average
plastic strain tensor of voxels adjacent to liquid zones (e.g. 350 < Tmax < TG); the aggregate
collision-induced plasticity parameter is computed as A = f(EP_)/cj, where (EfM) is the average
strain in voxels adjacent to liquid zones and 4 is the dose. .......................................................
103
Table 7.1: Model inputs for Fig. 7.3. The average stress predicted at various times is indicated, as
well as the actual stress measured in the material in the irradiated material. ............................. 111
Table 7.2: Predicting the maximum stress in thermal spikes......................................................
17
115
18
1
Introduction
The fundamental, atomic-scale mechanisms responsible for bulk property changes in
irradiated materials have received considerable attention over the past fifty years [1]. These
studies, motivated in large part by unanticipated property changes, such as void swelling in
irradiated structural metals in nuclear reactors, have revealed that radiation creates defects at the
atomic-scale. In some cases, such as focused ion-beam milling, radiation-induced defects can be
employed as a tool to engineer desirable material properties. In others, such as nuclear reactors,
radiation degrades critical material properties, reducing the lifetime of a material for its intended
application.
Most research effort on radiation effects in metals has focused on crystalline, structural
alloys. By contrast, another category of metals-metallic glasses-has received comparatively
little fundamental study. Metallic glasses are amorphous, meaning that no long-range order exists
at the atomic scale. As a consequence of their disordered atomic structure, amorphous metal
alloys have impressive mechanical properties, high temperature formability, and corrosion
resistance. Additionally, radiation has been found to yield property changes qualitatively
different from crystalline metals, but the fundamental radiation response mechanisms remain
poorly understood.
1.1
Fundamental radiation response mechanisms in crystalline metals
In crystalline metals, radiation in the form of high-energy particles like neutrons and ions
creates damage through atomic displacements caused by collisions between incident particles
and atoms in the irradiated material. Particle-atom scattering transfers energy to material atoms,
creating "Primary Knock-on Atoms" (PKAs) that may have sufficient energy to be displaced
from initial lattice positions. If the PKA energy exceeds a modest energy (~
25 eV), the
displaced PKA creates a single vacancy and terminate as a self-interstitial, creating a stable
Frenkel pair. However, as illustrated in Fig. 1.1, for PKAs with energies exceeding ~ 1 keV, the
PKA will initiate a chain reaction of atomic displacements ("collision cascade") that generates
multiple point defects [1] and, at high energies, can also yield defect complexes such as
interstitial clusters [2]. These atomic-level responses of crystalline solids to radiation yield large
changes to macroscale properties, including continuous void swelling due to clustering of
vacancies [3, 4] and embrittlement due to multiplication of dislocation obstacles (e.g. vacancy
19
A
B
14.4
14t=3.49
s
ps
7.3 nm
-Fig. 1.1: Molecular dynamics simulation of 1 keV self-ion irradiation of 32,000 atom FCC
copper configuration at 300 K (interatomic potential is copper Voter EAM potential splined to
ZBL at short-distances [8]). Atoms are colored by the number of nearest neighbors. Perfectly
coordinated (N = 12) FCC atoms are removed for clarity. (a) Ballistic stage of the cascade
results in numerous displacements. (b) The primary damage state includes vacancies (e.g.
indicated by under-coordinated atoms) and interstitials (e.g. indicated by over-coordinated
atoms).
clusters and precipitates) [5, 6]. Understanding atomic-scale radiation damage mechanisms is
therefore critical to science-based design of engineering alloys resistant to radiation damage [7].
Displaced PKAs lose energy through discrete scattering events with material atoms
("nuclear stopping"), as well as through interactions with the electronic
sub-system of the
material ("electronic stopping") [9]. Electrons slow PKAs and enhance
thermal conductivity,
dissipating energy away from collision cascades [1]. The average PKA energy in irradiated
alloys is well above the threshold for cascade formation (e.g. 1 MeV neutrons in nickel produces
an average PKA energy of 35 keV [9]) but in the energy range where most PKA energy is
lost
through nuclear stopping [10].
Collision cascades occupy volumes approximately ten nanometers (nm) in diameter and
exist for times on the order of ten picoseconds (ps) [Fig. 1.1]. Current experimental techniques
lack the spatiotemporal resolution to analyze defect production in-situ and are limited to postmortem analysis of radiation-induced damage. Therefore, theory and simulation provide the only
means of revealing the actual atomic-scale mechanisms responsible for damage production in
irradiated materials [1].
20
Extensive computer modeling demonstrates that collision cascades in metals proceed
through two distinct stages [1]. For the first ; 0.5 ps of the cascade, the PKA collides with other
atoms in discrete, two-body scattering events, so-called the "ballistic stage," which sets the
spatial distribution of displacements and subsequent energy deposition. Second, for the next
~ 10 ps, the energy of displaced atoms decreases, the scattering cross-section increases, and
energy is dissipated in the lattice through many-body interactions in "thermal spikes." During
the thermal spike stage, energy dissipation causes local melting, followed by rapid quenching to
a defective crystal state [11, 12]. Rapid melting leads to pressurization of the liquid zone, which
emits an elastic stress pulse into the surrounding unmelted material [2]. If the energy of a PKA
exceeds a material-dependent threshold, the collision cascade splits into multiple spatiallydistinct "sub-cascades." For example, computer simulations demonstrate 200 keV PKAs in iron
split into smaller sub-cascades that are individually comparable in size and energy to those
resulting from a single 10 keV PKA [13]. Although some Frenkel pairs recombine in the
disordered core of the collision cascade, numerous defects remain, including Frenkel pairs,
vacancy clusters, and self-interstitial clusters [2, 14]. The subsequent long-time evolution of the
damage state depends on the defect mobility and proximity to defect sinks.
1.2
Knowledge gaps-the radiation response of metallic glasses
Unlike in crystalline alloys, only a handful of modeling studies exist to guide
interpretation of experimental results of irradiated metallic glasses. As summarized in Chapter 2,
all fundamental studies in realistic models of metallic glasses have focused on changes to local
topological order, and no study has yet explored the dynamics of defect production. Knowledge
of collision cascade dynamics is lacking, both with respect to the possibility of sub-cascade
formation and concerning the types of damage created in individual cascades.
This Thesis resolves these knowledge gaps through a systematic simulation study of the
radiation response of amorphous metal alloys. Through the use of massive parallel computing
techniques, a series of
/2
Irradiation with
ions is subsequently simulated, revealing that ions lose energy through
/2 MeV
billion atom, realistic models of metallic glasses are constructed.
binary collisions that terminate in spatially distinct liquid zones (thermal spikes). Additionally,
novel damage mechanisms in the form of "super-quenched zones" (SQZs) and plastic
deformation are revealed at the level of individual collision cascades.
21
1.3
Thesis outline
In Chapters 2 and 3, I lay the groundwork for the Thesis results. In Chapter 2, I present a
detailed review of the literature on the properties of metallic glasses and their radiation response,
demonstrating that metallic glasses respond to radiation in ways qualitatively different than
crystalline alloys. I summarize experimental findings, present insights from computer modeling,
and describe the key knowledge gaps in the current understanding of the radiation response of
metallic glasses. In Chapter 3, I review the computational materials science methods utilized to
simulate the radiation response of metallic glasses.
In Chapters 4 - 8, I present the core results of the Thesis. In Chapter 4, I describe the
analysis methods developed to process the large simulation dataset (~ 100 Terabytes) produced
through the course of this research. In Chapter 5, I report the glass transition physics in the alloy
system employed in this research (Cu-Nb), as well as the mechanical properties. Identification of
the glass transition temperature in each of the studied alloys (Cu 2 5Nb 7 5 , Cu5 oNb 5o, and Cu 7 5Nb 2 5)
is critical to identification of liquid zones in irradiated alloys in Chapters 6 and 7. In Chapter 6, I
report the atomic-scale damage mechanisms in irradiated metallic glasses-super-quenched
zones (SQZs) and collision-induced plasticity-in the model system a-Cu5oNb5o. I expand the
investigation in a-Cu5oNb5o to other Cu-Nb alloy systems and discuss the role of free-volume and
composition in radiation response. In Chapter 7, I derive a figure of merit for selection of
metallic glasses with optimal radiation damage resistance. Conclusions are provided in Chapter 8
and references listed in Chapter 9.
22
2
Review of metallic glasses and their radiation response
Amorphous metals are a class of metal alloys with no long-range order at the atomic-
scale. While some early studies showed promising radiation damage resistance (e.g. swelling
without void formation [15, 16] and radiation-enhanced ductility [17]) and novel radiation
responses (e.g. ion-induced plasticity [18]) compared to crystalline alloys, the fundamental
mechanisms responsible for bulk radiation responses have received little investigation. This
Thesis resolves many of the knowledge gaps on fundamental radiation response mechanisms in
irradiated metallic glasses. In order to place my findings (Chapters 4 - 7) within the context of
previous work, this Chapter begins with an introduction to glasses generally and metallic glasses
specifically, before moving to a thorough review of the experimental and simulation-based
findings on radiation response mechanisms in irradiated metallic glasses.
2.1
Introduction to glasses
Many materials found in nature are crystalline. Crystalline materials are ordered at the
atomic scale, with atoms or molecules arranged on a periodic lattice. A crystal lattice has
translational symmetry extending to length-scales much greater than atomic distances, and single
crystal specimens have been prepared with dimensions approaching 1 meter (e.g. single crystal
aircraft turbine blades prepared from nickel based super-alloys and single crystal silicon wafers).
While crystalline materials are ubiquitous, it is not the only form of solid matter. In fact, noncrystalline-amorphous-materials, possessing no long-range topological order beyond a few
nanometers, can be found in nature (e.g. obsidian glass formed from volcanic lava), as well as
manufactured. Amorphous materials are employed in diverse applications, including oxide
glasses in window panes and amorphous silicon in solar cells. While the compositions,
chemistries, and material synthesis procedures vary widely, amorphous materials share the
common feature of a complete lack of long-range topological order at the atomic scale.
2.1.1
Glass transition
As shown schematically in Fig. 2.1, many slowly cooled liquids crystallize at the melting
temperature [19]. Crystallization is a first-order phase transition, marked by a discontinuous
change in the material volume (and enthalpy), as well as derivative quantities like specific heat
and thermal expansion, at the melting temperature (TM). While crystallization may be
thermodynamically preferred, crystallization is inherently affected by kinetics since a finite time
23
is required to nucleate a seed crystal in a liquid. While the thermodynamic driving force for
crystallization grows with increased cooling below TM, undercooling causes atomic diffusion to
decrease, slowing the formation of a critical crystalline nucleus. If cooled quickly enough, a
liquid may bypass crystallization completely. Because of the kinetics involved in the glass
transition, glasses are more easily formed in viscous liquids with a glass transition temperature
close to the melting temperature.
For a rapidly quenched liquid, in a relatively narrow temperature window, so-called the
glass transition, the viscosity of a quenched liquid is found to increase many orders of
magnitude. Below the glass transition temperature (TG), the material is a solid and resistant to
flow on the timescale of an experiment, although the structure is reminiscent of the liquid state,
without any long-range topological order [20]. As illustrated in Fig. 2.1, the properties of a glass
depend on its quench rate, with more rapid quench rates resulting in higher glass transition
temperatures and lower density. Annealing a rapidly quenched glass close to TG results in aging,
with relaxation towards the properties of a glass quenched at a slower rate (e.g. increased density
and lower TG).
L
E
Tga Tgb
Temperature
TM
Fig. 2.1: Variation of volume (or enthalpy) with temperature in a quenched liquid. Sufficiently
slow cooling causes crystallization at the melting temperature (TM). Fast cooling causes
undercooling below TM, suppressing crystallization and leading to formation of glass "a." Faster
cooling leads to glass "b." Reprinted by permission from Macmillan Publishers Ltd: Nature 410:
259-267, C 2001.
24
2.2
Overview of metallic glasses
While oxide-based glasses have been used for centuries, the discovery of amorphous
metal alloys occurred comparatively recently. Amorphous metals are a class of metal alloys with
no long-range order at the atomic-scale, resulting in a range of interesting and useful material
properties such as high yield stress [21], corrosion resistance [22], and excellent formability [23].
Amorphous metals can be prepared through a number of synthesis routes, including rapid
quenching of a liquid metal [24], vapor deposition [25], and ion-beam mixing [26]. Amorphous
alloys that are synthesized via quenching are termed "metallic glasses," reflecting the fact that
these alloys exhibit glass transition and relaxation behavior found in more traditional glassy
materials such as polymer and silicate glasses.
2.2.1
Synthesis
The first amorphous metal alloy-
Au 75 Si 2 5-was
reported by Duwez et al. in 1960 [27]
and was synthesized using a specially designed apparatus for rapid liquid quenching [28]. The
apparatus consisted of a graphite crucible for melting the material, separated by a Mylar
membrane from a chamber filled with pressurized helium. Upon bursting the membrane, a shock
wave propelled the liquid droplet onto the interior of a rotating copper cylinder [28], which
ensured good wetting for optimal heat conduction and produced quenching rates on the order of
109 K/s. Synthesized with this method, Au7 5 Si 2 5 , remained amorphous during characterization
with x-ray diffraction [27], but was unstable and crystallized within twenty-four hours.
Since Duwez et al. synthesized this first amorphous metal alloy by rapid quenching, a
variety of alloy systems, such as Pd-based and Zr-based metallic glasses, have since been
discovered that can be synthesized with conventional metallurgical casting techniques at cooling
rates as low as 1 K/s [24]. These so-called "bulk metallic glasses" (BMGs) can be formed into
fully-glassy ingots with diameters on the order of centimeters [24]. Development of BMG alloys
has been guided by the principal of "frustrating" the liquid state, such that the kinetics of crystal
nucleation in an undercooled melt are slow and therefore easily bypassed by rapid cooling.
Empirically, it has been found that good glass forming alloys meet the following criteria: (1)
Multiple components and a composition near a deep eutectic; (2) atomic size ratios excess of
15%, and (3) negative heats of mixing [24].
25
Structure
2.2.2
Metallic glasses are disordered at the atomic-scale, as evidenced by a diffuse X-ray
diffraction spectrum without any sharp Bragg reflection peaks [27]. However, determination of
the structure factor-S(q) -or
the real-space analog of the pair-correlation function -g(r)
-
reveals that the structure is not random. In fact, both topological and chemical order exists in
amorphous metals for distances out to
1 nm. As illustrated by the atomistic modeling results in
Fig. 2.2(a), a typical pair correlation function in amorphous metals has a well-defined first
nearest-neighbor shell (first peak), as well as inter-penetrating shells of atoms at the second
nearest-neighbor position (split second peak) [Fig. 2.2(b)]. In crystalline alloys, diffraction data
can be indexed to a unique 3-dimensional structure [29]. However, in non-crystalline, amorphous
metals, no unique atomic arrangement exists and therefore to understand what topological and
chemical ordering produces the g(r) signal it is necessary to use complimentary analyses
beyond the pair correlation function.
Development of models for the structure of amorphous materials has been guided by
Bemal's description of the structure of monatomic liquids (e.g. liquid argon) through physical
models [30, 31]. Bernal produced three-dimensional physical models for the structure of liquids,
including compressing ball bearings in paint, allowing the paint to harden, and then physically
picking apart the structures to determine the coordination of spheres [31]. The physical models
approximated an assemblage of randomly packed hard spheres and revealed a well-defined firstb
a
-------- --- --- ---- ------- --- ---0.5
0
.0
9 nm
2
4
6
Radial distance (A)
8
10
Fig. 2.2: Metallic glasses are disordered, but not random, at the atomic scale. (a) Atomic
configuration of an amorphous metal alloy-Cu5 oNb 5o-produced with atomistic modeling (See
Chapter 5 for details). Nb atoms colored in dark gray; Cu atoms colored with light gray. (b) Total
pair correlation function computed for the visualized structure shown in (a).
26
nearest neighbor shell in good agreement with that found in liquid argon [31]. A high fraction of
nearest-neighbor coordination contained five-fold symmetry, although the coordination geometry
was irregular and in need of a statistical description [30, 31].
In the spirit of Bernal's early work, Miracle proposed a solute-centered cluster model for
metallic glasses for alloys with low solute concentrations [32]. Miracle postulated that solvent
atoms surround solute atoms in a proportion dictated by the relative size ratios, with each of
these individual clusters in-turn forming a close-packed, cubic configuration of interpenetrating
clusters. Subsequent atomistic modeling demonstrated that while solute-centered clusters are a
distinguishing feature of metallic glasses, the packing of these "quasi-equivalent" clusters has
primarily icosahedral, rather than cubic, symmetry [33].
In support of this modeling work, more advanced experimental techniques have recently
emerged that provide direct evidence for ordering at the nanometer scale in metallic glasses.
Hirata et al. employed nano-beam electron diffraction in Zr66 .7Ni33 .3 to reveal short-range local
topological order in the form of atom clusters-atoms clustered around a central atoms-as well
as medium range order in the form of interpenetrating atom clusters [34]. Supporting this picture
of interpenetrating atom clusters, Hwang et al. investigated Zr5 oCu 4 5Al5 through a "hybrid" RMC
simulation that optimized an atomic configuration by fitting both to fluctuation electron
microscopy data and the system potential energy, as determined from an EAM interatomic
potential, finding evidence for crystal-like symmetry, in addition to icosahedral order, in the
medium-range cluster packing [35].
Modeling has also been successful in describing the atomic structure of immiscible
metallic glasses, such as the amorphous Cu5 oNb5o alloy visualized in Fig. 2.2(a). For example,
while phase separating binary systems such as Cu-Nb [36] or Ni-Ag [37] are poor glass formers,
calorimetry showed that sputter deposited amorphous immiscible binary metals exhibit a lower
than expected crystallization enthalpy (~~10 kJ/mol) [36], suggesting that some form of atomic
ordering stabilizes these amorphous solids [25]. He et al. employed Reverse Monte Carlo
modeling on the immiscible system Ag 4oNi6 o to reveal that nanoscale, "spinodal-like"
compositional order develops and stabilizes the amorphous structure against crystallization [37].
More recent experiments [36] confirmed the presence of "spinodal-like" patterns of nanometerscale compositional enrichment, with simulations predicting percolating networks of local
27
icosahedral atom packing [38}, located at the interfaces between regions of compositional order
[39] (See Chapter 5).
2.2.3
Mechanical properties
At low-temperatures and moderate stresses (e.g. T/TG < 0.8 and 1 > 0.02p, where r is
the shear stress and p is the shear modulus), most metallic glasses exhibit inhomogeneous plastic
flow, with shear localizing in narrow bands (shear bands) that lead to catastrophic failure [40].
At high temperatures and low stresses (e.g. T/TG > 0.8 and r < 0.003p), plastic flow is
uniform, and large strains can be achieved [40]. The unit process responsible for deformation is
the shear transformation zone [41]. Unlike flow-defects in crystalline solids-dislocations-that
can intersect and lead to strain hardening, shear transformation zones are accompanied by
dilatation, which increases the propensity for flow, and ultimately leads to shear localization
[41].
Under some loading conditions, such as uniaxial tension, shear localization leads to
failure in a brittle-like manner. However, a few specially engineered amorphous metals show
properties comparable (or better) than many crystalline alloys. For example, tensile ductility has
been achieved in metallic glass composites containing crystalline precipitates [42]. Precipitates
act as barriers to the propagation of shear bands and greatly improve ductility. Additionally, a
remarkable combination of toughness and yield strength has been demonstrated in a special
palladium-based
metallic glass (Pd 79Ag 3 .5 P 6Si 9 .sGe2 ), rivaling the properties of the best
crystalline alloys [43].
Experiments demonstrate that local variations in structure in metallic glasses yield large
local variations in the deformation response of metallic glasses. For example, Wagner et al. used
atomic force microscopy to map local variations in elastic constants of Pd77. 5 Cu6 Sii6 .5 metallic
glass and found a wide distribution (full-width half maximum of 33%) of contact moduli on
length-scale of 10 nm [44]. Simulations suggest that percolating networks of "soft" (i.e. negative
elastic constants) and "stiff' material exist at the ~ 1 nm scale [45]. Finally, dynamic nanopillar
compression experiments yield results consistent with percolating network of rigidly bonded
clusters [46]
Plastic deformation has been found to trigger crystallization in deformed metallic glasses.
For example, Chen et al. deformed amorphous alloys and found crystalline precipitates formed
inside the shear band of three alloys (AlgoFe 5 Gd5 , AlgoFe 5 Ce5 , A187Ni8 .7Y4.3), but not in a fourth
28
(Al 8 5NilOC 35 ), demonstrating that deformation-induced crystallization is composition dependent
[47]. Deformation induced-crystallization has also been observed in simulations of deformed
amorphous silicon (a-Si) [48], suggesting that plasticity-induced crystallization may be a general
feature of the amorphous state.
2.3
Radiation response of metallic glasses: Experiments
Since the primary effect of radiation is to generate atomic-scale defects, it is reasonable
to expect that the disordered structure of metallic glasses might render their response to radiation
different from crystalline alloys, if not superior. Indeed, as I show below, experiments
demonstrate that the macroscale behavior of metallic glasses under irradiation is markedly
different than in crystalline metals-they swell without voids to a finite limit [15, 16] and
become more ductile [17, 49]-suggesting distinct atomic-level responses to radiation. Similar to
crystalline alloys, radiation enhances diffusion and points to the kinetics of radiation-induced
defects. Finally, high-energy ion irradiation causes anisotropic plastic flow in irradiated stress
free samples.
2.3.1
Radiation-induced swelling
Void swelling in irradiated crystalline alloys results from radiation-induced vacancy
production and subsequent agglomeration [9]. By contrast, neutron irradiation of amorphous
materials results in density reduction [15] with no void formation [15, 16], suggesting that
radiation-induced increases in free-volume remain diffuse and non-localized [16].
The key test for void swelling in amorphous materials is the evolution of density with
radiation dose. This approach was taken by Gerling and Wagner, who demonstrated a ~0.9%
density reduction in neutron-irradiated amorphous Fe 40Ni 4OB 20 with the rate of density change
going to zero at high doses [15]. Electron microscopy revealed no clear evidence of void or
helium bubble formation [16], in stark contrast to the agglomeration of vacancies into voids in
crystalline materials. Annealing of neutron-irradiated amorphous Fe 4oNi 4OB 20 does not enable a
full recovery to the non-irradiated, annealed density, demonstrating that permanent, irreversible
structural changes do accompany swelling [15]. However, the exact nature of these irreversible
structural changes is unclear.
Swelling has also been probed through surface profile measurements of ion-irradiated
amorphous nickel-based alloys [50, 51]. While distinct changes in the surface profile have been
29
measured following irradiation, no void formation was observed [50], suggesting that a
qualitatively different mechanism is operating than the void swelling reported in crystalline
metals. Indeed, Chang and Li note in an early study that surface swelling could be due to
inhomogeneous plastic deformation [51], as is well known to result from ion irradiation of
metallic glasses [18]).
While other studies have claimed to discern the presence of voids either during annealing
[52, 53] or through ion irradiation [54], those results are not convincing. For example, Tiwari
and von Heimendahl [55] argue that the "voids" observed by Morris [52], are in fact crystallites,
rather than voids. While Shibayama et al. observe voids in ion-irradiated amorphous alloy,
crystallization occurred, obscuring whether voids form in the amorphous or crystalline matrix.
2.3.2
Radiation-enhanced ductility
While irradiated crystalline alloys become brittle, irradiated metallic glasses become
more ductile [17, 49]. Radiation-induce ductility is reversible and subsequent annealing returns
the alloy to its pre-irradiated brittle state [17, 49, 56].
Gerling et al. annealed a series of amorphous alloys, FeNiB, FeNiP, and CuTi, resulting
in a systematic decrease in the strain to fracture with increasing annealing temperature [17], and
subsequently irradiated the embrittled alloys with neutrons, resulting in a complete restoration of
ductility. While the neutron dose required for complete ductilization varied with composition, for
a sufficiently high neutron dose, all alloys returned to the pre-annealed ductile state. Alloys
subject to longer annealing times (i.e. more brittle) required larger neutron doses to restore
ductility. Finally, the authors performed a cyclic thermal annealing and post-annealing radiation
experiment and found that the transition from brittle to ductile was completely reversible [17].
Magagnosc et al. also found reversible radiation-induced ductilization in Ga+ ionirradiated Pt5 7.5 Cu 1 4. 7Ni. 3P 2 2 .5 metallic glass nanowires [49]. As-fabricated metallic glass
nanowires are initially brittle and marked by inhomogeneous flow. Following irradiation,
however, the nanowires exhibit an increase in tensile ductility, marked by homogeneous flow.
Post-radiation thermal annealing returns the samples to the initially brittle state, demonstrating
reversibility
Raghavan et al. irradiated Zr 4
2 Ti1
.8 Cu 1 2.5 NiioBe 22 .5 with Cu ions up to doses of 100 dpa,
and found in subsequent micro-compression testing that the irradiated glasses had reduced yield
strength and a significant decrease in the energy release per shear plane [56]. These results
30
suggest a brittle to ductile transition, and subsequent nanoindentation tests confirmed a
homogeneous flow in the irradiated metallic glass [57].
2.3.3
Radiation-enhanced diffusion
Irradiation of amorphous metals has been observed to enhance diffusivity of tracer
elements [58, 59]. In their review of radiation-enhanced diffusion, Faupel et al. propose two
distinct mechanisms for radiation enhanced diffusivity: 1)
Radiation produces diffusion-
mediating defects and 2) Radiation creates a secondary phase with high diffusivity [60]. The first
mechanism is based upon diffusivity measurements in ion-irradiated amorphous metals, such as
reported by Averback and Hahn in the amorphous Ni-Zr system [58]. For such systems,
enhancements in diffusivity (above the ballistic mixing regime) follow an inverse square root
dependence of diffusivity upon ion flux. This relationship is characteristic of recombination
reaction kinetics found in crystalline materials, prompting the authors to suggest that irradiation
creates vacancy and interstitial-like defects [58].
The second mechanism is supported by diffusivity measurements under low-energy (i.e.
400 keV protons) irradiation conditions [59], where enhancements to diffusivity are found to
effect only the Arrhenius pre-exponential factor and not the activation energy for diffusion.
Unchanged activation energy eliminates the possibility of radiation-induced defects that act as
diffusion carriers and can be explained by radiation-induced structural changes with enhanced
diffusivity [59, 60]. Molecular dynamics simulations showing structural relaxation via localized
displacement chains, after 100 eV collision cascades, further confirm this experimental
interpretation [59]. These observed displacement chains only occur in the regions affected by the
collision cascade, emphasizing that local radiation damage does enhance atomic mobility.
These results on radiation-enhanced diffusion suggest that different types of structural
changes result for low and high-energy irradiation. Low-energy irradiation may yield unrelaxed
structural regions that enhance diffusivity [59], while high-energy irradiation may create pointdefect like entities which mediate diffusion [58].
2.3.4
Ion-induced plasticity
Irradiation of stress-free amorphous thin metal films with penetrating fast, heavy ions
(i.e. 360 MeV Xe ions at ~ 1016 ions-m-2 [18]) leads to expansion of the specimen in the direction
perpendicular to the ion beam, with dimensional changes of up to 20% reported [18]. This shape
change appears to be volume-conserving [18], leading to the conclusion that the dimensional
31
change is in-fact ion-induced plasticity. Ion-induced plasticity is ubiquitous within a wide range
of amorphous materials, including insulators [61], and has not been observed in crystalline
metals [18].
For electronic stopping powers > 1 keV/nm, ion-induced plasticity is well explained by the
mechanism of electronic excitations and attendant local melting/solidification in the cylindrical
wake of a penetrating ion [18, 62, 63, 64]. At stopping powers < 1 keV/nm, defect production
has been proposed as a more likely mechanism [64]. Point defect analogs were proposed to
function as flow defects in a manner similar to that proposed by Spaepen within the free-volume
theory for homogeneous flow [40, 65]. However, ion-induced plasticity occurs for stress free
films, making it unclear what physical meaning to attach to the radiation-induced defects
proposed by Mayr et al [64].
2.3.5
Radiation-induced crystallization
Radiation-induced crystallization has been demonstrated in a broad variety of alloys over a
wide range of radiation conditions, including irradiation with ions [66] and electrons [67].
Results suggest that devitrification does not proceed directly from localized melting but is rather
facilitated through radiation-induced defects. For example, Carter et. al. find that the specific
crystalline precipitates produced by 1 MeV Cu ion irradiation of amorphous Cu5 oZr 45Ti5 are not
consistent with direct quenching from a liquid core and instead likely result from enhanced
diffusion from radiation damage [66]. Similarly, Nagase and Umakoshi found that irradiationinduced formation of nano precipitates in Zr-Cu alloys due to electron irradiation is temperature
dependent, suggesting crystallization due to free volume creation as opposed to direct atomic
displacements [67].
Radiation-induced
devitrification has been demonstrated to be sensitive to alloy
composition. For example, Rechtin et al. investigated the stability of Nb 4oNi6 o (Tx = 940 K) to 3
MeV Ni+ ions for doses of up to 20 dpa (1.5x 1021 ions m 2 ) for T = 900 K [68] and found that
the Nb-Ni alloy is completely stable to irradiation (20 dpa) at 900 K. By contrast, Brimhall et al.
performed a similar investigation on amorphous Mo-Ni (Tx > 1000 K) using 5 MeV Ni++ ions
up to 20 dpa and found that at 875K, 20 dpa irradiation causes complete devitrification of the
initially amorphous Mo-Ni alloy, with minimal radiation damage in the crystalline Mo-Ni
sample [69]. It is not clear what accounts for such marked differences in similar alloys.
32
Finally, I note that Dunlop et al. found crystallization in the vicinity of amorphous ion
tracks formed by 5 GeV Pb ion irradiation in the metallic glass Fe 7 3 .5 Cu 1Nb 3 Si 13 5B 9, suggesting
that shock-fronts induced by the ion track may produce crystallization [70]. However, in an alloy
with different crystallization mechanism (Fe 4oNi 35 Si 1oB
1i),
no evidence of ion-induced
crystallization is observed, suggesting that composition is important. Observations of shear band
formation adjacent to ion tracks in different irradiated iron-based amorphous
alloys
(Fe73.5CuiNb 3 Si 3 sB 9 and FegoZr 7 B 3) [71] leads to the possibility that plasticity could drive
crystallization in irradiated alloys.
2.3.6
Summary
Experiments demonstrate that metallic glasses respond differently to radiation than
crystalline alloys and enable the following inferences to be made concerning possible
mechanisms for property degradation in irradiated amorphous alloys. First, irreversible radiationinduced swelling-without voids-suggests that defects are diffuse, non-localized increases in
free volume. Second, radiation-enhanced diffusion suggests that defects can either be point
defect-like entities or perturbations in structure, depending upon radiation energy. Finally,
radiation-induced crystallization appears to be driven by perturbations in structure, instead of
melting and subsequent crystallization.
2.4
Radiation response of metallic glasses: Simulations
Radiation effects in amorphous metals have been simulated with MD simulations in
several monatomic model systems, including the dense random packed hard sphere (DRPHS)
system [72], the Lennard-Jones system [73, 74], and the Dzugutov potential [75, 76], as well as
more realistic models of amorphous metals like a-Ni3 P [75], a-CuTi [64, 77], and Cu-Zr based
alloys [78, 79]. These studies demonstrate that collision cascades change the distribution of free
volume [73, 74, 75], the local topological order [59, 75, 77], and mechanical strength [77, 78,
79].
2.4.1
Increased free volume
Free volume changes were identified by cavity analysis-computing the largest sphere
that fits between atoms with fixed hard sphere radii [74, 75]-and with computation of voronoi
volume per atom [73]. Both Chaki and Li and Mattila et al. find interstitials, identified as region
of high compressive hydrostatic stress, to relax out faster than vacancies, identified through
voronoi volume [73] or cavity analysis [75], perhaps explaining previously reported non-void
33
swelling results [15, 16, 50, 51]. These results are also in accord with the defect stability studies
performed by Chaudhari et al [80]. Laakkonen and Nieminem claim the opposite, namely that
vacancies anneal out faster than interstitials in the a-LJ system [74], despite using the same
defect identification method as Mattila et al. [75]. The reason for this discrepancy is unknown.
2.4.2
Reduced short-range topological order
Analyzing the first nearest neighbor shell of irradiated a-Ni 3 P, a-Ni, and the monatomic
Dzugutov potential system for icosahedra, four-fold, or six-fold bonds, Matilla et al.
demonstrated permanent local structural changes of a decreased number of five-fold
bonds/icosahedra in response to radiation [75]. Mayr demonstrated that topological order in
irradiated a-CuTi is independent of the initial structure (quench rate), as multiple collision
cascades cause the structural order, characterized by the number of icosahedra, to converge to a
universal value [77]. For the case of an optimal quench rate, no changes are observed upon
irradiation, suggesting that specific amorphous structures may be indifferent to radiation-induced
structural changes. Similarly, Avchaciov et al. simulated multiple collision cascades in
amorphous Cu 64Zr36 and found that the reduction in icosahedral short-range order saturates at a
constant value, independent of dose [79], however, the authors do not report whether this change
can be mapped to thermal processing.
2.4.3
Enhanced plasticity
Xiao et al. studied the deformation response of irradiated Zr5oCu 40AlIo metallic glass with
molecular dynamics and found that radiation damage causes a transition in deformation response
from shear localization to homogeneous flow [78], in agreement with the experimental results of
irradiation induced ductilization in Ga+ irradiated Pt 57 .5Cui4.7Nis. 3 P2 2.5 [49]. Furthermore, Xiao et
al. demonstrate that the mechanical properties of the irradiated metallic glass can be mapped to
an unirradiated sample synthesized with a more rapid quench rate, supporting the equivalence
between radiation damage and thermal processing [78].
Avchaciov et al. simulated deformation of irradiated amorphous Cu6 4 Zr 36 and
surprisingly found an increase in strain localization in the irradiated alloy [79]. While they
attribute these results destruction of the icosahedral "elastic backbone" of the alloy and
promotion of STZ nucleation, it is unclear how to reconcile these results with the work by Xiao
et al. [78].
34
2.4.4
Summary
Simulations demonstrate that radiation perturbs the structure of metallic glasses through
reduced topological order, as well as through increases in free volume. These "defects" can be
correlated with some experimentally measured radiation-induced changes in mechanical
properties, in particular radiation-enhanced ductility. Furthermore, simulations suggest that
structural changes have a thermal origin, as structural changes can be mapped to quench rate.
2.5
Open research questions
All fundamental studies in realistic models of metallic glasses have focused on changes
to local topological order, and no study has yet explored the dynamics of defect production.
Knowledge of collision cascade dynamics is lacking, both with respect to the possibility of subcascade formation and concerning the types of damage created in individual cascades.
In this Thesis, I therefore seek to answer the following open questions:
1.
What is the spatial distribution of damage resulting of collision cascades in metallic
glasses?
2. What is the nature of the defects left in radiation damage zones?
Answering these questions successfully requires detailed analysis of collision cascades and
associated property changes, in addition to the more common measures of aggregate changes in
structural order parameters, such as icosahedral order, and mechanical strength. Through such
detailed analysis, a mechanistic framework for understanding property changes in irradiated
metallic glasses will be developed.
35
36
3 Atomistic simulation methods
3.1
Molecular dynamics
Atomistic simulations of collision cascades in metals are frequently studied using classical
molecular dynamics (MD) simulations with empirical interatomic potentials, due to the fact that
the time and length scale of the collision cascade (picoseconds in tens-of-nanometer sized
regions) is completely resolvable with classical MD [1].
The computational technique of
molecular dynamics numerically integrates F, = mai for each atom i, where the force is given by
the negative gradient of the interatomic potential: F = -VV(r). Two critical approximations are
made in this computational method: 1) neglect of electronic effects and 2) the description of the
metal with an empirical interatomic potential. The first concern is sometimes treated
phemonologically through the addition of a viscous drag term to the equations of motion for
atoms with a kinetic energy greater than, for example, 10 eV [1]. Empirical potentials can be
constructed to reproduce bulk properties of metals like melting temperature, defect formation
energies, and crystal structure cohesive energies, and have been used successfully to probe
structure in alloys [81].
3.2
Molecular statics
Given an atomic system of size N and system potential energy function V(R), where R is
the Nx3 matrix of atomic positions, an atomic configuration that minimizes V(R) is sought. This
is a standard optimization problem, which can be solved through iterative methods like steepest
descent and conjugate gradient energy minimization. In the method of steepest descent energy
minimization, the negative gradient of V(R), F = -VV(R) is computed, which yields the
direction of steepest descent within the potential energy landscape, V(R), and subsequently the
new atomic configuration is found by performing a one dimensional line minimization along the
steepest descent direction,
Rnew =
a. f +ho , with respect to a [82].
Energy minimization
methods are useful in structure analysis, as potential energy minimization removes thermal
effects and leaves only the "inherent structure" for analysis.
37
3.3
Atomic structure analysis
Structural order in simulated atomic configurations can be studied with two different
approaches: (1) aggregate measures of the average structural order and (2) local measures of
structural order. Within the first category, the pair-correlation function (or radial distribution
function)-g(r) -is
the most common and is presented below. In the second category, a wide
variety of methods have been developed to analyze local ordering in crystalline and amorphous
metals. In this Thesis, the common neighbor analysis method is employed and presented below.
3.3.1
Analysis of average structural order with the pair correlation function-g(r)
As schematically illustrated in Fig. 3.1(a), the pair correlation function-g(r)-measures
the probability of finding an atom within a spherical shell of width Ar at a distance r from a
given atom, relative to the bulk number density [83]. When averaged over all atoms in the
configuration, g(r) is computed as:
g
1 fi'
= N(r)
V\
/1
- rij, Ar)
A(r
(4/3wr[(r + Ar)
i jti
3
-
r3])
where 17(r - rj,Ar) is an indicator function equal to I for any atom within a spherical shell of
width Ar at distance r, N is the total number of atoms in the configuration, and V is the total
volume of the configuration. The shell width Ar is typically Ar ~ 0.05
a
A. The
typical output
b
2
1.5
0
9cr
40.5
0
0
5
Radial distance
(A)
10
Fig. 3.1: Calculation of the pair correlation function. (a) g(r) computed as the number of atoms
within a spherical shell of width Ar at a distance r from a given atom, relative to bulk density.
(b) Pair-correlation function computed in liquid Cu 5oNb5 O at 4000 K. The dashed line indicates
the value for the normalized bulk density.
38
found from a g(r) calculation is illustrated in Fig. 3.1(b), while shows the total pair-correlation
function in liquid Cu 5 oNb5o simulated at 4000 K. At short-distances, a well-defined first-nearest
neighbor cage is identified (indicated by a peak value approximately twice the bulk density),
although the ordering quickly decays to the bulk density (indicated by the dashed line). The g(r)
is well-suited for identifying system-wide ordering and discriminates between liquid, glassy, and
crystalline states. However, since g(r) is not able to reveal local variations in topological
ordering, it is also necessary to employ localized analysis approaches.
3.3.2
Classification of bond topology with common neighbor analysis (CNA)
Common neighbor analysis (CNA) is a topological analysis method that classifies the
symmetry of local atomic structure on the basis of bond connectivity [84, 85]. From the first
minimum in the pair-correlation function, a cutoff distance is established to identify "bonded"
atoms, with typical cutoff distances on the order of 3.5 A. Using this cutoff distance, the firstnearest neighbor shell is identified for every atom. To quantify the topology of each bonded atom
pair, the overlap in nearest neighbor atoms is found and the bond connectivity in the atoms
common to the bonded pair is characterized using three indices, jkl. The first index, j, is the
number of common first-nearest neighbors to the root pair. The second index, k, corresponds to
the number of bonds among the common neighbors. The third index, 1, is the longest contiguous
chain of bonds among the common neighbors [38, 84, 85].
The CNA procedure is illustrated in Fig. 3.2. As shown in Fig. 3.2 (a), a bond is identified
between two atoms, highlighted in light gray. All nearest neighbors for these two atoms are
a
b
Fig. 3.2: Application of common neighbor analysis (CNA). (a) Bonded atoms are identified on
the basis of a cutoff distance (here, 3.5 A). The charcoal and red colored atoms are the nearest
neighbors to the light gray atoms. (b) The nearest neighbors common to the two light gray atoms
are highlighted in red and correspond to a 5-5-5 CNA index for the root pair bond.
39
Structure
BCC
FCC
HCP
Full
Icosahedra
CNA Indices
6-6-6)
4-2-1 (12)
4-2-2(6)
5-5-5 (12)
Broken
Icosahedra
Table 3.1: Common neighbor analysis (CNA) indices for different atomic structures [38, 86].
shown in the charcoal and red atoms. As highlighted in Fig. 3.2 (b), the red atoms are the atoms
that are common to the nearest neighbor lists of the bonded pair of atoms. For the common
neighbor atoms, there are 5 common neighbors (j = 5), 5 total bonds (k = 5), and the longest
contiguous chain of bonds has a length of 5 (1 = 5). Thus, the CNA index for the root pair in Fig.
3.2 (b) is 5-5-5.
As summarized in Table 3.1, it has been found that local atomic structures can be
uniquely identified on the basis of connectivity of the neighbor atoms common to a root pair. For
example, if an atom has 12 nearest neighbors and has a 5-5-5 index with each nearest neighbor
atom, the central atom has icosahedral (i.e. five-fold) symmetry. Crystalline configurations can
be likewise identified on the basis of the CNA indices of a central atom with each of its
neighboring atoms.
40
4
Parallelized atomistic data analysis
The continued growth in computing resources has greatly expanded the possibility for
large-scale atomistic simulations. In the past, most MD simulations of more than 100 million
atoms employed an in-situ, on-the-fly data analysis approach. In this paradigm, atomic structures
are analyzed during the simulation and only predefined "interesting" features are saved for
further analysis and visualization. In the case of crystalline materials, defects are well-defined
and topological analysis can be readily employed to extract these quantities.
In the case of glasses, however, a different analysis paradigm is needed. Radiation
damage mechanisms are at best ill-defined and more likely, unknown altogether. I therefore
utilize a post-processing approach where complete system snapshots-atom positions, velocities,
forces, and atomic-stresses-are saved for subsequent analysis. The benefit of this approach is
that an iterative data analysis procedure can be utilized to identify mechanisms and use
preliminary insights to define subsequent analysis procedures, enabling convergence on the
previously unknown mechanisms.
To support this post-simulation data analysis paradigm, I developed a fully-parallelized
analysis code that uses a coarse-grained analysis approach that discretizes atom positions to a 3dimensional array of cubic volumes (voxels) and aggregates per-atom information into voxelbased nanoscale field quantities. For example, my simulation of
2 MeV
ion irradiation of a
'/2
billion atom Cu5oNb5 o amorphous alloy (Section 6.2), I save complete system snapshots (detailed
information for all atoms) every 1,000 timesteps. The resulting dataset is large (~15 TB) and I
employ the parallelized post-processing approach to identify regions of interest for subsequent
visualization and quantitative analysis. Subsequent atom visualizations are performed with Ovito
[87] and field visualizations are performed with VisIt [88].
The discretization of atomistic data into nanometer scale fields has been previously used
successfully in diverse studies, including: revealing the spatial heterogeneity in elastic properties
and plasticity in deformed glasses [89]; characterizing spatial variations in density and stress in a
40 million atom simulation of nanoindented silica glass [90]; and identifying shock fronts in a
220 million atom simulation of shock loaded AlN [91]. In the spirit of these previous
approaches, I compute voxel field quantities of temperature (T), density (p), potential energy
(PE), diffusivity (D), stresses (on), and strains (Eij). Below, I first summarize the coarse grained
analysis procedure and explain the calculation of each field quantity.
41
4.1
Voxel field calculations
The discretization of atomistic data into nanoscale fields has been previously used in
diverse studies [89, 90, 91]. Below, I describe the calculation of each voxel field quantity andin the case of tensor fields-derived scalar quantities.
4.1.1
Density and potential energy
Following Nomura et al. [90], I compute the voxel density field by summing the atomic
mass of voxel constituent atoms and normalizing by the voxel volume. Similarly, I compute the
potential energy voxel field by summing the potential energy of voxel atoms and normalizing by
the total number of atoms.
4.1.2
Temperature
Following Zhu et al. [12], I determine temperatures by fitting the kinetic energy
distributions of atoms in every voxel to the Maxwell-Boltzmann distribution [92]:
ke (1\3/2
/ke
2- exp
p(ke) = 2
where ke is per-atom kinetic energy, kB is the Boltzmann constant, and T is temperature. Prior to
the fit, the center of mass velocity of the voxel is first subtracted from the atomic velocities. The
fitting is performed by minimizing the mean squared difference between the simulated
distribution of kinetic energies and the predicted distribution with temperature as the fitting
parameter.
4.1.3
Diffusivity
Following Hsieh et al. [93], I compute the time dependent diffusivity from the mean
squared displacement (MSD) of voxel atoms with respect to a reference configuration at 1 ps,
after the ballistic phase of the collision cascade has ended. The instantaneous diffusivity is
computed from the slope of the linear fit to MSD(t) in 2 ps time intervals centered at t,
D(t)
4.1.4
1 dr(t)2
at
Stress tensor
I use built-in LAMMPS functions to compute the per-atom virial stress tensor [94] and
obtain voxel stress tensors oai by summing the per-atom stresses and dividing by the voxel
42
volume. I compute the voxel pressure as P = -1/
3/2o;bu', where the deviatoric stress
'=
aij -
3 Ukk
and the von Mises stress as amU
11/3Ckk8
-
and Sj is the Kronecker delta
[95].
4.1.5
Strain tensor
Using a similar approach to ones used previously for computing atomic-level strains [96,
97], I find the best-fit uniform deformation gradient F1 connecting an initial (t = 0 ps) and final
configuration of atoms in a voxel. All atom positions are referenced to the center of mass of the
voxel. From the deformation gradient, I compute the Lagrangian total strain tensor as E=
1/ 2 (FiFkj - 6Sd). In the small strain approximation, the total strain may be decomposed into
elastic (E -) and a plastic strains (Er)
Hooke's Law as E - =
Sijkl(Tkl
+a
as
E
E + e+ . The elastic strains are calculated via
, where ar, is the independently determined voxel
stress, S 1 ikl is the compliance tensor, a is the linear thermal expansion, and AT is the change in
temperature from the initial temperature. I assume that the model is isotropic on the voxel length
scale and described by the bulk elastic constants. I therefore obtain the plastic strain tensor for
every voxel E
2/3E1
E
= Eij - c<,. From it, I find the tensile work equivalent plastic strain
, where the deviatoric strain E' = E - 1/ 3 Ekk Sij-
43
EPM =
44
5 Atomistic modeling of metallic glasses
In this chapter, I employ atomistic modeling to construct realistic model atomic structures
of the amorphous binary alloy system Cu-Nb at compositions of Cu 2 5Nb 7 5 , Cu5oNb 5 o, and
Cu 7 5Nb 2 5. These atomic structures are subsequently irradiated and the resulting damage state is
characterized in Chapters 6 and 7. In Section 5.1, I discuss the chosen model system, report
simulation results, and demonstrate that the molecular dynamics simulation protocol produces
realistic model atomic structures. In Section 5.2, I report a detailed investigation of the glass
transition mechanism in a-Cu5 oNb5o. In Section 5.3, I extend the methods employed in Section
5.2, to obtain the glass transition temperatures in Cu 2 5Nb75 and Cu 75Nb 2 5 , and report the
mechanical properties in Section 5.4. Finally, in Section 5.5, I use large parallelized simulations
to obtain 2 billion atom configurations of Cu 25Nb75 , Cu5 oNbso, and Cu 75 Nb 25 .
5.1
5.1.1
Modeling of amorphous Cu-Nb alloys with molecular dynamics
Cu-Nb as a model amorphous alloy system
In atomistic modeling of metallic glasses, all structural and thermo-physical properties
are embedded in the interatomic potential. At the most basic level, the interatomic potential
should reproduce the phase diagram of the system, which is typically complex in the alloy
systems of good glass formers. For example, even the "simple" binary bulk metallic glass Cu-Zr
has numerous line compounds in the phase diagram and Cheng et al. employed 600 atomic
configurations in order to produce a Zr-Cu-Al EAM potential and no demonstration is given that
it reproduces the system phase diagram [98]. In seeking to uncover the radiation response of
metallic glasses, it is therefore beneficial to work with amorphous alloys with simple phase
diagrams that can be modeled with straightforward interatomic potential fitting procedures.
One class of alloys that satisfies this goal of phase diagram simplicity is the amorphous
alloy system formed between the copper (Cu) and niobium (Nb), which has no line compounds
and exhibits very limited solid solubility. Amorphous copper-niobium (a-CuxNb.x) has been
experimentally synthesized via ion-beam mixing [99, 100] and sputter deposition [25, 36, 101,
102] and has been found to be amorphous between composition ranges of approximately 3575% copper, in the case of magnetron sputtering on water cooled substrates [25]. Atom-probe
tomography reconstructions in a-Cu5 5Nb 4 5 demonstrate that the structure of these amorphous
films is characterized by composition modulations between 25 and 75%, with a characteristic
45
length of 2-3 nm [36, 102]. Annealing of a-CuxNbi.x, leads to the formation of nanoscale
precipitates (200 0 C, [102]) or complete devitrification (350'C, [102, 103]). These properties and
compositional features make amorphous Cu-Nb representative of the general class of immiscible
binary alloys [104].
Previous studies demonstrate that the structure of immiscible binary alloys can be
captured successfully through atomistic modeling. For example, immiscible Ni-Ag alloys were
modeled with Reverse Monte-Carlo [37, 38], revealing "spinodal-like" compositional ordering
and icosahedral topological order. Icosahedral order is a ubiquitous topological signature of
metallic glasses (e.g. [98]), suggesting that immiscible metallic glasses are topologically similar
to their miscible, bulk glass forming counterparts. Additionally, Wang et aL. simulated
amorphous alloy formation in Cu-Nb with an EAM potential and found good agreement with
their predicted composition limits on alloy formation and experimental synthesis results
(between 28 and 85% copper) [99]. All these results suggest that modeling a-CuxNbijx via a
classical EAM potential will successfully capture the system details. Additionally, the
topological order will likely be of sufficient similarity to more conventional metallic glasses to
make the results of radiation damage in amorphous Cu-Nb representative of all metallic glasses.
Demkowicz and Hoagland reported an EAM potential for Cu-Nb based upon firstprinciples calculations of defect formation energies, heats of mixing, and cohesive energies [8].
Additionally, the potential provides an accurate representation of short-range interactions (i.e.
displacement cascade conditions) through spline fitting to the Ziegler, Biersack and Littmark
(ZBL) universal potential at small distances. The ZBL potential is given by a universal screening
function times the Coulomb potential [105], while the general form of an EAM potential is a
pair-wise energy sum plus a non-linear contribution from the electron density [81]. This potential
is therefore well-suited for modeling the structure and thermodynamics of amorphous Cu-Nb and
its radiation response. I therefore employ the Demkowicz Cu-Nb potential in this Thesis.
5.1.2
Construction of amorphous Cu-Nb alloy configurations with molecular dynamics
Previous molecular dynamics studies of metallic glasses have successfully employed
molecular dynamics with empirical interatomic potentials-embedded atom method (EAM)
potentials [81] are common-to simulate metallic glass alloys. Models are typically 3dimensional, -10 nm on an edge length, contain approximately 50,000 atoms, and are generated
in two steps. First, the ensemble of atoms is simulated with molecular dynamics under periodic
46
boundary conditions above the system melting temperature until melting occurs and a uniform
liquid forms. Secondly, the temperature of the liquid is decremented to room temperature,
typically with a barostat applied so that the total system pressure is zero. Under this "virtual
quenching" procedure, the melt is cooled through the glass transition to a vitrified (amorphous)
solid [98, 106]. The procedure has produced amorphous structures with pair-correlation
functions in quantitative agreement with experimental results (e.g. [33, 34]), suggesting that the
"virtual quenching" procedure is capable of resolving much of the structural and chemical
ordering present in metallic glasses.
The principal drawback of the virtual quenching molecular dynamics method is that the
MD quench rates (typically 1010 - 1013 K/s) are orders of magnitude higher than experimental
quench rates used to cast typical metallic glasses (below 106 K/s [24]). Molecular dynamics
quench rates are so high because current computational techniques limit molecular dynamics
simulations to simulations times less than 1 ps. For example, for a liquid quenched from 2300 K
to 300 K over 1 ps, the effective quench rate is 2 - 109 K/s. Thus, the structural and chemical
order found in metallic glass atomic configurations generated with MD rapid quenching will be,
at best, a lower bound on the degree of ordering in real quenched metallic glasses [33, 35, 98].
However, for poor glass formers, such as immiscible amorphous Cu-Nb alloys, the
drawbacks of rapid quenching are less severe since the material can only be synthesized through
vapor deposition [25, 36, 101, 102], which may be characterized with an "effective" quench rate
of ~101 K/s [107]. Thus, for the amorphous Cu-Nb alloy system, I employ rapid quenching of
uniform liquids to produce amorphous atomic configurations. The MD synthesis procedure
yields atomic configurations in good agreement with experimental results [39].
MD "virtual quenching" at 1013 K's
I employ the following virtual quenching MD simulation procedure, illustrated in Fig. 5.1,
using the open-source MD code LAMMPS [94]:
1.
I initialize a crystal configuration to the desired system size and composition, with the
physics of the system dictated by chosen interatomic potential. I typically start with
-50,000 atoms in a BCC, FCC, or CsCl crystal structure, with the Demkowicz Cu-Nb
EAM potential [8].
47
2.
I initialize the velocities to a temperature above the system melting temperature and run
MD until melting occurs. I start with a temperature of 4000 K and relax the system with
a combination of conjugate gradient energy minimization and molecular dynamics to
produce a liquid at zero pressure.
3.
I repeatedly decrement the temperature and equilibrate the liquid until 300 K is reached,
with a barostat of P = 0 GPa applied. For an effective quench rate of 10" K/s, I use
decrements of 25 K, followed by 2.5 ps MD runs in the NPH ensemble. Temperature is
controlled by the velocity rescaling to the target temperature every 25 timesteps [83].
Slower quench rates employ the NPT ensemble.
The application of this synthesis procedure is illustrated in Fig. 5.1 for the melting of
Cu5oNb 5 o and quenching at 101 K/s to 300 K. In Fig. 5.1(a), I plot the simulation temperature
versus time. As indicated by the temperature inset, the temperature is decremented by 25 K,
every 2.5 ps, yielding an effective quench rate of 1013 K/s. The temperature is controlled by
velocity rescaling every 25 timesteps. During the quench the pressure is close to zero, due to the
a
b
4000
-
50
Lo3000
35
a2000
345
350
W5380
3530
E 1000
-1
IC-
0
C
0
100
d
-2
200
300
0
400
Time (ps)
1000
2000
3000
Temperature (K)
e
4000
t =0 ps, 4000 K
f
E -4.4
% 20-
-4.6.
E
ai
19.
18
S-5E
-5
17
-5.2.
a.S-5.41-I
0
-
16.1
1000
2000
3000
Temperature (K)
4000
0
1000
2000
3000
Temperature (K)
4000
t
370 Ps, 300 K
Fig. 5.1: Molecular dynamics "virtual quenching" procedure for synthesis of amorphous metal
structures. (a) Simulation temperature versus time, with inset showing the stepwise cooling
procedure. Simulation pressure, potential energy, and volume (b, d, e, respectively) are plotted
against the simulation temperature during the quench. The initial crystalline and final amorphous
configuration (c and f, respectively). Cu atoms are shown in light gray; Nb atoms in dark gray.
48
applied NPH barostat (P = 0 GPa) [Fig. 5.1(b)]. The initial structure is 50-50 Cu-Nb in a CsCl
structure, containing 48,778 atoms in a cubic simulation cell with an edge length of 9 nm [Fig.
5.1(c)]. During the quench, the potential energy and volume decrease continuously, without the
discontinuities expected in crystallization [Fig. 5.1(d) and (e)]. The final, as-quenched structure
appears disordered with some compositional patterning [Fig. 5.1(f)].
To quantify if the quenched configuration, visualized in Fig. 5.1(f), is amorphous, I
quantify the degree of topological ordering with partial pair correlation functions and the
structure factor. Computing the partial pair-correlation functions gf (r) for the Cu-Cu, Cu-Nb,
and Nb-Nb correlations [Fig. 5.2 (a)], a well-defined first-nearest neighbor peak can be seen
between 2.5-3 A and a split second neighbor peak is visible at distances 4-6 A. However, by 10
A the pair correlation functions decay to the value expected from the bulk density, demonstrating
that no long-range topological order is present and that the systems are fully amorphous.
The visualized quenched configuration of amorphous Cu 5 oNb5 o in Fig. 5.1(f) has clear
regions of compositional enrichment. To probe for long-range compositional ordering, I compute
the total g (r) out to a distance of r = 4.5 nm and, from it, I compute the total structure factor:
S..(q) = 1 + 4 7'f(g
q fo
(r) - 1) rsin(qr)dr
As can be seen in Fig. 5.2 (b) and expected for glasses, S,,(q) displays interatomic density
correlations at medium q-values [q = 2 - 8 (2w/A)]. However, an anomalous pre-peak signal
can be see at low-q values [q < 2 (2w/A)]. I thus employ the Bhatia-Thornton compositioncomposition structure Sec(q) factor to test if compositional order accounts for the anomalous
pre-peak signal in S. (q).
The composition-composition structure factor Sec(q) is computed as [108]:
Scc (q)
= XAXB1
+
XAXB (SAA
+ SBB
-
SAB)
where So (q) is the structure factor computed for each of the individual partial-pair correlation
functions. The partial structure factors are related to the total structure factor as:
Sun(q)
= XASAA
+
2
49
xAXBSAB
+
SBB
As can be seen in Fig. 5.2 (c), at medium q-values [q = 2 - 8 (2w/A)], the partial structure
factors Sp(q) are qualitatively similar to the total structure factor. However, at low-q values
[q < 2 (2w/A )], Sap (q) are very different. Both ScUcj(q) and SNb-Nb(q) display large,
positive values at q = 0.2 (2w/A), while SCU-Nb(q) has a large negative value at the same q
value. Positive values in Saf (q) above 1 indicate ordering, demonstrating Cu-Cu and Nb-Nb
a
b
41
2+gCu-Cu(r)
+1_+gCu-Nb(r)
3
3
-O-9Nb-Nb (r)
2
C
1
02
4
6
Radial distance (A)
C
60
12
8
1
40,
10
20-,
a.
0O
10
1- A(
1)Nb-Nbi
-40
0.4 IT.J6
q (2~A
0.8
=
21r/q,,,
::: 3.1 nm
Ur
1
M
2
-------- -----
10
6
00.2
4
0
8
8
-2x
6
6
10
0
8
4
d
s)Cu-Nb
20,
2
4
II
11
-
2
2
0
0.2
0.4
0.6
0 (2n/A)
0.8
1
C
6
8
10
0
2
4
6
8
10
q (2 n/A)
q (2;/A)
Fig. 5.2: Topological and chemical ordering in a-CuMoNb 5 o quenched at 1013 K/s. (a) Pair-pair
correlation functions for Cu-Cu, Cu-Nb, and Nb-Nb interactions. (b) Total structure factor,
computed from the total g (r), computed for r values out to r=4.5 nm. (c) Partial structure factors
Sap (q) computed for each of the individual g(r)ap curves in (a), but with radial distances out to
r=-4.5 nm. (d) Composition-composition structure factor factor Scc(q), computed from the partial
structure factors (shown at low q-values in the inset).
2
4
50
compositional ordering at q = 0.2 (2w/A). By contrast, the large negative value in SCU.-Nb(q)
reflects avoidance of Cu-Nb interactions at q = 0.2 (2w/A). These observations are captured
with a single curve, Scc(q). As can be seen in Fig. 5.2 (d), like the partial structure factors,
Sec(q) displays a single, high intensity peak at q = 0.2 (2w/A). To obtain the real-space lengthscale, I compute Ac as Ac ~~2; / qmA , where qmAx is the wavevector at the maximum of Sec(q)
[108], yielding a wavelength of Ac = 27/0.2 ~ 3.1 nm. Thus, it is clear that the pre-peak signal
in S,,(q) is due to the compositional medium range order (CMRO) first identified through
visualization.
Critical quench ratefor glass formation
To determine the critical quench rate for crystallization, I repeat the quenching procedure
over five decades in quench rate, finding that complete compositional demixing occurs for
a
b
10 10 K/s
C
1011 K/s
d
-9nm
1012 K/s
.
Nb atoms
O Cu atoms
101 K/s
1014 K/s
Fig. 5.3: Cu5 oNb5 Ovia molecular dynamics quenching. (a) Quenching at 1010 K/s yields a phase
separated structure with crystallization in the Nb phase. Quenching at 10" K/s and 1012 K/s
yields phase separated amorphous structures, (b) and (c) respectively. Quenching at 1013 K/s and
1014 K/s amorphous structures with interpenetrating networks of compositionally enriched
material, (d) and (e) respectively. All structures shown at 300 K, after quenching from 4000 K
liquid under P = 0 GPa at the indicated quench rate. Atomic structure visualizations performed
with OVITO [87].
51
quench rates below 10" K/s and that crystallization occurs at a quench rate of 1010 K/s. The
clear onset of crystallization at a critical quench rate, coupled with structural analysis of the
quenched structures, demonstrates that rapidly quenched structures are amorphous.
Following the procedure outlined above, I melt a configuration of 50-50 Cu-Nb
(Cu 5 oNb 5 o) at 4000 K and quench to 300 K using stepwise temperature decrements, at quench
rates varying between 1010 - 1014 K/s, with a constant pressure of P = 0 GPa (See Table 5.1
for synthesis procedure for each quench rate). As illustrated by Fig. 5.3, the quenched structures
(300 K) are very sensitive to the applied quench rate. For quench rates between 1010 10" K/s, complete compositional demixing occurs into distinct Cu and Nb regions. Visual
inspection of Fig. 5.3(a) suggests that for the system quenched at 1010 K/s crystallization has
occurred in the Nb region, although the Cu material remains disordered. For quench rates
between 1013 - 1014 K/s, an interpenetrating network of Cu-rich and Nb-rich
material forms,
with the length-scale decreasing with increasing quench rate [Fig. 5.3(d) and (e)].
To quantitatively test for the presence of long-range topological order in the quenched
structures in Fig. 5.3, I compute the pair-correlation function for each configuration. As can be
seen in Fig. 5.4(a), the total g(r) of the quenched configurations changes markedly between the
quench rates of 1010 K/s and 1011 K/s. At a quench rate of 1010 K/s, total g(r) exhibits longrange order, characteristic of a crystalline materials, while the configuration quenched at
ab
4-4.9
3
2
-
1010 K/s
-5
10 K/S
0
0
2
-5. 13
4
5
6
7
8
0
4
,C
= -5.3-
2
3
4
5
6
7
Radial distance (A)
8
-5.5
9
10
500
1000
1500
Temperature (K)
2000
2500
Fig. 5.4: Critical quench rate for crystallization in rapidly quenched Cu oNb o. (a) Pair5
5
correlation functions-g(r)-at quench rates of 1011 K/s (top) and 1010 K/s (bottom). (b)
Potential energy versus temperature in Cu5 oNb5 o quenched from 4000 K liquid to 300 K solid at
rates of 1011 K/s (dashed line) and 1010 K/s (solid line).
52
Quench Rate
Quenched Cu5ONb5O
10" K/s
1012 K/s
CsCI
CsCI
CsCI
(CuNb)
(CuNb)
(CuNb)
48,778
48,778
48,778
4,000 K, 2.5 ns 4,000 K, 250 ps 4,000 K, 25 ps
T
2T
p
40
4
1010 K/s
Initial Structure
Number of Atoms
Liquid Annealing
Temp/Time
Ensemble
Temperature
Decrement/Relaxation
Time
25 K, 2.5 ns
NPT
25 K, 250 ps
NPT
25 K, 25 ps
NPT
Ensemble
_________
Result
Phase separated; Phase separated; Phase separated;
amorphous
amorphous
Nb crystallizes
___
__
1013 K/s
1014 K/s
CsCl
(CuNb)
48,778
3,975 K, 2.5 ps
NPH+Velocity
Rescaling
CsCI
(CuNb)
48,778
3,900 K, 1 ps
NPH+Velocity
Rescaling
25 K, 2.5 ps
NPH+Velocity
Rescaling
100 K, I ps
NPH+Velocity
Rescaling
CMRO;
amorphous
CMRO;
amorphous
____
Table 5.1: Variable quench rate synthesis procedure for 50k atom model glasses and resulting
properties.
10" K/s shows no signs of long-range topological order. Supporting this interpretation, I find
that the potential energy versus temperature profile for the two alloys looks markedly different
[Fig. 5.4(b)], with a sharp discontinuity present in the system quenched quench rate of 1010 K/s,
as expected in the first-order phase transition of crystallization.
Optimizing quench ratefor compositional order
Having established a critical quench rate (10" K/s) for synthesis of amorphous
configurations of Cu5 oNb5o, I next proceed to optimize the quench rate to reproduce the
experimentally observed compositional order in vapor-deposited amorphous Cu-Nb films
[36][36]. Atom probe tomography (APT) reconstructions on vapor-deposited amorphous
Cu55 Nb 45 were reported by Banerjee et al. [36] and composition modulations were quantified by
computing the local composition along the axial distance of a 2 nm diameter cylinder (Fig 2(d) in
100
80
60
0
0 40
20
-Nb
(MD)
-Cu (MD)
50
60
30
40
distance, nm
Fig. 5.5: Length-scale of compositional medium range order (CMRO). Variation of local
composition as a function of distance along a 2 nm diameter cylinder in 300 K amorphous
0
'o
20
Cu5 5Nb 4 5 produced by MD quenching. Compare with Fig. 2(d) in Ref. [36].
53
[36]). Examining the atomic-configurations shown in Fig. 5.3, I see that a rate greater than 1012
K/s is needed to avoid complete compositional demixing. I therefore compare the compositional
order in configurations obtained at 1013 K/s with the experimental data.
To compare directly with the experimental results in Cu5 5Nb 4 5 [36], I quenched a 5M
atom Cu55 Nb 45 structure from a liquid at 5000 K to a 300 K amorphous solid at a quench rate of
1013 K/s, employing the same quench procedure described above. In the 300 K Cu5 5Nb 4 5
quenched amorphous structure, I compute the variation in local composition along a 2 nm
cylinder, 60 nm in length, and plot the results in Fig. 5.5. I compute the local concentration in
intervals of width I nm. Using a composition of Cu5 5Nb 4 5, I am able to confirm the good quality
of our model our by direct comparison to the data reported in [36].
From the amorphous Cu55 Nb 45 strutures obtained with MD quenching, I find that local
composition fluctuates between ~25% and 75% Cu, with a composition modulation length-scale
between 3-5 nm. Banerjee et al. reported maximum copper concentration variations of 25% and
75% with a length-scale of 2-3 nm [36]. The results of Fig. 5.5 are thus in excellent agreement
with the APT data reported by Banerjee et al. and I conclude that my MD synthesis approach
produces amorphous structures with CMRO consistent with the experimental findings.
5.2
Glass transition by gelation in Cu5 oNb 5 O
Having established a critical quench rate (10" K/s) for synthesis of amorphous
configurations of Cu5 oNb 5o, I next use molecular dynamics simulations to show that glass
transition in Cu5 oNb5 o occurs by gelation. At the glass transition, a mechanically stiff, percolating
network of atoms with icosahedral local packing forms at the interfaces between compositionally
enriched regions. This low-energy network halts coarsening of the phase-separated structure and
imparts shear resistance. These features of glass transition are remarkably similar to gelation
processes in polymeric and colloidal gels.
5.2.1
Introduction
The existence of amorphous metals in alloy systems with positive heats of mixing (
AHMIX
>0 ) is surprising in the face of traditional metallic glass design guidelines, which
identify compositions near deep eutectics with negative heats of mixing as the best glass formers
[24, 104]. While phase separating binary systems such as Cu-Nb [36] or Ni-Ag [37] are
admittedly poor glass formers, calorimetry shows that sputter deposited amorphous metals with
54
these compositions exhibit a lower than expected crystallization enthalpy (~
10 kJ mol- 1 ) [36],
suggesting that some form of atomic ordering stabilizes these amorphous solids [25].
Experiments [36] and simulations [37] indeed show that these alloys contain "spinodal-like"
patterns of nanometer-scale compositional enrichment as well as percolating networks of local
icosahedral atom packing [38]. However, the relationship between icosahedral short-range order
(ISRO), compositional medium-range order (CMRO), and glass transition has not been
determined.
Using molecular dynamics (MD) simulations in a model phase separating amorphous
metal alloy-Cu5 oNb 5 o-I show that a percolating network of ISRO forms at interfaces between
compositionally enriched regions and leads to glass transition. Below the glass transition
temperature TG, the ISRO network is mechanically stiff, imparts shear resistance, and halts
coarsening of the CMRO. The ISRO network constrains the dynamics of surrounding atoms and
leads to anomalous diffusion. This ISRO network and its influence on the physical properties of
the system bears striking resemblance to gelation in colloidal systems, in which a systemspanning, dynamically arrested network of locally preferred structures imparts stiffness [109,
110]. I discuss the potential technological implications of these findings for the synthesis of more
conventional metallic glasses.
5.2.2
Methods
The "virtual quenching" model building procedure-detailed in Section 5.1.2-is
employed with an effective quench rate of 1013 K s-1 to produce 48,778 atom amorphous
configurations of Cu5 oNb 5 o. Configurations saved during the quench are annealed for 20 ns to
investigate their thermal stability, while smaller systems (9,826 atoms) were annealed for up to
100 ns to quantify diffusion properties. All equations of motion are integrated with a timestep of
2 fs under periodic boundary conditions in cubic simulation cells. As demonstrated in Section
5.1.2, radial distribution functions confirm that as-quenched and annealed Cu5oNb 5 o structures
are fully amorphous. The temperature dependence of volume and enthalpy shows no evidence of
a first order phase transition.
55
5.2.3
Result -Glass transition temperature is 1500 K
Arrest of compositional order coarsening at glass transition
The Cu5oNb5 o system undergoes a pronounced change in properties between 1500 K and
1600 K. Fig. 5.6(a)-(b) show representative atomic structures obtained after 20 ns anneals.
Atoms are colored by local copper concentration, _, determined by counting the number of
copper and niobium atoms in a sphere of radius 0.7 nm, centered at each atom. Concentration
regions are colored on a gray color scale, with copper-rich regions (F >75% Cu ) colored in light
gray and niobium rich regions (F <25% Cu ) colored in black. Visual inspection of a 1 nm thick,
two-dimensional slice of the annealed structures at 1400 K and 1600 K reveals pronounced
differences in the length scale of local composition fluctuations. At 1400 K, CMRO varies
between 25 - 75% Cu with a characteristic length-scale 2C = 4 nm (see Section 5.1.2 for
calculation of Ac). At 1600 K, however, 2C =7 nm, suggesting that the CMRO length-scale is
approaching the simulation cell dimension. As shown in Fig. 5.7(a), )c increases sharply when
the annealing temperature exceeds 1500 K. Similar results are found in annealed Cu5oNb 5 o
systems with different simulation cell sizes ( LO = 5.4 nm and LO = 12.8 nm ). The weak
temperature dependence of Ac below 1500 K shows that the atomic mobility required for
diffusion and coarsening of the compositionally patterned structure is sharply reduced in the
temperature range 1500-1600 K. Thus, I conclude that TG is between 1500 K and 1600 K.
Previous simulations of the phase separating system Ni-Ag reveal the emergence of a
percolating network of ISRO below TG, in addition to stable, nanometer-scale CMRO [38].
Thus, I seek to establish whether ISRO networks might play a role in stabilizing CMRO at the
glass transition. Following Luo et al. [38], I use common neighbor analysis (CNA, described in
detail in Section 3.3.2) to probe for topological order in the Cu5oNb 5 o system. Consistent with the
results in Ni-Ag [38], below TG,
icosahedral local packing-
I find a system-spanning network of atoms with fully
atoms having a 5-5-5 CNA index with 12 first nearest neighbors-
centered on the smaller atom (Cu) and with neighboring atoms a mixture of Cu and Nb. A cutoff
radius of 0.35 nm is used in the CNA calculation.
56
1400 K
(a)
1600 K
(b)
(c)
(d)
(e)
(f)
.3nm
3
1.5
2
1
1
0.5
Z> 75%Cu
c < 25%Cu
40 < e < 60%Cu
.0
-2
-1
0
1
2
-2
r [nm]
-1
0
1
r [nm]
2
Fig. 5.6: Visualizations of Cu5 oNb5 o at 1400 K (left) and 1600 K (right) after 20 ns of annealing.
(a and b) A 1 nm thick slice is shown with Cu-rich regions colored light gray and Nb-rich
regions colored black. (c and d) Atoms at the CMRO interface, 40 < J < 60% Cu, in the slice of
the top panel are visualized. Atoms participating in ISRO packing are colored red and
emphasized with a 20% larger radius. Bonds between ISRO nearest neighbor atoms are colored
red. (e and f) Probability paff"(r) of finding an atom in the icosahedra network at distance r
from CMRO interfaces.
Following Cheng et al. [98], the fraction of atoms contained in either the center or
vertices of icosahedra is denoted f"'"". As shown in Fig. 5.7(b), f t '"" rises sharply at TG .
Furthermore, the spanning length Lc,0 of the largest cluster of
fi'""" atoms
possible in a simulation cell under periodic boundary conditions,
has the largest value
V3 / 2LO
, at temperatures
below TG, demonstrating that system-spanning clusters have formed in the system [Fig. 5.7(b)].
57
(b)
(a)
8
6i
0.4
TG -
0.75
.0.3
0.5
0.15
-15-0-1000 1500 2000 2500
(c) 4
4
0.25
*
900 1000 1500 2000 2500
(d)
1.21
0.9
2 2
::
0.6
=
0.5
0.
%0 1000 1500 2000 2500
00
1500- 2000 2500
Teiiiperattire [I]
Temiperature [K]
Fig. 5.7: Properties in annealed Cu oNbso.
Temperature
dependence of (a) CMRO wavelength
5
Ac; (b) percent of atoms in full icosahedra f"'" and size of largest ISRO cluster divided by
simulation cell edge length L = L / LO ; (c) flow stress U-F ; and (d) diffusion exponent n . The
vertical lines at 1500 K correspond to the glass transition temperature. All quantities computed
after 20 ns annealing at indicated temperature.
The spanning length LC0 is the radius of the largest sphere necessary to contain all atoms in a
cluster of f"'"' atoms [Ill].
Icosahedra network concentrated at interfaces between compositional order
Atoms with icosahedral order (fO'"
atoms) form a continuous, percolating network
below TG and are concentrated at the interfaces between Cu and Nb-rich regions [Fig. 5.6 (c)(d)], clearly demonstrating a coupling between CMRO and ISRO in Cu oNb o. In Fig. 5.6 (c)5
5
(d), I visualize all atoms at the CMRO interfaces (40% < C < 60% ) contained in the planar view
in Fig. 5.6 (a) and (b). Atoms at the CMRO interface with icosahedral order are colored
red and
emphasized with a 20% larger radius. Bonds between ISRO nearest neighbor atoms are colored
red. At 1600 K, the CMRO interface contains only isolated icosahedra in Fig. 5.6 (d). By
contrast, at 1400 K, the icosahedra form a connected network on the CMRO interface, Fig. 5.6
(c). Distributions of icosahedra as a function of distance from interfaces p""'"(r) are plotted in
Fig. 5.6 (e)-(f) and quantitatively demonstrate that ISRO order is concentrated at the CMRO
58
interface, both below and above
TG
. The total fraction of icosahedra increases by a factor of
-
3
as temperature decreases from 1600 K to 1400 K [Fig. 5.7 (b)] and p"""(r) shows a clear
increase in the concentration of icosahedra at the CMRO interfaces at a temperature of 1400 K
[Fig. 5.6 (f)] reflecting the visual observation in Fig. 5.6 (c) of a connected network of icosahedra
on the CMRO interfaces at 1400 K.
The presence of icosahedra at CMRO interfaces lowers the energy of the system and
stabilizes CMRO against coarsening. I compute the enthalpy of formation of icosahedra,
FO,,
=-19 kJ mol- . My method for computing the enthalpy of mixing is
and find AHRMc
illustrated in Fig. 5.8. First, I compute the average local concentration and corresponding
potential energy for atoms in the Nb-rich (U < 40% Cu ), CMRO interface (40% < < 60% Cu ),
Cu-rich regions (U > 60% Cu ), and J""" atoms. Local concentration for each atom is computed
as average over the atoms within a sphere of radius 0.5 nm centered at each atom. The potential
energy for each atom is obtained after minimizing the potential energy of the configuration via a
Fit, Regions w/o Ico
-3- *Ico
-0-Data, Regions w/o Ico
---
-5,_
-6
-7
0
-
25
-''
AH
.m= -19 kJ/mol
50
% Cu
75
100
Fig. 5.8: Variation of potential energy as a function of local composition in 300 K amorphous
Cu5 oNb5 o. Open data points correspond to the average local composition and potential energy in
the Nb-rich (triangle), interface (squre), and Cu-rich region (diamond). Dashed line is the
interpolated value of potential energy versus local concentration based on the values of the Nbrich and Cu-rich data points. Filled star corresponds to the average local composition and
potential energy for ISRO atoms. Uncertainty on the mean values reported of order of the size of
the symbols and therefore not shown.
59
steepest descent energy minimization. Second, I extrapolate the potential energy versus local
concentration based on the terminal values obtained for the Nb-rich and Cu-rich atoms. The
independently computed average local composition and potential energy for CMRO interface
atoms falls exactly on the interpolated line, demonstrating the utility of this interpolation.
Finally, I compute the difference in potential energy between the average value for f""" atoms
and the corresponding potential energy at the same composition. I find that
J;"o,"""atoms
are on
average 19 kJ mol- 1 lower in energy than non-icosahedral atoms at the same local composition,
demonstrating that the formation energy of icosahedral atoms is negative. Although the
equilibrium state of the system is phase separated, with a positive heat of formation, the
icosahedra network has a negative heat of formation with respect to CMRO interfaces, thus
stabilizing the compositionally patterned structure. Coarsening would reduce the area of CMRO
interfaces and therefore also the number of atoms in the icosahedra network, causing-at least
initially-a net rise in energy.
Admittedly, the determination of AHF5OJRm relies upon multiple binning and averaging
steps. While the computed values of potential energy and local composition [Fig. 5.8] have
uncertainty on the mean values on the order of the symbols themselves, suggesting that the value
found for AH"O'Rm is statistically significant, a more direct approach to validating the stability of
icosahedra might be found through studying the energy of individual icosahedra clusters, as a
function of varying composition. This approach has the potential to demonstrate the importance
of relative size ratios of Cu and Nb atoms for icosahedra formation. This alternative approach is
a promising research opportunity and left for future study.
5.2.4
Result - Glass transition mechanism is gelation
Icosahedral network is mechanically stiff
Previous studies in miscible metallic glasses demonstrate that icosahedra form a
mechanically stiff "elastic backbone" [112], that icosahedra are less prone to irreversible
rearrangements under elastic loading than non-icosahedral atoms [113], and that the glass
transition coincides with the percolation of mechanically stiff material [45]. Consistent with
these findings, I find that the steady state flow stress rises abruptly below 1500 K, as shown in
Fig. 5.7(c), demonstrating that below the glass transition there is a strain range within which
60
CusoNb 5 o deforms elastically. The flow stress is computed under volume-conserving deformation
(extension in z and equal contractions in x and y directions) at a strain rate of tz = 2 x 10 9 s' in
strain increments of Aeg =2x 10-4, followed by 0.1 ps NPT ensemble MD run between each
strain step. Additional simulations at strain rates as low as tz =2 x 10' s- yield similar results
for the temperature dependence of the flow stress. I therefore test whether the icosahedra
network described here is in fact a load-bearing, "elastic backbone" below the glass transition.
Below the elastic limit, the potential energy of the icosahedra network in the deformed
configurations (.e
= 2 x 109 s') exhibits the harmonic dependence on applied strain expected of
linear elastic solids, as shown in Fig. 5.9. In contrast, the potential energy of atoms outside the
icosahedra network is nearly strain-independent, except in the initial stages of loading, when it
actually decreases due to local, irreversible relaxations. I compute these changes in potential
energy with respect to a configuration at zero applied strain and identify icosahedral atoms from
their strain-free CNA type. While the initial deformation is applied uniformly to the system, the
MD relaxation allows all atoms to undergo independent displacements that reduce the total
energy of the system. To remove thermal noise, potential energies are calculated after steepest
descent potential energy minimization.
A
U
CD
1000
1100
@1200K
v 1300K
1400K
W7
2.5
>_
vvy
o.30AA.
AA
AA
000 0
-
-2.5,-VVV
-3
-2
-2
8(
O
-1
0
1
-1
0
1
0 0
2
3
Ezz x 100%
Fig. 5.9: Harmonic elastic response of icosahedra network. Change in potential energy for the
icosahedra network and non-icosahedral atoms (closed and open symbols, respectively) as a
function of applied strain e. below the elastic limit.
61
The nearly strain-independent potential energy of non-icosahedral atoms demonstrates
that atoms outside the icosahedra network accommodate applied strain through liquid-like,
inelastic relaxations [Fig. 5.9]. In contrast, the increase in potential energy with strain for the
icosahedral atoms is only possible if these atoms form a connected, load-bearing network. If
icosahedral clusters were disconnected and embedded in the liquid-like material, strain would be
accommodated through the relaxation of the liquid-like matrix and no energy increase would
occur. Therefore, I conclude that the icosahedra network is connected and load-bearing.
Icosahedral network deforms reversibly
The increase in potential energy of the icosahedra network in Fig. 5.9 is consistent with
elastic deformation. However, a definitive claim of elasticity requires demonstration of
reversible deformation. First, I show that the energy changes observed in Fig. 5.9 cannot be
accounted for by destruction of icosahedra. Second, I show that atomic displacements and
potential energy changes are reversible under deformation. Similar to the procedure in [113], to
demonstrate that the deformation of the icosahedra network is reversible, I deform a zero strain
configuration at 1400 K (denoted Cl) to a prescribed applied strain EAPP at a rate of
ez = 2 x 109 s-' and subsequently unload the deformed configurations to 0% strain (denoted C2)
at the same strain rate.
In Fig. 5.10(a), I plot the ratio between the fraction of icosahedra f"""" at applied strain
Ztof atoms
En t
'Co
falm~satm
at Ez, = 0. I find that "'"
changes less than 5% up to global yield and f""i"
recovers completely upon unloading [Fig. 5.10]. The change in the fraction of icosahedra is less
than 5% up to the global yield strain, suggesting that changes in the fraction of icosahedra are not
responsible for the measured increases in potential energy. In Fig. 5.10(b), I plot the ratio
between
atoms
in Cl and C2
as a function of applied strain
f
atoms
8
AP.
~atoms (l
(C)1.
icosahedra are found in the two configurations, f"'o""(C2)/
If the same number of
.U
otegoa
il
Up to the global yield
strain, I find that any changes in fiaO"" [Fig. 5.10(a)] are completely reversible.
The average (a) displacement magnitude IAr I and (b) potential energy difference APE
between C1 and C2 icosahedral atoms are computed after steepest descent potential energy
minimization of each configuration, with icosahedra identified based on their type in the C1
configuration. For perfectly reversible deformation, I expect both of these quantities to equal
62
(a)
(b)
0.95
"-,0.95
0.9
0.9
N
S0.85
.83
____
0.8 0.025 0.05 0.075
IfZZ
0.1
0
0.025 0.05 0.075 0.1
'A 'i'
Fig. 5.10: Variation in the fraction of icosahedra f"'""" in Cu 5 oNb o deformed at 1400K. (a)
5
Ratio of f"" at applied strain EZ tof ao"" at ea = 0. (b) Ratio of f'0"" between two zerostrain configurations, Cl and C2. Cl is the initial, zero strain configuration. C2 is the final
configuration after Cl has been loaded to a total strain of e,, at ta = 2 x 109 s' and unloaded
to zero strain at the same rate. Error bars represent the uncertainty on the mean value, determined
by averaging over 5 (a) and 30 (b) independent simulations.
zero. The mean value and uncertainty were computed by repeating the calculation with
30
independent initial configurations at 1400 K.
As shown in Fig. 5.11(a) and 4(b), both IAr I and APE for icosahedra atoms are small (
IAr 1<0.03 nm and APE < 0.005 eV/atom , respectively) for eAP, <EY and only increase
markedly after the onset of global yielding. The reversible displacements demonstrate that
the
deformation of the icosahedra network is elastic. The reversible changes in potential energy
demonstrate that the network is mechanically stable and stiff. On the basis of Fig. 5.10 and Fig.
5.11, I therefore conclude that the icosahedra network is load-bearing, elastic, and mechanically
(a)
(b)
6
2
74
1
0
0
0.05
( APIP
0.1
0
i 0
0.05
0.1
f APP
Fig. 5.11: Reversible deformation. (a) Average displacement magnitude IAr I and (b) average
difference in potential energy APE between two zero-strain configurations, Cl and C2, as
a
function of applied strain E,, (see text for details). The vertical lines indicate the global yield
strain. Error bars represent the uncertainty on the mean value, determined by averaging over 30
independent simulations.
63
stiff. It is responsible for the stiffening of the system below the glass transition temperature.
Icosahedral network constrains diffusion dynamics
The presence of the load-bearing, elastic, mechanically stiff icosahedra network at the
CMRO interfaces should prevent atoms from passing through it, effectively restricting diffusion
to CMRO regions. Visual inspection of the CMRO region geometry suggests that the CMRO
regions are system-spanning, interpenetrating ligaments. Consistent with the idea of an
impermeable diffusion barrier at the CMRO interfaces that restricts diffusion to a fractal
subspace, I find the diffusion exponent [Fig. 5.7(d)] is sharply reduced at the glass transition,
with diffusion exponent n ~0.5 for temperatures between 700
T
1400 K .
The Mean-Squared Displacement r 2 (t) (MSD) plot for a typical supercooled liquid
plateaus during cage breaking, before converging to the long-time limit of Browning motion,
r 2 (t)
Oc
t" where n = 1 [60]. A diffusion exponent of n <1 after cage breaking is therefore an
indication of anomalous diffusion [114]. To compute the diffusion exponent n , I perform
constant temperature and pressure (P = 0 GPa) NPT ensemble anneals in a 9,826 atom Cu5 oNb5o
system for times up to 100 ns and performed linear fits of the form log 10[ r2 (t)I = n log 101 t + B
to the measured r 2 (t) curves in a fitting interval of a lower and upper time [Fig. 5.12]. The
lower time bound is set at a time after cage breaking. Because annealing at temperatures above
the glass transition yields complete phase separation after a sufficient annealing time, the upper
bound time is set by a time prior to complete phase separation.
In Fig. 5.12, I plot the MSD r 2 (t), where r 2 (t) is measured as the average for all atoms
with respect to the initial configuration. I compute r 2 (t) at all temperatures between 500 K and
2500 K in 100 K increments. Visual inspection reveals that at high temperatures (T > 1700 K),
the MSD transitions from a ballistic diffusion regime, r 2 (t)
Mt 2
, to Brownian motion, r 2 (t)
c
t',
in times less than 10 ps. At temperatures below the glass transition temperature (T < 1500 K), a
distinct plateau in r2 (t) emerges, consistent with previous reports of cage breaking [60]. Visual
inspection reveals that the cage-breaking regime extends to times of approximately 100 ps for
500 K. Beyond the cage-breaking regime, the MSD displays anomalous diffusion, i.e. r 2 (t)
where n <1.
64
Oc
t"
10
10
5
4
3n=1
2500 K
2
10
10
10~
n
2
10
10 2 10~ 100
101
102
103
10 4
10
t [PS]
oNb
of
annealed
Cu
Fig. 5.12: Diffusion behavior
5
5o. Variation of mean-squared displacement
with temperature for 9,826 atom Cu5oNb5o annealed at temperatures between 500 K and 2500 K
(100 K increments). The cage breaking interval is shaded in blue; the fitting window is shaded
in cyan; and the phase separation interval is shaded in red.
5.2.5
Discussion - Glass transition by gelation
A liquid-to-solid transition due to the formation of a system-spanning, load-bearing
network in a phase separating liquid mixture is the canonical description of gelation [109].
the
Gelation is common in colloidal [109] and polymeric systems [115]. Similar to Cu oNbso,
5
percolating network that leads to gelation in some colloidal systems consists of particles packed
in a preferred topology [110]. The formation of a system-spanning, load-bearing network of
icosahedra along interfaces between compositionally enriched regions, coincident with the
abrupt arrest of coarsening and increase in system flow stress, shows that glass transition in
Cu5 oNb5 o may also be described as a liquid-gel transition in a phase separating metallic-rather
than colloidal or polymeric-liquid.
Gelation has not been used to describe glass transition in more conventional metallic
glasses composed of compound-forming elements. Several previous findings, however, suggest
that such a description may be warranted in some cases. Icosahedra have been identified as the
most common form of structural short-range order in several metallic glasses [33, 34, 116] and
correlated with low mobility atoms at temperatures near the glass transition [117]. Dynamic
heterogeneity has been shown to couple to composition in such materials [118]. Icosahedra are
65
the building blocks of system-spanning networks in these metallic glasses [35, 98]. Finally, I
again note that icosahedra have been demonstrated to be mechanically stiff [112], that icosahedra
are resistant to irreversible rearrangements under loading [113], and that glass transition has been
correlated with percolation of mechanically stiff phases [45].
The Cohen-Grest free-volume theory for glass transition predicts that glass transition
occurs due to the percolation of a "solid-like" phase within an otherwise "liquid-like" (i.e. high
free volume) material [119]. This theory makes direct use of percolation theory, and therefore
predicts that the glass transition is a first-order transition. My identification of glass transition by
gelation, due to percolation of stiff icosahedra, thus bears some similarity to assumptions in the
Cohen-Grest theory. While my results do not suggest a first order phase transition, additional
investigation of percolation of icosahedra clusters in the Cu 5 oNb 5o system at the glass transition,
compared to the predictions of the Cohen-Grest theory, is a promising research direction for
future study.
CMRO-albeit more compositionally complex than that in amorphous metal alloys with
positive heats of mixing-has also been observed in bulk metallic glasses, such as
Zr 4 1.2 Ti 13 .8Cu 12.5 NijoBe 2 2 .5 (Vitreloy 1) quenched at 10 K s-1 [120, 121]. In addition to quench
rate [122, 123] and annealing time near TG [121], the length-scale and morphology of such
compositionally enriched regions are thought to reflect the proximity of TG to a critical
temperature below which spinodal decomposition may occur [124]. Because secondary phases
generally arrest shear band propagation and improve mechanical toughness [42], metallic glasses
with tailored composition modulations are of technological interest. My finding that CMRO
couples with ISRO suggests that altering the ISRO network by chemical means may provide a
route to controlling CMRO in these materials, thereby influencing their mechanical properties.
5.3
Glass transition temperatures in Cu2 sNb 7 5 , Cu5 0 Nb5 O,and Cu75Nb 25
The successful identification of the glass transition mechanism in Cu 5oNb 5o [39] provides
the methods needed to characterize the glass transition in other compositions in the Cu-Nb alloy
system. Here, I report the glass transition temperature in Cu 25Nb 75 and Cu75Nb 25 and provide
values from Cu 5 oNb5 o for comparison. I find that the glass transition temperature increases with
increasing Nb content, with TG = 1400 K for Cu 7 5 Nb 2 5, TG = 1500 K for Cu5 oNb5 O, and
66
9 nm
d
C25 Nb75 1700 K
C25 Nb 75 1500 K
CsoNbso 1600 K
e
f
CsoNbso 1400 K
C75 Nb 25 1500 K
C75 Nb 25 1300 K
Fig. 5.13: CMRO versus temperature and composition. Visualization of alloys (Cu Nb ,
25
75
Cu5 oNb 5 o, and Cu75 Nb 25 at top, middle, and bottom, respectively) following 20 ns annealing. A 1
nm thick slice is shown with Cu-rich regions colored light gray and Nb-rich regions colored
black.
TG =
1600 K for Cu 25 Nb 75 [Fig. 5.14(d)]. These glass transition temperature values are utilized
for subsequent analysis of the irradiated Cu 25Nb 75 and Cu 75Nb 25 systems.
5.3.1
Length-scale of compositional order
The variation of compositional medium range order (CMRO) with temperature was
studied in the three alloy systems-Cu2 5Nbs, Cu oNbso,
5
and Cu 75Nb 2 5 -between
the
temperatures of 1000 K and 2000 K by annealing quenched (1013 K/s) liquid atom configurations
(50k atoms) for 20 ns. As can be seen below in Fig. 5.13, pronounced changes in the length-scale
of CMRO are found in a relatively narrow temperature window (200 K) for all three alloys. For
Cu2 5Nb 7 5, this transition occurs between 1500 K and 1700 K; for Cu5 oNb 5 o, this transition occurs
between 1400 K and 1600 K; and for Cu 75Nb 25 this transition occurs between 1300 K and 1500
K. Within a representative 1 nm slice, the CMRO is visualized by coloring each atom by its local
Cu concentration, j, computed as the fraction of copper atoms within the sphere of radius 0.7
nm, centered at each atom.
67
a
b
10
3[
QQ"75
2.5
8
71
6.
400
1200
160
c
1600
1800
so
io
2000
-
60
1800-200
d
0..4
0.5r
iRo
-1700
1600
4
0.3r
1500
0.2
E"
1400
0.1
1000
1200
1400
1600
Temnperatur (K)
1800
2000
1300
Cu25Nb75
Cu50Nb5O
Cu75Nb25
Fig. 5.14: Variation of properties with composition and annealing temperature. (a) Variation of
the length-scale of compositional medium range order (Ac) with temperature. (b) Variation of the
flow stress with temperature. (c) Fraction of atoms in full icosahedra as a function of
temperature. (d) Glass transition temperature as a function of composition. All values computed
after annealing for 20 ns at the indicated temperature.
Supporting this visual analysis, I find that the temperature-dependent CMRO length-scale
(Ac) diverges in a similar temperature window. At the conclusion of each of the annealing runs,
the radial distribution function was computed and the composition-composition structure factor
was subsequently calculated. The characteristic length scale of compositional medium range
order (CMRO) was computed from the maximum in the structure factor and plotted as a function
of annealing temperature. As can be seen in Fig. 5.14(a), the temperature-dependence of the
CMRO length-scale (Ac) is weak below a critical temperature (Tc), suggesting that the Tc signals
the onset (upon cooling) of a temperature range where the atomic mobility required for diffusion
and coarsening of the compositionally patterned structure is sharply reduced.
Following the procedure discussed above, I identify the range in which Ac sharply
decreases with decreasing temperature. For Cu 2 5Nb 75, coarsening of Ac is reduced in the
temperature range of 1600 -1800 K. Based upon visual inspection of annealed structures, I
68
identify the onset of coarsening at 1600 K and take TG = 1600 K. For Cu5oNb 5o, Ac is sharply
reduced in the temperature range 1500-1600 K and I take TG = 1500 K. Finally, for
Cu7 5Nb 2 5 , Ac is sharply reduced in the temperature range 1300-1500 K and I take TG = 1400 K.
5.3.2
Flow stress
Following the 20 ns anneal, each alloy was deformed under volume-conserving
deformation at a rate of 109 1/s to a total von Mises tensile work equivalent strain of 22% for
temperatures between 1000 K and 2000 K. At each temperature, the von Mises stress was
computed as a function of strain and the flow stress was found as the average of the von Mises
equivalent stress between 20-20% strain. In Fig. 5.14(b), the flow stress is plotted as a function
of temperature for each alloy. Above a critical temperature, the flow stress is low (af <
0.5 GPa). This transition temperature distinguishes liquid-like and solid-like flow and therefore
should be coincident with the glass transition temperature.
As expected, I find transition temperatures that are exactly the same as the glass
transition temperatures identified on the basis of coarsening of compositional order. For
Cu7 5Nb 2 5, T - 1400 K; for Cu5oNb 5o, T ~ 1500 K; and For Cu 2 5Nb75 , T = 1600 K. These
values build confidence in the identified glass transition temperature.
5.3.3
Icosahedral short-range order
Icosahedral order has been identified in numerous amorphous metal systems as the most
common form of short-range topological order and the fraction of atoms in full-icosahedra has
been shown to increase markedly at the glass transition. It is therefore of interest to investigate
the temperature dependence of the fraction of atoms in full-icosahedra (at the center and in the
first nearest neighbor shell). As shown in Fig. 5.14(c), the fraction of atoms in full icosahedra
increases dramatically for all three alloys between 1600 K and 1400 K, consistent with my
identification of the glass transition for all three alloys in this temperature range.
5.3.4
Thermal expansion
The linear thermal expansion coefficient was computed for the three alloys systems from
the thermodynamic output of the 1013 K/s quench of the V2 billion atom systems (see Section 5.5)
used in the collision cascade simulations. In Fig. 5.15(a), I plot the temperature dependence of
thermal expansion. There are two key trends that emerge from the thermal expansion data: (1)
thermal expansion is highest in systems rich in copper (Cu 75Nb 2 5) and lowest in the niobium rich
alloy (Cu 25Nb 75 ); and (2) the inflection point in the thermal expansion versus temperature curve
69
follows the previously identified trend in the critical temperature of the CMRO length-scale, with
a systematic increase in the inflection point temperature (TI) with increasing niobium content.
For Cu 75Nb 25, T, = 1200 K; for Cu5 oNb 50 , T, = 1400 K; and For Cu 25Nb 75, T, = 1500 K. The
correspond of the inflection point temperature in the thermal expansion with the temperature
dependence of the CMRO length-scale supports the identified glass transition temperatures.
The thermal expansion is computed as the first derivative (slope of the linear fit to a
temperature interval of 100 K centered at the temperature of interest) of the system cell length
with respect to system temperature. The inflection point is computed as the temperature at which
the second derivative of thermal expansion with respect to temperature is zero (centered finite
differences, with a temperature interval of 200 K).
5.3.5
Heat capacity
Like the linear thermal expansion, the constant-pressure heat capacity (Cp) was computed
a
b
-
_
40
-
Cu25Nb75
Cu5ONb50
30
--
Cu75Nb25
x 10"
0.5
/,0
Thih..
W-
A
V
201
S10
-1
1000
2
3"1000
2000
Temperature (K)
c
d
40
1
35
0
3025-
3M0
Temperature (K)
/I_
*'
Cu25Nb75
-2
---- Cu50Nb50
---
Cu75Nb25
1000
2000
3000
Temperature (K)
1000
2000
3000
Temperature (K)
Fig. 5.15: Variation of properties with composition and temperature. (a) Variation of thermal
expansion with respect to temperature for three alloy systems. (b) Temperature dependence of
the second derivatives of the curves in (a). The inflection point is where the second derivative
equals zero. (c) Variation of heat capacity with respect to temperature for three alloy systems. (d)
Temperature dependence of the second derivatives of the curves in (c). The inflection point is
where the second derivative equals zero.
70
for the three alloys systems from the thermodynamic output of the 1013 K/s quench of the
/2
billion atom systems used in the collision cascade simulations. From classical thermodynamics,
CP = d'T
where H = U + PV. The quench was performed under zero pressure and to good approximation
is nearly zero throughout the quench. I therefore neglect the PV term and consider only U =
PE + KE, where PE is the system potential energy and KE is the system kinetic energy. In Fig.
5.15(b), I plot the temperature variation of the heat capacity for the three alloys. The temperature
dependence is very similar to that found in for the linear thermal expansion.
5.4
Properties of amorphous Cu 2 5 Nb 75, Cu5 0 Nb5 0 , and Cu7 5 Nb 25
Following the successful characterization of the glass transition temperatures in
Cu 2 5Nb75 , Cu5 oNb 5o, and Cu 75 Nb2 5, I next probe the mechanical properties of these systems in
both the quenched (1013 K/s) and relaxed (~0.5 ns below the glass transition temperature)
systems. The synthesis procedure and measured properties are summarized below in Table 5.2. I
subsequently employ the computed elastic constants and yield stresses for analysis of plastic
deformation in irradiated metallic glasses (Chapter 6 and 7), as well as for predicting the
radiation damage resistance of these materials (Chapter 7).
71
5.4.1
Elastic constants
The elastic constants of each configuration are computed using the stress-strain response
during quasi-static loading at 0 K. After setting the temperature of each alloy to 0 K, I relax the
systems to a stress free state using a combination of deformation and energy minimization. I
subsequently deform the system uniformly with uniaxial tensile deformation in the z-direction in
small strain increments (AEzz = 10-), with periodic boundary conditions applied in all three
directions and the strain in the x and y-directions set to zero. Following the application of each
strain increment, I relax the energy of the system with steepest descent energy minimization. As
an example, in Fig. 5.16(a), I plot the system stress as a function of applied strain for quasi-static
deformation the as-quenched Cu5oNb 5o system at 0 K. As expected, the system as whole has a
linear stress-strain response, and the stress response a , and ou,
Initial
Structure
Numbers
C
Quenched
Cu25Nb75
Cu3Au
(Nb3Cu)
48,668
Relaxed
Cu25Nb75
Cu3Au
(Nb3Cu)
48,668
Quenched
Cu50Nb5O
CsCI
(CuNb)
48,778
Relaxed
Cu50Nb5O
CsCl
(CuNb)
Quenched
Cu75Nb25
Cu3Au
(Cu3Nb)
Relaxed
Cu75Nb25
Cu3Au
(Cu3Nb)
48,778
48,668
48,668
4000 K
1,000 ps
4000 K
1,000 Ps
4000 K
1,000 ps
4000 K
1,000 ps
4000 K
1,000 ps
4000 K
1,000 ps
101 K/s
101 K/s
101 K/s
10'
K/s
101 K/s
10'
-
1500
--
1200
--
1250
--
500
--
400
--
500
Liquid
Tealing
Temp/Time
Quench Rate
Anal
< Temp [K]
Annealing
o
p [g cm 3 ]
PE [eV/atom]
a [10~6 1/K]
C, [J/(mol K)]
ay [GPa]
Cy
E [GPa]
TG[K]
K/s
____
Time [ps]
Properties
Cl I [GPa]
C12 [GPa]
p [GPa]
[GPa]
p [g cm~]
are approximately equal. The
500__400_500
Quenched
Relaxed
Quenched
Cu50Nb5O
Cu25Nb75
Cu25Nb75
229.3 ± 0.1 231.5 0.1 227.4 0.1
131.3 + 0.04 131.4 + 0.06 130.8 ± 0.03
49.0 ± 0.1
50.0 + 0.1
48.3 ± 0.1
131.3 + 0.04 131.4 + 0.06 130.8 ± 0.03
8.2575
8.287
8.282
Relaxed
Cu50Nb5O
228.6 + 0.1
131.5 ± 0.1
48.6 ± 0.2
131.5 0.1
8.2642
Quenched
Relaxed
Cu75Nb25
Cu75Nb25
212.1 ± 0.1 215.3 0.1
0.03
129.7 ± 0.1 130.1
41.2 ± 0.2
42.6 ± 0.1
129.7
0.1 130.1 ±0.03
8.324
8.320
Quenched
Relaxed
Quenched
Relaxed
Quenched
Relaxed
Cu25Nb75
8.2347
-6.3259
15.3
Cu25Nb75
8.2415
-6.3325
15.3
Cu50Nb5O
8.1958
-5.3696
20.4
Cu50Nb5O
8.2039
-5.3772
20.4
Cu75Nb25
8.2337
-4.4101
25.5
Cu75Nb25
8.2387
-4.4171
25.5
35.9
3.51
0.0377
98.4
Quenched
Cu25Nb75
1600
35.9
3.31
0.0325
108.3
Relaxed
Cu25Nb75
1600
34.9
2.97
0.0315
100.8
Quenched
Cu50Nb5O
1500
34.9
3.05
0.0307
106.5
Relaxed
Cu50Nb5O
1500
35.6
2.37
0.0272
94.3
Quenched
Cu75Nb25
1400
35.6
2.51
0.0286
94.5
Relaxed
Cu75Nb25
1400
Table 5.2: Synthesis procedure for 50k atom model glasses and resulting properties.
72
linear fit of oxx = mEz + B yields C12 = m, while the linear fit of oz = mezz + B yields
C11 = m. Similar calculations were performed in the other five systems and the resulting
properties are tabulated in Table 5.2.
5.4.2
Yield stress
I compute the finite temperature yield stress at 300 K using volume-conserving
deformation with strain increments of AEVM = 2 -10-4,
followed by molecular dynamics
relaxation for At = 0.1 ps, for an effective strain rate of e = 2 - 10' 1/s. As illustrated in Fig.
5.16(b), the yield stress is determined from the 0.2% strain offset, and for a-Cu5oNb5o deformed
at 300 K, found to be o = 100.8 GPa. Similar calculations were performed for the other alloys
and the results are tabulated in Table 5.2.
5.5
Synthesis of % billion atom amorphous alloy configurations
To probe the radiation response of metallic glasses at ion irradiation energies of
/2 MeV
(Chapter 6 and 7), it is necessary to synthesize 3-dimensional atomic configurations with
simulation cell edge length of -200 nm (see Section 6.1), yielding configurations with
-2
billion atoms. Running LAMMPS [94] on the unclassified BlueGene/L (uBGL) supercomputer at
Lawrence Livermore National Laboratory with 16,384 cores, I create crystalline configurations
of Cu 2 5Nb 7 5 , Cu5 oNb 5 o, and Cu 75N 25 with the initial structures indicated in Table 5.3. Following
a
b
0.8
6
--
/
0.6- o
0.4 ---
3
02
S2-
c2=3.0 GPa
E(300 K,2E9 1/s)
=100.8
GPa
U),
0 00 1Strain,
E
0.002
0.003
00
0
0.05
.0.2
Stran, EvM
Fig. 5.16: Atomistic calculation of mechanical properties of of a-Cu5 oNb5 o. (a) Stress versus
strain computed under quasi-static uniaxial tension. Solid lines indicate linear fits. (b) Von Mises
stress versus work equivalent strain under volume conserving deformation at 300 K with a strain
rate of e = 2 - 10' 1/s. Solid line indicates 0.2% strain offset.
73
the rapid quenching procedures described above in Section 5.1, I first melt the material at 4000 K
and subsequently quench the liquid at an effective quench rate of 1013 K/s using stepwise cooling
in 25 K decrements and 2.5 ps equilibration runs to 300 K, with a velocity rescaling thermostat
and Nose-Hoover NPH barostat. Relaxed configurations are prepared by selecting an asquenched configuration at the desired annealing temperature and relaxing with NPT annealing at
the chosen temperature for -0.5 ns. Following annealing, the relaxed configuration is quenched
to 300 K at 1013 K/s. This approach yields six unique atomic configurations, enabling an
investigation of the role of composition and structural relaxation on the radiation response of
metallic glasses. The exact synthesis procedure and resulting alloy properties are summarized for
the six configurations in Table 5.3.
To validate that the
/
billion atom models are in-fact amorphous, I compare the
thermodynamic output during quenching to the output obtained during quenching the smaller,
50k atom configurations. As example, I show the potential energy (a) and volume per atom (b) as
a function of simulation time for both a 50k atom and 474M atom configuration of quenched in
Fig. 5.17. On average, the output is identical, giving confidence that the properties of the 474M
atom configuration are the same as the 50k atom configurations. On this basis, I therefore
assume that alloy properties computed in 50k atom configurations (reported in Table 5.2) are
representative of the properties of the
-4.4
'-
9 y.0
'2'
-5.075
E0
-4.
billion atom alloys.
E
-5.0
21
16.84
16.8
16.x
.085-
-4.8
16.88
-5-4
18
40
242
244
240
242
244
0
O
16
0
-e-474M
-5.4
''
50
100
'
'
'
'
'
150 200 250 300 350
151
50
5ok atoms
atoms
100 150 200 250 300 350
time, psec
time, psec
Fig. 5.17: Comparison of thermodynamic output between 50k and 474M atom configurations of
quenched Cu5 oNb 5 o. (a) Potential energy versus simulation time. (b) Volume per atom versus
simulation time.
74
Quenched
Cu25Nb75
Cu3Au
(Nb3Cu)
Cu25Nb75
Cu3Au
(Nb3Cu)
Quenched
Cu50Nb5O
CsCI
(CuNb)
Cu50Nb5O
CsCI
(CuNb)
Quenched
Cu75Nb25
Cu3Au
(Cu3Nb)
Cu75Nb25
Cu3Au
(Cu3Nb)
0.352
0.352
0.352
0.352
0.352
0.352
500,000,000
500,000,000
474,353,318
474,353,318
500,000,000
500,000,000
4000 K
4000 K
4000 K
4000 K
4000 K
2.5 ps
4000 K
2.5 ps
Initial
Structure
Lattice0.5032
Parame [nm
Nu eof
Atoms
A
o
Liq g
Temp/Time
Quench Rate
Annealing
Relaxed
2.5 ps
2.5 ps
25 ps
1013 K/s
1013 K/s
1013 K/s
1013 K/s
1013 K/s
1013 K/s
-
1500
--
1200
--
1250
--
500
--
400
--
500
Relaxed
Quenched
Cu50Nb5O
8.1932
Relaxed
Cu25Nb75
8.2410
Cu50Nb5O
8.2012
Quenched
Cu75Nb25
8.2323
Cu75Nb25
8.2386
-6.3332
-5.3695
-5.3800
-4.4099
-4.4171
Time [ps]504050
Quenched
Cu25Nb75
8.2329
p [g cm 3]
PB [eV/atom]
Relaxed
25 ps
Temp [K]
Annealing
Relaxed
-6.3259
Relaxed
Table 5.3 Synthesis procedure for 2 billion atom model glasses and resulting properties.
75
76
6 Atomistic simulations of irradiated metallic glasses
In Chapter 2, I demonstrated that radiation response of metallic glasses under irradiation
is markedly different than in crystalline metals-they swell without voids to a finite limit [15,
16] and become more ductile [17, 49]. These dramatic differences suggest that the atomic-scale
radiation response mechanisms of amorphous metals are qualitatively different from crystalline
alloys. However, these fundamental mechanisms are poorly understood and several open
research questions remain. In this Chapter, I use
/2billion
atom molecular dynamics simulations
in a series of Cu-Nb alloys to reveal the answer to the two primary questions of this Thesis. First,
I reveal the spatial distribution of radiation damage in irradiated metallic glasses to be
qualitatively similar to that found in irradiated crystalline alloys. Second, I show that the
fundamental radiation response mechanism is rapid localized melting and quenching in isolated
thermal spikes. This rapid quenching leads to the formation of "super-quenched zones" (SQZs):
rapidly quenched amorphous regions formed where thermal spikes occurred. SQZ properties are
determined by the local quench rate and correspond to those of uniform liquids quenched at the
same rate. Irradiation of the amorphous alloy also gives rise to polarized collision-induced
plasticity, which occurs due to intense stress pulses emitted during the melting stage of thermal
spikes. New parallelized analysis techniques are employed to investigate these radiation response
mechanisms in detail.
In Section 6.1, I justify the choice of the PKA energy utilized in the MD collision cascade
simulations. Next, in Section 6.2, I report a detailed analysis of the radiation response
mechanisms in a 475 keV Nb ion irradiation simulation of quenched Cu 5 oNb 5o. In Section 6.3, I
study irradiated amorphous Cu 25 Nb75 , Cu5 oNb5 o, and Cu 75Nb 2 5, in both the quenched and relaxed
configurations, and report the role of composition and free volume in the radiation response
mechanisms.
6.1
Design of
/2 MeV
molecular dynamics collision cascade studies
As discussed in Chapters 2 and 3, molecular dynamics is well suited to investigation of
the fundamental radiation response mechanisms in irradiated alloys. After selecting the
interatomic potential and constructing realistic atomic configurations, the final modeling choice
is the energy of the initial PKA. The choice of the PKA energy is dictated by the first question of
interest, namely revealing the spatial distribution of radiation damage.
77
a
b
E 10
5
- Cu PKA
Nb PKA
4-
100
444
0
0-
I> 0
--
,7
-2
C 10
S,---Se
Sn Cu
Z
-- S~eCu
Sn Nb
1- - - - - - -
- - - -
Nb
*' 10-,(O
103210 210
0 10 1102 103 104
PKA energy (keV)
0
0
500
1000
1500
2000
PKA energy (keV)
Fig. 6.1: (a) The nuclear and electronic stopping powers (Sn and Se, respectively) as a function
of PKA energy for Nb and Cu (black and red, respectively), as computed in an a-Cu oNb O
5
5
system of density p=8.193 g/cm with standard SRIM [128]. (b) Ratio of Sn and Se as a function
of PKA energy, for Cu (red) and Nb (black) PKAs.
6.1.1
Primary knock-on atom energy selection
Previous atomistic modeling of collision cascades in irradiated crystalline metals has
demonstrated that for PKAs with energies exceeding a threshold (e.g. 10 keV in Fe [125], 25
keV in Cu [126] and 65 keV in Au [127]), the PKA creates multiple, isolated thermal spikes.
Therefore, to test for the formation of isolated thermal spikes in irradiated metallic glasses, it is
necessary to select a PKA with an initial energy of at least 25 keV. The upper bound of the PKA
energy is limited by the energy at which electronic stopping becomes the dominant stopping
mechanism.
To optimize the PKA energy within these two bounds, I employed the Monte Carlo
binary collision computer simulation code SRIM to simulate the projected range of ions in CuNb alloys, both with [128] and without electronic stopping [129]. SRIM models the binary
collisions resulting from a PKA impinging on a material target of a specific composition and
density, treating binary collisions via a screened columbic ZBL potential and accounting for both
electronic and nuclear stopping. Monte Carlo steps are used to determine if collisions occur. The
simulation tracks the positions and resulting energies of atoms during collisions, but does not
resolve the dynamics of collisions. SRIM is able to predict projected ranges of ions in a target
material, although it cannot reveal changes to the target atomic structure.
78
Using tabulated stopping powers in SRIM, I plot the nuclear and electronic stopping
powers for Cu and Nb ions in a Cu5oNb5o target material of density p=8.193 g/cm 3, as a function
of PKA energy, in Fig. 6.1(a). The ratio of the nuclear and electronic stopping is plotted in Fig.
6.1(b) and reveals that electronic stopping becomes equal to nuclear stopping at Cu PKA
energies of 900 keV and Nb PKA energies of 1700 keV. Within the constraint of classical
molecular dynamics, which excludes electronic interactions, it is necessary to employ a PKA
energy well below the PKA energy at which nuclear and electronic stopping are equal. To
achieve my goal of providing the opportunity for multiple collision cascades to form in the
irradiated amorphous Cu-Nb alloys, while still remaining in a PKA energy regime in which
nuclear stopping is the dominant energy loss mechanism, I chose a Nb PKA with 475 keV. This
Nb PKA corresponds to a ratio Sn/Se = 3.7, meaning that electronic stopping accounts for 20%
of the energy loss. This ratio of nuclear to electronic stopping is consistent with the energy range
studied previously with classical molecular dynamics simulations of 50 keV PKAs in Fe [125],
which from TRIM corresponds to 20% of the energy lost to electronic stopping.
To quantify the effect of excluding electronic stopping on the spatial distribution of
damage, I performed SRIM simulations of 475 keV Nb PKA irradiation of Cu5 oNb5 o (p= 8 .193
g/cm 3). I computed the distribution of the final position of the PKA (the "projected range"), both
a
b
E--10
-
S8,
o
40
SRIM NO SE
SRIM NO SE
- - - SRIM w/ SE
C
0 30
-
- - - SRIM w/ SE
6
20
4
0
0
0
0- 10
100
200
Range (nm)
300
10
10
10
102
10
Primary recoil energy (keV)
Fig. 6.2: (a) Distributions of final positions of 475 keV Nb PKAs in an a-Cu5oNb 5 o system of
density p=8.193 g/cm computed with SRIM. The solid line and filled circles are for no electronic
stopping (modified SRIM [129]) while the dashed line and open circles include electronic
stopping (standard SRIM [128]). (b) Histogram of primary recoil energies due to 475 keV Nb
ions, averaged over 1,000 Nb PKAs, computed using SRIM without and with electronic stopping
(solid and dashed lines, respectively).
79
with [128] and without electronic stopping [129], from 1,000 independent collision simulations.
As shown in Fig. 6.2(a), SRIM predicts that, on average, ions without electronic stopping travel
14% farther in the material (the average projected range is 123 nm), compared to ions with
electronic stopping (average projected range of 108 nm). However, the distribution of primary
recoil kinetic energies measured in SRIM [Fig. 6.2(b)], with and without electronic stopping, is
very similar. These insights suggest that excluding electronic stopping will have a relatively
small impact on the distribution of damage and that qualitative insights concerning the
distribution of damage obtained with molecular dynamics will be correct.
6.1.2
Selection of simulation cell size
To ensure that the PKA does not exit and re-enter the simulation cell through the periodic
boundaries, it is necessary to construct a model configuration with a simulation cell dimension
exceeding the largest possible PKA projected range. From the distribution of projected ranges of
475 keV Nb ions [Fig. 6.1(b)], the maximum projected range without electronic stopping is 300
nm. To accommodate this range within molecular dynamics, I chose a simulation cell size of 196
nm, giving a simulation cell diagonal of 339 nm. This simulation cell size corresponds to a
system of nearly
'/2
billion atoms. As reported in Chapter 5, realistic
'/2
billion atom
configurations were obtained by rapid quenching liquids from 4000 K to 300 K at an effective
quench rate of 1013 K/s.
6.2
Radiation response mechanisms in metallic glasses: Isolated super-quenched
zones and polarized plasticity
6.2.1
Simulation setup
In order to reveal the spatial distribution of radiation damage in irradiated metallic
glasses, as well as the fundamental mechanisms of radiation response, I performed a molecular
dynamics simulation of 475 keV Nb ion irradiation of a 474 million atom amorphous Cu5 oNb5 o
alloy. Using the
1/2
billion atom as-quenched amorphous Cu5oNb 5o configuration, constructed
with the model building procedure outlined in Section 5.5, I initiate a single collision cascade by
giving one Nb atom 475 keV of kinetic energy, directed along the simulation cell diagonal.
During the collision cascade, the equations of motion are integrated in the NVE ensemble.
Beginning with a timestep of 5x10- 7 ps, the timestep is adjusted every 100 timesteps so that no
atom displaces more than 5 x 10- nm between subsequent timesteps. The variable timestep
80
method allows the integration timestep size to increase as the kinetic energy of atoms decreases
and enables the simulation to cover 13.5 ps in only 496,000 timesteps while conserving energy to
within 2.1x10- 7 % (See discussion in Section 6.2.2).
Result 1 - Simulation output is reliable
6.2.2
Before analyzing the simulation output in detail (Sections 6.2.3-6.2.5) it is first necessary
to demonstrate that the simulation output is reliable. Below, I provide three independent
validations of the reliability of the simulation output. First, I demonstrate that the variable
timestep method accurately integrates the equations of motion, with excellent energy
conservation for both individual elastic scattering events and the system as a whole. Second, I
demonstrate that the input PKA is contained within the simulation. Third, I demonstrate that the
center of mass velocity is small.
Energy conserved during NVE molecular dynamics
Accurate finite-differences time integration of Newton's equations in the NVE ensemble
yields conservation of the total system energy, U = KE + PE, by construction [83]. Energy
conservation is therefore a necessary condition for simulation reliability. Here, I demonstrate that
the total system conserves energy and that the integration of the PKA trajectory is accurate.
Accurate time integration demonstrates that the variable timestep method is successful.
As shown in Fig. 6.3(a), the simulation timestep remains small, dt < 10-5 ps, up to a total
a
b
10
30
1
-
-
-
-
-
C
-
20
__0._
_4_0.045__
0___
5
10
Time (ps)
100
-
010
0
.
10
0.05
0.1
015
02
0.25
0.3
-
0.4
0.45
0.5
5001
500
-
- -
-
-,
-
-
4--
400
10
0.35
-6
460
300
W
440
042.
2000
0.045
1
1
10
Time (ps)
10
102
0
0.05
Time (p)
100o
0.1
0.2
0.3
0.4
0.5
Time (ps)
Fig. 6.3: Energy is conserved in MD simulation of 475 keV Nb ion irradiation of a Cu5 oNb5 o. (a)
Variation of simulation timestep size with total simulation time. (b) PKA energy and change in
total system energy as a function of simulation time.
81
a
b
-(150p)
E
Az
~0
150n
150
10nmTime
10~
10
100
(ps)
Fig. 6.4: Trajectory of 475 keV Nb ion in a-CuMoNbMo, computed with NVE molecular dynamics.
(a) Visualization of PKA trajectory. The simulation cell boundaries are indicated. The dashed
line indicates the specified PKA direction. (b) PKA position as a function of simulation time.
The simulation cell has an edge length of 196 nm.
simulation time of t=0.5 ps, the point at which all high-energy (ke > I keV) collisions are
complete. Following the initial ballistic stage, with energy exchanges predominantly in the form
of binary elastic collision, the kinetic energy per atom decreases and a larger timestep is allowed
by the variable timestep scheme. As shown in the top panel of Fig. 6.3(b), the variable timestep
method yields excellent total energy conservation, with a total system energy change of -5.3 eV
out of an initial energy of -2.5282 - 109 eV, representing a fractional energy change of 2 10-7%.
Comparing the total system energy change with the energy of the PKA [bottom panel,
Fig. 6.3(b)], it is evident that most of the system energy change occurs due to high-energy,
binary scattering events. However, even in these individual scattering events the time integration
yields excellent energy conservation. For example, during the first large energy drop in the PKA
energy-an energy change of 55 keV-the total system energy changes by 3.2 eV, yielding an
error in the energy of the binary scattering event of 0.006%. These results demonstrate that the
simulation methods yields excellent energy conservation, both for individual atom trajectories
and the system as a whole, and provides strong evidence that the simulation output is reliable.
PKA is contained within simulation cell
In order to reveal the radiation response mechanisms of metallic glasses, it is necessary
that the PKA is contained within the simulation cell. If the PKA passes through previously
82
2
-Ay
+1- o5 A
-Az
+ 2 - 10-5A
1.5-
0.5-
0
0
2
4
6
8
10
12
Time (ps)
Fig. 6.5: Change in the simulation center of mass position as a function of simulation time.
irradiated material, it becomes impossible to resolve my primary research questions, namely the
spatial distribution of radiation damage and the details of radiation response mechanisms. While
SRIM was employed to predict the range of 475 keV Nb PKAs in Cu oNbso,
it is necessary to
5
demonstrate that the PKA is in fact contained within the simulation cell.
As illustrated in Fig. 6.4, the Nb PKA is initially oriented along the simulation cell
diagonal. Subsequent time integration within the NVE ensemble yields the visualized PKA
trajectory, with the abrupt changes in PKA direction suggestive of elastic scattering events. With
respect to its initial position, the PKA has a total displacement vector of
A? = [161
58
291 nm, with an integral path distance of 200 nm. From both the visualization
and these quantitative measures, it is clear that the PKA is contained within the simulation cell
and that it only interacts with non-irradiated material. Furthermore, the projected range of the
PKA, IA&| = 174 nm is in excellent agreement with the predicted projected range computed
with SRIM [Fig. 6.2(a)], providing additional evidence that the MD simulation yields reliable
output.
Center of mass velocity
The introduction of the 475 keV Nb atom imparts a net momentum to the simulation. It is
therefore necessary to validate that the simulation is not a "flying ice cube" with a large center of
mass velocity. In Fig. 6.5, I plot the simulation center of mass position as a function of
83
simulation time. As expected, the PKA does cause a small, net drift in the simulation center of
mass. A linear least-squares fit to the to the displacement magnitude versus time yields a velocity
magnitude of 2.5e-5 A/ps. Converting the center of mass speed to an effective temperature via
3/2kBT = 1/2mv 2 , the center of mass velocity corresponds to a temperature of 9.2 K. This
effective temperature is small with respect to local increases in temperature (found to exceed
4000 K), and all local temperatures are computed only after first subtracting the center of mass
velocity of the voxel. Thus, the center of mass velocity of the system as a whole should not affect
the underlying physics of the system nor lead to errors in interpretation of the simulation output,
providing additional evidence that the simulation output is reliable.
6.2.3
Result 2 - PKA produces isolated thermal spikes without ion tracks
Collision cascade
As the PKA travels through the model, it undergoes collisions with surrounding atoms,
transferring kinetic energy to them and displacing them from their initial locations. Plotting the
PKA kinetic energy as a function of its path length [Fig. 6.6(a)] it is evident that the PKA kinetic
energy decreases through discrete scattering events. Cataloging every discrete energy drop of the
PKA and summing the energy of each collision that transfers more than 1 keV of kinetic energy,
I find that the PKA loses over 90% of its energy through these high-energy binary elastic
collisions. The distribution of these recoil energies is in good agreement with the predicted
distribution of recoil energies from SRIM [Fig. 6.6(a)]. These results demonstrate that binary
scattering is the dominant mechanism in determining the spatial distribution of energy transfers
in the irradiated amorphous alloy.
Supporting the insight that the PKA loses energy through discrete, binary elastic
collisions, the PKA (red line) trajectory is plotted in Fig. 6.7(a), along with the trajectories of
atoms acquiring at least 1 keV (KAs, black lines). Knock-on atoms terminate in spatially
disconnected regions with numerous displacements of 1 nm or less (displacement vectors,
computed between 0 - 12 ps, shown as a solid blue line connecting initial and final position).
The isolated regions of displacements are connected by straight, collision-free KA trajectories,
suggesting that inter-nuclear scattering does not generate ion tracks and that, on the length scales
84
a
b
500
25
----
-SRIM
NO SE
---SRIM w/ SE
-- MD
___
400.220
300
15
W'200
0 10
EM
10-
100
5
%
50
0
100
150
200
PKA trajectory distance (nm)
250
02
10
10
Recoil energy (keV)
102
Fig. 6.6: Quantifying PKA collision events. (a) PKA energy versus integral trajectory distance.
(b) Histogram of number of recoils created at a given energy, computed using SRIM without
[129] and with [128] electronic stopping (solid and dashed lines, respectively), as previously
shown in Fig. 6.2. Blue symbols with dashed line correspond to MD data, computed as the
histogram of PKA energy drops [from part (a)].
of hundreds of nanometers, the distributions of collision-induced damage in metallic glasses and
crystalline alloys are comparable [105].
To characterize radiation response in displacement zones, I evaluate temperature (T),
density (p), potential energy (PE), diffusivity (D), stresses (aij), and strains (Eci) on a 75x75x
75 array of cubic volumes (voxels) with edge length 2.6 nm (see Section 4.1 for details),
containing ~1,100 atoms each. The compositions of these voxels are normally distributed with a
mean of 49.9% Cu and a standard deviation of 5.6% and behave as valid representative volume
elements.
Computing the temperature as a function of time in each voxel between 1-12 ps, the
maximum temperature (Tmax) is found for each voxel. In Fig. 6.7(b), voxels whose maximum
temperature Tmax exceeds the glass transition temperature of a-Cu oNbso
(TG = 1500 K) are
5
shown as red cubes superimposed on the KA trajectories. Using cluster analysis, neighboring
voxels with Tmax > TG are binned to unique clusters. As can be seen in Fig. 6.7(c), these hightemperature regions are localized and coincident with the regions with numerous displacements
in Fig. 6.7(a).
85
Ranging in diameter from approximately 2-12 nm, these high temperature zones are
known as "thermal spikes." Tracking the trajectory and energy of each knock-on atom, the
energy deposited in each thermal spike is computed using the energy flux method illustrated in
Fig. 6.7(d). The KA energy at the time it enters and leaves the thermal spike is noted and the
difference computed. The sum of all energy fluxes yields the total energy deposited. As shown in
Fig. 6.8 the volume of the thermal spike scales linearly with deposited energy, with a best least-
a
b
-"""
475 keV Nb PKA
KE,=> 1 keV
0.5
[TN
= 350 K
ST. > 1500 K
< LO.J< 1 nm
150 nm
U
460,
440
420
N 40
380
2
320,
ClusterID
2
KAID
273455
kk_"r ke-leave
2.3
0.4
0'0 128MOA
AKE
Ta
AKE 8VW
-0.9
-
Fig. 6.7: Displacement zones and thermal spikes in irradiated a-Cu5 oNb5o. (a) Displaced atom
trajectories in a-Cu5 oNb5o: 475keV Nb PKA plotted in red, knock-on atoms acquiring at least 1
keV in black; atoms displaced between 0.5-1 nm in blue. (b) Temperature fields due to internuclear collisions. Red voxels have a maximum temperature greater than TG = 1500 K [39]. The
blue contour is for Tmax = 350 K after a total simulation time of 12 ps. (c) Isolated thermal
spikes identified on the basis of nearest-neighbor cluster analysis. (d) Energy flux into a single
representative thermal spike, boxed in (c).
86
800
-
0
fit
E 600
0
0
400
TE 200
E
I-
0
0
10
20
30
40
50
Thermal spike energy (keV)
Fig. 6.8: Thermal spike volume versus deposited energy. The straight line corresponds to the
linear fit: VTs = (15.1 ± 0.6 nm 3 /keV) ETS - (8.9 ± 11.2 nm 3 ).
squares linear fit of VTs = (15.1 + 0.6 nm3 /keV) ETS
-
(8.9 + 11.2 nm 3 ). Furthermore, no
thermal spike energy exceeds 50 keV.
Addressing the first Thesis research question, these findings reveal that the spatial
distribution of radiation damage is qualitatively similar to that found in crystalline alloys. Due to
binary elastic scattering of the PKA with material atoms, isolated thermal spikes form, similar to
previous findings of isolated "sub-cascades" in crystalline materials [1]. Thermal spikes vary in
energy and size, but the characteristic energy and size is on the order of 10 keV and 10 nm in
diameter, respectively, similar to crystalline materials.
6.2.4
Result 3 - Thermal spikes are liquids that quench to "Super-quenched zones"
The formation of spatially distinct thermal spikes, with a characteristic energy below 50
keV, is qualitatively similar to previous findings of "sub-cascades" in crystalline materials [1].
Sub-cascades have been extensively studied in crystalline solids due to the important role they
play in radiation-induced point defect production [1, 2, 130]. However, I find that thermal spikes
have a distinctly different effect on amorphous metals than on crystalline solids. Below, I show
that thermal spikes contain equilibrium liquids. These short-lived liquid zones subsequently
quench at rates approach 1014 K/s to produce "Super-quenched Zones" (SQZs) of low-density
and high potential energy, with local SQZ properties determined solely by local quench rate.
87
Thermal spikes are equilibriumliquids
The identification of thermal spikes with a maximum local temperature exceeding the
glass transition temperature [Fig. 6.7(b)] suggests that localized melting may have occurred.
While a local temperature in excess of the glass transition temperature is a necessary requirement
for localized melting in thermal spikes, it is insufficient for a conclusive identification of
localized liquid zones. I therefore compare the temperature dependence of two key propertiesvoxel diffusivity and density-with the values obtained in rapidly quenched, uniform liquid
Cu5 oNb 5o. Diffusivity is selected since high atomic mobility is a signature of liquids.
Temperature-dependent density is chosen since it is reflects the equation of state of the liquid.
For every voxel, the temperature, density, and diffusivity are computed at timesteps
between 1 and 12 ps. As described in Chapter 4, the temperature in each voxel, at every timestep,
is obtained by fitting the distribution of voxel atom kinetic energies to the Maxwell-Boltzmann
kinetic energy distribution. The density is obtained from the number of atoms in the voxel,
divided by the voxel volume. The time-dependent diffusivity of each voxel is obtained from the
time-dependent mean-squared displacement, computed for the atoms found in the voxel at t=1
ps.
As an example, I plot the time-dependent temperature, density, MSD, and diffusivity for
a single voxel contained in the thermal spike highlighted in Fig. 6.7(d). Suggestive of local
melting, the voxel temperature is well above the glass transition temperature for 10 ps. Initially,
the voxel density rapidly decreases, due to thermal expansion. As the voxel cools [note the
decreasing voxel temperature Fig. 6.9(a)], the density increases. Plotting the voxel density versus
voxel temperature at equal timesteps in Fig. 6.9(a), it can be seen that the voxel data follows the
values obtained in uniform liquid Cu5 oNb 5o, quenched at 6 - 1013 K/s, suggesting that the voxel
follows the equation of state of a rapidly quenched liquid. Confirming this interpretation, the
diffusivity likewise follows the dynamics of a rapidly quenched liquid. From the MSD as a
function of time, rz(t), the time-dependent diffusivity is obtained from the slope of r2 (t),
computed from the linear fit at time t, in a centered 2 ps fitting window. Plotting the voxel
diffusivity against the temperature at the same timestep, the voxel data is in excellent agreement
with the diffusion data obtained from a liquid quenched at 6 - 10" K/s.
88
The successful mapping of the temperature-dependent properties of the individual voxel
to those of a rapidly quenched liquid demonstrates that at least one voxel displays the properties
of a rapidly quenched liquid. To test if other candidate liquid voxels (Tmax > 1500 K) can also
be mapped to quenched Cu 5 oNb5o liquid, I compute the diffusivity, temperature, and density of
all voxels exceeding the glass transition temperature at a single timestep, 5 ps. In Fig. 6.10 (a), I
plot diffusivity versus temperature at 5 ps for each voxel with Tmax > 1500 K. Despite
considerable voxel-to-voxel variability, the dependence of diffusivity on temperature averaged
over all voxels collapses to that of an independently simulated, uniform liquid quenched at 6
a
1013
b
-
-4500
40-
2 3000
20-
E
1500
C
2
4
6
i
i
j
8
10
12
2
.
4
6
8
10
12
20
0
D(t) =
6
9)1-(t)
Ot
00
6
2
4
C
6
8
Time (ps)
10
0 VOXel
-Uniform
8
2
12
4
6
Time (ps)
8
10
12
3500
4000
10 nVoxel
Liquid
-Uniform Liquid
10
E
U5
10
.
6
1500
2000
I
2500
3000
3500
Voxel temperature (K)
4000
1
4500
10 -1011
1000
1500
2000
2500
3000
Temperature (K)
Fig. 6.9: Time-dependent properties of a single voxel inside thermal spike shown in Fig. 6.7 (d).
(a) Voxel temperature and density versus time. (b) Voxel mean-squared displacement (MSD)
and derivative diffusivity versus time. (c) Voxel density and (d) diffusivity versus voxel
temperature (open symbols), compared with values from uniform Cu5 oNb5 o liquid quenched at
6 - 10" K/s.
89
(a)
(b)
T8
10-8
_8.2
1500 K
78
(00
E
100
0
-10
-p-
10
500
7.6
0
74
Voxe
voxel average
orm liquid
Uny
0
-p
-
1000 1500 2000 2500 3000 3500
7.2
500
Voxe
VoxeI average
Uniform liquid
1000 1500 2000 2500 3000 3500
Temperature (K)
Temperature (K)
Fig. 6.10: Mapping of thermal spike properties to rapidly quenched, uniform liquid. Diffusivity
(a) and density (b) at t=5 ps plotted versus temperature for voxels with Tmax > TG (open symbols:
voxel data; blue line: binned average). The values for Cu5oNb 5 o liquid quenched at 6. 1013 K/s are
shown for comparison (black line).
K/s.
The temperature dependence of voxel densities, shown in Fig. 6.10 (b), also follows that
of the rapidly quenched liquid, although shifted slightly to higher densities, likely due to residual
pressure in the thermal spike (-1 GPa). These correspondences with a uniform liquid
demonstrate that voxels with Tmax > TG are not simply superheated, but rather contain genuine
Cu-Nb liquid, albeit only for times of the order of 10 ps.
Super-quenchedzones (SQZs)
Having demonstrated that voxels are liquids, with properties corresponding to a rapidly
cooled liquid, I next test the relationship between the voxel quench rate and resulting properties
in the solid, quenched state. In order to compare the properties of the thermal spike voxels,
before and after irradiation, it is necessary to anneal the system until voxels have quenched well
below the glass transition temperature. However, as discussed in Section 6.2.5 (below), elastic
waves are emitted by thermal spikes and travel at the system longitudinal speed of sound
= 5275 m/s). Waves reenter the simulation cell box through the periodic boundaries and
begin to interact at t=14.5 ps. Undamped, these waves could perturb the properties of the
(VL
quenching thermal spikes. Thus, to damp out the stress waves, I restart the simulation at 13.5 ps
with 3 perpendicular, intersecting planes of atoms located at the intersection of the stress pulses,
each 5 nm thick and containing a Langevin thermostat set to 300 K. I subsequently anneal the
90
system in the NVE ensemble for 416 ps. The absorbing boundaries successfully damp the stress
pulse and allow for accurate analysis of the properties of quenched thermal spikes.
After 400 ps, all voxels with T. >1500 K quench to temperatures less than 450 K, well
below TG for a-Cu 50Nb5o. I compare the properties of the rapidly quenched voxels to those of
uniform a-Cu 50 Nb5 0 created by quenching from the liquid with a range of different rates. To
facilitate the comparison, both irradiated and quenched models were relaxed to T = 0 K by
conjugate gradient energy minimization. Changes in voxel potential energies and densities with
respect to values prior to irradiation are plotted versus quench rates in Fig. 6.11 (a) and Fig. 6.11
(b) respectively. Voxel densities decrease and potential energies increase with increasing quench
rates. On average, the voxel properties collapse remarkably well onto the quench rate-dependent
changes in potential energy and density of uniform a-Cu 0Nb .
5
50
These insights demonstrate that, within thermal spikes in amorphous metals, the essential
effect of radiation is to form localized, nanoscale liquid zones that rapidly quench through the
glass transition to a vitrified solid. The physical properties of the solid are governed by the local
quench rate and map to properties of uniform liquids quenched at the same rate. By
contrast,
thermal spike-affected regions in crystalline metals recover to a defective crystalline structure
[1]. The simulations are consistent with continuum-level arguments for radiation-induced
localized melting and quenching [131, 132], but call into question previous attempts to describe
radiation damage in these materials through "point defect-like" entities [64, 73, 74, 75], at least
a
b
2
0
2
1
0
3
0
1
C
-1
-- Unifbrm
-3
MCI
A00
0
-2
a-Cusofba
-3
-200
0
Of
0
0
-1
Vxlaverage
0
00
0
0 -0-0
0
-2
Voxel
Vow average
Uniform a-CusoNb5o
MA
0
0
0
t
0
0
-150
-100
-50
0
-200
-150
-100
-50
0
Quench rate (K ps~ )
Quench rate (K ps-)
Fig. 6.11: Changes in voxel potential energy (a) and density (b) between the initial and postirradiation SQZs are plotted versus voxel quench rate. Property changes for a-Cu oNb
o
5
quenched at various rates, with respect to 1 - 1013 K/s, are shown for comparison (black line). 5
91
for thermal spike energies larger than 1 keV. To distinguish the damage mechanism described
here from interpretations based on point defects, I refer to the thermal spike-affected regions in
amorphous metals as "super-quenched zones" (SQZs). This designation highlights that the key
characteristics of these regions are their extremely high local quench rates and the equivalence of
their properties to those of rapidly quenched liquids.
SQZ-based models for radiation-inducedswelling and ductilization
Radiation-induced production of isolated SQZs is schematically represented below in
Fig. 6.12. With continued radiation, SQZs occupy an increasing volume fraction of the material.
Since SQZ quench rates are approximately constant, the properties of the material, in the limit of
complete coverage with SQZs, will converge to that of the SQZs alone.
Writing the volume fraction of SQZs fsQz = VQz / V, , the effect of reduced SQZ density
on the bulk material density can be described within the framework of composite theory as
Pf = po + fsQz(PsQz - po). In the limit of complete coverage of the material with SQZs, the
relative density change of the material can be written Xs = (PsQz - po)/po. On the basis of the
average SQZ quench rate for rapidly quenched Cu 5oNb5o, Zs = (8.15-8.2)/8.2= -0.6% (density
in units of g cm-3), in quantitative agreement with a saturable density change of 1% in a neutron
irradiated Fe-based amorphous metal alloy [15, 16]. Thus, this SQZ model for swelling is
consistent with experimental investigations that have demonstrated saturable volumetric swelling
without voids in irradiated metallic glasses [15, 16, 50, 51].
Fig. 6.12: Schematic representation of radiation-induced SQZ formation, responsible for
radiation-induced swelling and ductilization.
92
Additionally, my identification of SQZs with high free volume provides a natural
explanation for radiation-enhanced ductility. It is well-known that increased free volume leads to
homogeneous flow in amorphous alloys [40]. Thus, SQZs with reduced density (increased free
volume) provide a mechanistic interpretation for previous MD simulations of irradiated metallic
glasses demonstrating that mechanical properties are affected by irradiation [77, 78, 79] and
experiments showing radiation-enhanced ductility [17, 49].
6.2.5
Result 4 - Thermal spikes produce stress pulses that trigger polarized plasticity
As demonstrated above, thermal spikes contain equilibrium liquids. However, the
material outside the thermal spike remains solid and constrains the thermal expansion of the
a
b
-
Thermal spike
15
Adjacent, r<4 nm
10
62.
LO5
-""
475 keV Nb PKA
KE,=> 1 keV
< l9.l<
1 nm
0
0.5
d
C4000
-- Thermal spike
3500
2
4
6
8
Time (ps)
10
8
-Thermal
Adjacent, r<4 nm
12
spike
Adjacent, r<4 nm
a.
8.4
3000
E
LD 2500
%,2000
*57.8
E 1500
1000
/.0
500
7.4
2
4
6
Time (ps)
8
10
0
12
2
4
6
Time (ps)
8
10
12
Fig. 6.13: Confined melting leads to pressurization of thermal spike and initiation of a stress
pulse. (a) Close-up view of thermal spike. (b) Pressure, (c) temperature, and (d) density plotted
versus simulation time for thermal spike (black line) and adjacent material within 4 nm of
thermal spike surface (gray line). Shaded band indicates uncertainty of the mean.
93
liquid. As a result, pressures in excess of 10 GPa build in the liquid thermal spike, leading to the
propagation of a stress pulse away from thermal spikes at the longitudinal speed of sound. While
the stress pulse amplitude decays with distance traveled, the magnitude exceeds the system yield
stress close to the thermal spikes. As a result, plastic deformation accumulates with 3-4 nm of
thermal spikes. These plastic strains are aligned with the thermal spike orientation.
Thermal spikes emit stress pulses
To demonstrate the mechanism of stress pulse induced plasticity, I analyze one
representative thermal spike in detail, shown with a close-up view in Fig. 6.13 (a). The thermal
spike is shaped as a prolate ellipsoid, with the major axis along the PKA trajectory and
approximately equal dimensions along the other two principal directions. Its temperature
increases to well above the glass transition temperature in less than 1 ps [Fig. 6.13 (c)]. However,
the material immediately adjacent to the thermal spike remains below TG and does not melt. As
shown in Fig. 6.13 (b), melting leads to an associated pressure excursion to 15 GPa inside the
thermal spike. After reaching its maximum pressure, the density of the thermal spike decreases
rapidly [Fig. 6.13 (d)], while the pressure and density of the adjacent material simultaneously
increases [Fig. 6.13 (b) and (d)], demonstrating that pressurized thermal spike has initiated a
stress pulse.
a
b
1.9 Ps
50
'v.=
-+-2.9 ps
v
, p=
.5275 is
401,,
3 -4-5 ps
+
30
10.1 ps
02-
10
1Time
%0
0 Data
4
6
(pe)
--Unear Fit
8
10
P=0.5GPa
0-E
ST.>1500KI
0
10
40
20
30
Distance from TS (nm)
50
150 nm
Fig. 6.14: Liquid thermal spikes emit stress pules. (a) Pressure as a function of distance from the
surface of the thermal spike visualized in Fig. 6.12 (a). Inset plot is position of the peak of the
pressure pulse as a function of time. (b) Stress pulse front after 5 ps.
94
To quantify the speed and amplitude of the stress pulse, the average pressure as a
function of distance from the thermal spike is plotted in Fig. 6.14 (a) at times between 2 and 10
ps. The stress pulse has a positive (compressive) front and is trailed by a negative (tensile) pulse.
The amplitude of the pulse decays from 2 GPa (5 nm, 1.9 ps) to below 0.5 GPa (38 nm, 8 ps)
the
over a distance of 33 nm and a time interval of 6.1 ps. Plotting the position and time of
of
maximum in the stress pulse [inset of Fig. 6.14 (a)], a linear fit yields a wave speed
v = 5042 ms- 1 . Using the zero temperature elastic constants (Chapter 5), the longitudinal speed
1
the
of sound VL = -ftCii/p = 5275 ms- . While the measured speed of the wave is lower than
b
a
3.U
XI
1
3
cy = 3 GPa
2. 5
'U
2
0.
1. 51
y
0. 5
2
6
d
C
10
8
6
Time (ps)
4
12
60
T
40
1.5
0
20
C
40
0ii
W
'L
0
1
-20
0'
0.5[
-40
-60
'
-1A
5
A
5
0
0
2
2
4
4
6
8
8
6
Time (ps)
10
10
12
12
z (nm)
in a radiation damage zone. (a) Close-up view of thermal spike
response
Fig. 6.15: Material
the
boxed in Fig. 1(a). A cylindrical coordinate system is defined along the major (z) axis of
time.
versus
voxels
thermal spike. (b) Average temperature in the thermal spike and in adjacent
(c) Variation of diagonal components of plastic strain in cylindrical coordinates with location
along the thermal spike major axis, with uncertainty indicated by shaded bands. (d) Von Mises
stress and tensile work equivalent plastic strain versus time, averaged over all adjacent voxels
within 4 nm of the thermal spike.
95
speed predicted from the zero temperature elastic constants, it is expected that the highfrequency response at finite temperature will change the system elastic response, lowering the
elastic constants, and produce a slower wave speed. These results demonstrate that melting of
thermal spike yields a stress pulse traveling at the longitudinal speed of sound. As shown in Fig.
6.14 (b), at times between 5-10 ps, individual stress pulses superimpose and yield a stress front
that is approximately cylindrical and axisymmetric with the PKA trajectory.
Stress pulse triggersplasticity adjacent to thermal spikes
While the stress pulse amplitude decays, close to the thermal spike, the stress pulse
exceeds the material yield stress. Using the thermal spike studied in Fig. 6.13 as an example, I
plot the von Mises equivalent stress amplitude in the material adjacent to the thermal spike
(within 4 nm) as a function of time. As shown in Fig. 6.15 (b), the stress briefly exceeds 3 GPathe stress required for yielding in a-Cu5oNb5 o at 500 K at the strain rate of the pressure pulse
(1010 s 1 ). The stress amplitude only exceeds the yield stress in voxels within 4 nm of thermal
spikes. Von Mises tensile work equivalent plastic strains EM in these voxels increase rapidly to
-1.5%
E,
after the yield stress in them is exceeded. The plastic strain is partially driven back to
~ 1% by elastic stresses in surrounding material after the pulse has propagated away [Fig.
6.15 (d)].
EP
4
40
4t-J-
o
20
4W
0
cc
-20
-40-
15
.
T
-o'
Highlighted TS
25
35
45
Thermal spike energy (keV)
Fig. 6.16: Voxel average plastic strain components in material adjacent to the seven largest
thermal spikes as a function of the thermal spike energy. Arrow indicates the thermal spike
analyzed in Fig. 6.14.
96
Plastic strains near the thermal spike in Fig. 6.15 (a) exhibit approximately cylindrical
symmetry about the major axis. I transform the strain tensor of each adjacent voxel to the
cylindrical coordinate system defined by the thermal spike axes and find that the off-diagonal
components are close to zero. The variation of the diagonal components
(err, E%,
Ezz) as a
function of location along the thermal spike major axis is shown in Fig. 6.15 (c). Averaging over
all voxels within 4 nm of the thermal spike surface, I find that 00 = (31.5 ± 4.0) - 10-',
Err = -(13.0 ± 4.9)
-10-4,and Ezz = -(17.8 ± 4.8)
.10-4,
indicating elongation along the
hoop direction and compression along the radial and axial directions. These values are consistent
with plastic strains expected near an elongated internally pressurized inclusion.
I repeat the analysis described above for the seven largest thermal spikes in the
simulation and find that they are also shaped as prolate ellipsoids with major axes along their KA
directions and with cylindrically symmetric plastic strain distributions. The voxel average plastic
strains for each thermal spike, shown in Fig. 6.16, satisfy Err < f
<e
and are nearly
independent of thermal spike volume. I attribute this fact to the linear dependence of thermal
spike volume on deposited knock-on energy, as previously shown in Fig. 6.8. The similar strain
magnitudes and orientations in these thermal spikes reflects a corresponding narrow distribution
of maximum pressures inside thermal spikes [average of 13 GPa, Fig. 6.17(a)] and a similarly
narrow distribution of maximum stresses in the adjacent solid material [average of 2.5 GPa, Fig.
6.17(b)].
I determine the net effect of plastic deformation around all the thermal spikes by
A
B
10
'
'
10
-
8
C
04
8
6-
C
04
6-
2.
2
0
0
10 11 12 13 14 15 16 17 18
1.5
Maximum pressure (GPa)
2
2.5
3
3.5
Maximum stress (GPa)
Fig. 6.17: (a) Distribution of maximum pressure inside thermal spikes; (b) Distribution of
maximum average von Mises equivalent stress (Oum) in material within 4 nm of thermal spikes.
97
averaging over all plastic strains in voxels adjacent thermal spikes (350 < Tmax < 1500 K) and
scaling the resulting strain values by this region's volume fraction. Computing the principal
values of the average plastic strain tensor, I find that one of the principal directions is well
aligned with the cascade direction (with a deviation of only 170). The principal values reveal that
plastic flow is compressive (-2.5 - 10-1) along the principal direction aligned with the cascade
direction, tensile (3.9- 10-') in one direction normal to the cascade direction, and compressive
(-0.7 - 10-') in the second direction normal to the cascade direction. The cascade direction is
determined from the major axis of the best-fit ellipsoid to voxels with Tm ax > 350 K. Thus, I
expect that, in experiments, collision-induced plasticity will lead to contraction along the beam
direction and a net expansion perpendicular to it. The radiation-induced deformation includes a
hydrostatic component el = 0.2 - 10-,
which is small compared to the tensile work equivalent
strain ejM = 3.9 - 10- and consistent with shear-induced dilatation in metallic glasses [21].
Using these results, I compute the plastic strain per fluence expected in irradiation
experiments. The effective fluence in the simulation is P = 1 ion/SA = (1 ion)/(3.84x
10-1 0 cm 2 )=2.6x 10' ion/cm 2 , where SA is the cross-section area of the model. The radiationinduced plastic strain per fluence is A = fEcM/# = 1.5x10
1
scm 2 /ion,
where E7" is the
average strain in voxels adjacent thermal spikes and f = 1.13% is the fraction of the total
volume taken up by these voxels. Thus, barring any recovery or devitrification processes that
may occur on longer time scales, I would expect a-Cu5 oNb5 o to deform to ~ 15% plastic strain
for a fluence of 10'4 ion/cm 2 of 0.5 MeV Nb ions.
Analysis of the collision-induced plasticity requires an accurate determination of the
plastic strain. As discussed in Section 4.1, the plastic strain is computed by first computing the
total strain. From the total strain, I subtract the predicted elastic strains from voxel stress and the
zero temperature elastic constants. Additionally, I subtract the predicted strain from thermal
expansion due to local heating. The assumption of uniform elastic constants, described by the
zero temperature elastic response of a uniform system, is a possible source of error in the
prediction of elastic strain. However, use of the finite temperature, high frequency elastic
constants will result in a decrease in the expected elastic strain, thus, in the worst case, I am
actually underestimating the plastic strain. Computing the ratio of the predicted elastic strains
and the measured total strain, I find that for voxels with E
98
M
> 0.01, the predicted elastic strains
are less than half of the measured total strain. An additional confirmation of the validity of the
method is found in comparing the hydrostatic strain before and after subtracting for thermal
expansion. Initially, a finite hydrostatic strain is present, but subtraction of the predicted thermal
strains leads to nearly zero hydrostatic strain.
Identification of plasticity should ideally be based upon demonstration of irreversibility
in strain. However, demonstrating reversibility is challenging in this system, due to the constraint
of the constant volume ensemble. This leads to a backstress, obscuring what reversibility is truly
present. However, relaxation of the configuration to zero total stress, following irradiation,
would provide an opportunity to probe the reversibility of strains. This is an interesting topic and
identified for future research. However, even in the absence of a full treatment of reversibility,
the analysis of the voxel-level strain response demonstrates that the plastic strains identified here
are statistically significant.
6.2.6
Discussion
Comparisonwith ion-inducedplasticity
The collision-induced plasticity mechanism described above is distinct from the wellknown phenomenon of ion-induced plasticity, in which metallic glasses irradiated with swift,
heavy ions, e.g. 360 MeV Kr+, exhibit volume-conserving plastic flow due to electronic
excitations, with a strain increment per dose of A-5x10-s cm 2 /ion [18]. While ion-induced
plasticity occurs due to viscoelastic relaxation in ion tracks generated by electronic excitations
[62, 133], collision-induced plasticity is due to plastic deformation adjacent to thermal spikes
created by nuclear scattering at relatively low energies (<1 MeV). Unlike ion-induced plasticity,
collision-induced plasticity does not require electronic excitations and therefore may occur under
neutron irradiation or low-energy heavy ion bombardment.
Electronic effects
Electron-phonon coupling enhances thermal conductivity and would therefore lead to
higher quench rates in thermal spikes than observed in our simulation. Since plasticity is
triggered by local melting in the thermal spikes, not quenching, it is likely to be unaffected by
the neglect of electron-phonon coupling. On the other hand, the degree of density reduction in
SQZs increases with quench rate. Therefore, our simulation is actually a lower-bound on the
99
density reduction in SQZs, since any additional heat conduction due to electronic thermal
conductivity will increase local quench rates.
Recent work has demonstrated that fully quantum mechanical calculations via timedependent density functional theory capture electronic stopping for small systems and low
energies (e.g. 1 keV proton in Aluminum) [134]. More computationally efficient approaches are
mostly ad-hoc additions to classical simulations (e.g. two-temperature models), although semiclassical methods are being developed [10].
Applications
As demonstrated in experiments [17, 49, 56], irradiation can be used as a processing tool
to introduce SQZs and engineer metallic glasses with excess free-volume and correspondingly
improved ductility. Additionally, there has been considerable interest in finding approaches for
forming of small-scale amorphous metal components [23]. It may be possible to make use of
collision-induced polarized plasticity for this purpose.
The outstanding corrosion resistance of metallic glasses [22, 135] has prompted
exploration of metallic glasses for use as coating materials for nuclear waste storage [22, 135].
Collision-induced plasticity may limit the performance of metallic glasses in such applications.
However, our findings suggest that it may be possible to limit collision-induced plasticity by
identifying amorphous alloys with minimal liquid thermal expansion (to reduce the amplitude of
stress pulses emitted from thermal spikes) and high yield stress (to reduce plasticity near SQZs).
6.3
6.3.1
Role of composition and free volume in radiation response of metallic glasses
Introduction
Radiation damage due to a 475 keV Nb ion was simulated in three,
alloys-Cu 2 Nb 7 5, Cu5oNb 5o,
Cu 75Nb 25-each
1/2
irradiated in the as-quenched
billion atom
and relaxed
configuration (6 systems total). The synthesis procedure for each alloy is summarized in Table
5.3. In each alloy, a single 475keV Nb knock-on atom is initiated and the equations of motion are
solved in the NVE ensemble.
6.3.2
Thermal spike size
As can be seen in Fig. 6.18, a branched cascade structure forms in each alloy, with
knock-on atoms terminating in spatially distinct thermal spikes. The entire collision cascade in
each alloy is contained in a volume approximately 100-150 nm in length and some 50 - 100 nm
100
in diameter. As in the case of the previously studied as-quenched CuMoNb 5 o alloy [included here
for reference in Fig. 6.18 (g)-(i)], the maximum temperature in each voxel is measured, at least 1
ps after the start of the simulation, and those voxels with Tmax > TG are taken to be liquids and
indicated with red cubes (center column in Fig. 6.18).
As suggested by the visualizations in Fig. 6.18, the size of thermal spikes increases in the
annealed alloys. As shown in Fig. 6.19, fewer numbers of clusters comprise more of the total
thermal spike volume in the relaxed systems, demonstrating that clusters are larger in the relaxed
systems. Additionally, it is clear that thermal spikes are smaller in the systems with higher glass
transition temperatures, in both the relaxed and the as-quenched systems.
6.3.3
Collision-induced plasticity
Averaging over all the strains in voxels adjacent to liquid zones, I find that the average
total strains in the collision-affected zones are aligned with the collision cascade direction. To
test alignment between the total strain orientation and the cascade affected region direction, I
first compute the best-fit ellipsoid of all voxels with Tmax > 350 K and take the major axis as
the direction of the collision cascade. Next, I compute the principal values of the strain tensor
computed as the mean of all voxel strain tensors in the cascade affected region (350 < Tmax <
TG). I finally compare the direction of the strain eigenvectors with the collision cascade direction.
101
A
B
C
475keV Nb in quenched
E
D
500M
atom a-Cu25Nb75
F
475keV Nb in relaxed 500M atom a-Cu25Nb7 5
H
150 nm
475keV Nb in quenched 474M atom a-CusoNbso
K
L
100nm
iEnm
M
475keV Nb in relaxed 474M atom a-CusoNbw
N
0
P
475keV Nb in quenched 500M atom a-Cu7 FNbs
R
475keV Nb in relaxed 500M atom a-Cu7rNb2 6
Fig. 6.18: Summary of radiation damage in irradiated Cu-Nb alloys. Left column, PKA
trajectories shown with red lines; KA (ke>lkeV) shown with black lines. Center column, red
cubes correspond to regions with Tmax > TG; blue contour corresponds Tmax = 350 K. Right
column, voxels adjacent to liquid zones ( Tmax > 350 K ) with plastic strains
2 ps)> 0.01
E(t-1
are shown as black cubes.
102
As shown in Table 6.1 one of the principal strain directions is typically well aligned with
the direction of the collision cascade (misalignment of less than 200). The principal strain best
aligned with the cascade direction is always negative. Approximately normal to the cascade
direction, a principal strain component is always found to be positive and larger in magnitude
than the strain aligned with the cascade direction. The third principal strain, also approximately
normal to the cascade direction, is generally the smallest in amplitude. This asymmetry in the
strain values corresponds to a stress-free contraction in the direction normal to the beam and a
net expansion in the direction perpendicular to the beam.
Using the average strain in the cascade affected region, I compute the tensile equivalent
strain EPM, normalizing by the region volume fraction f and total effective dose 0 to obtain
A = fEgM/4. As shown in Table 6.1, A has a similar magnitude for all alloys. However, as
shown in Fig. 6.20, the collision-induced plasticity parameter A tends to decreases with
increasing glass transition temperature. For all alloys, less plasticity accumulates in the relaxed
state. These findings suggest that relaxed alloys with higher glass transition temperatures may be
more resistant to collision-induced plasticity.
Properties
Cascade Direction
Alignment
Quenched Relaxed Quenched Relaxed Quenched Relaxed
Cu25Nb75 Cu25Nb75 Cu50Nb5O Cu50Nb5O Cu75Nb25 Cu75Nb25
14
37
18
17
42
11
-1.65
-1.85
-2.46
-2.84
-3.09
-4.62
0.25
2.24
0.57
-1.01
-0.78
2.64
5.36
3.80
3.87
3.89
3.16
0.31
[Degrees]
Eigenvalue parallel
to cascade
direction
[1O~4]
Eigenvalue normal
to cascade
direction [10-4]
Eigenvalue normal
to cascade
direction [10- ]
1.64
1.80
1.48
1.55
1.16
1.62
A 10-15 cm 2 /ion]
Table 6.1: Plasticity in irradiated Cu-Nb alloys. Cascade direction determined by the major axis
of the best-fit ellipsoid to voxels with Tmax > 350 K; eigenvalues computed from the average
plastic strain tensor of voxels adjacent to liquid zones (e.g. 350 < Tmax < TG); the aggregate
collision-induced plasticity parameter is computed as A = f(EPM)/, where (eM) is the average
strain in voxels adjacent to liquid zones and 4 is the dose.
103
15
o Quenched
* Relaxed
0
0
10
0
0
0
F
z
5
0*
1'IL
1900
1400
1500
1600
1700
Glass Transition Temperature
Fig. 6.19: Number of clusters, sorted largest to smallest, comprising 80% of the total thermal
spike volume, versus glass transition temperature of irradiated alloys (Cu 25Nb 75, TG=1400 K;
Cu 5oNb 5 o, TG=1500 K; Cu 75Nb 2 5, TG=1600 K).
2
1.8
0
C F
0
0
V
E 1.6
1.4
1.2
~1I
0 Cu25Nb75
0
11 Cu50Nb5O
0 Cu75Nb25
1 00
1400
1500
1600
Glass transition temperature (K)
1700
Fig. 6.20: Variation of collision-induced plasticity with material glass transition temperature and
annealing. Open symbols correspond to as-quenched state and filled symbols indicate relaxed
state.
104
7 Micro-mechanical model for collision-induced plasticity
7.1
Introduction
In the previous chapter, I demonstrated that the fundamental radiation response
mechanisms of amorphous metal alloys are radiation-induced thermal spikes, which give rise to
the formation of "super-quenched zones" (SQZs) and to polarized collision-induced plasticity
adjacent to SQZs. Identification of collision-induced plasticity, qualitatively distinct from ioninduced plasticity, which arises from electronic excitations, was unexpected and could prove a
serious limitation to application of corrosion-resistant metallic glass coatings in radiation
environments. Furthermore, predicting the susceptibility of numerous metallic glasses to
collision-induced plasticity is challenging, due to the considerable effort and computational
resources required to formulate realistic interatomic potentials, perform simulations, and to
analyze simulation results. Experimental investigations are also expected to be resourceintensive.
In this Chapter, I develop a predictive analytical model for the susceptibility of metallic
glasses to collision-induced plasticity. Using equilibrium material properties, the susceptibility
parameter X successfully ranks the relative resistance of six Cu-Nb amorphous alloys to
collision-induced plasticity. The X parameter may prove a valuable tool for identifying metallic
glasses with optimized radiation tolerance using readily available materials data.
7.2
Micro-mechanical model
As illustrated previously in irradiated as-quenched Cu 5oNb 5 o, confined melting leads to a
rapid, nearly instantaneous increase in the pressure of liquid to values above 10 GPa (see Fig.
6.4). As illustrated in Fig. 6.14, the dramatic rise in pressure is accommodated by rapidly loading
of the surrounding, unmelted material with a stress pulse. Because the local stress approaches or
exceeds the material yield stress, plasticity occurs (Fig. 6.15). This mechanism can be
quantitatively reproduced with a simple micro-mechanical model: step-function pressurization of
a spherical cavity in an infinite elastic medium. Below, I develop the elastic model and compare
it with the results from MD. In Section 7.3, I use the model to rank the susceptibility of each
irradiated alloy to collision-induced plasticity.
7.2.1
Transient analytical solution to pressurized spherical cavity
105
For simplicity, I neglect the ellipsoidal shape of thermal spikes and study the transient
solution to the stress and strain in material surrounding a thermal spike modeled as a spherical
cavity of radius a pressurized with a pressure step pulse of amplitude P at t = 0 (See Fig. 7.3). I
assume that the cavity is embedded in an infinite elastic medium. For this simplified geometry,
the stress response of material adjacent to the spherical cavity, pressurized with a step function,
is described with a transient analytical solution [136].
Displacement field
As given by Graff [136], the boundary conditions and geometry require that the
displacements are purely radial. The displacement field at distance r from the cavity center is:
0
U (r, t) = a3P
uP[12
4pr
(.for
2r
1)0 sin
--
- exp (- T)
Wr
-
cos
orJI
T < 0
T > 0
T>O
where
=2c2
/ ac
r-a
CI
a
b
P
0
Time
Fig. 7.1: Schematic of the micro-mechanical model for the onset of collision-induced plasticity.
(a) Spherical cavity of radius a loaded with an internal pressure P at time t = 0. An analytical
solution describes the transient stress response at a material point r. (b) Schematic of the
assumed step-function loading pressurization of the spherical cavity.
106
The longitudinal speed of sound is c and the speed of sound of shear waves is denoted c ,
2
where cl = [(A + 2p)/p]1/ 2 and c2
=
[(y)/p]1/2 . The material shear modulus is and P and A is
Lame's first parameter. The amplitude of the pressure impulse is denoted P and a is the radius of
spherical cavity at the uniform pressure P. The shifted time -r equals zero when the stress pulse
arrives at the material point r.
Strain field
In spherical coordinates with a purely radial displacement field, the strain displacement
relations are [95]
_du
dr
and
U
=O
=
-
r
Thus, for T > 0 I obtain the radial strain as:
Sr(r,t)=
rr ()
=
a 2P
r
3
{ +exp(- ,)Kac
L+
r2 COS(W)+ 1 (2
r
2() (2r (r - c, + a(2c,
r
c
+ ) 2 )) sin(wyr)
-c)+a(c4
r(
and the tangential strains are:
E0P(r,t)= a 2
-exp(-[r)f(2r
4Mr _
1 -sinw-cosw l
a
)j
Stress field
The constitutive relations for an isotropic, linear elasticity in spherical coordinates are:
Q,,
= (2p + A))err + 2 Ae0
and
(70 = Aerr +(2p + A)ee
where the expressions for the radial and tangential strains are given above.
107
7.2.2
Model-based predictions of transient stress adjacent to thermal spikes
The analytical model described above is able to quantitatively reproduce the transient
stress fields identified adjacent to thermal spikes, without use of any adjustable parameters. As
an example, I model the stress response of material adjacent to the thermal spike studied in detail
in Chapter 6 (Fig. 6.14). To evaluate the expressions for stress, arr and ago, I compute the
needed parameters directly with MD. The size and pressure of the spherical cavity is obtained
directly from the simulation results of the 475 keV Nb atom collision cascade in Cu 5oNb 5 o
(Chapter 6). The material parameters are obtained from previously computed properties in
Cu 5oNb 5 o (Chapter 5).
Model geometry
The geometry of the spherical cavity is obtained directly from the MD simulation results.
The thermal spike of interest, from the irradiated Cu 5oNb 5o system (Fig. 6.15), is reproduced
below in Fig. 7.2 (a). The geometry of the thermal spike is well described as a prolate ellipsoid,
with a total length of 19.6 nm along the major (z) axis and radii of 5 and 3.3 nm in the two
directions normal to the z-axis. To identify the radius of a sphere approximately equivalent to the
thermal spike, I first compute the average of the two minor directions, which yields a radius of
a=4.1 nm. Second, I compute the total volume of the thermal spike is 463 nm 3 and find the
corresponding radius of a sphere of the same volume, yielding a radius a=4.8 nm. Both
approaches yield similar results, and I model the thermal spike with a radius of a=4 nm [see Fig.
7.2 (b)].
Model evaluation with single step function approximation to cavity pressurization
The time-dependent pressure of the thermal spike, averaged over all voxels in the thermal
spike, is plotted as the dashed blue line in Fig. 7.3 (a-c). The short-time behavior is highlighted in
the plot inset. Between the times of 0.3 - 0.5 ps, the pressure abruptly increases from P=0 to
P=15 GPa, before unloading to a residual pressure of 1 GPa at times larger than 3 ps. Using the
expressions for the stress adjacent to the step-function pressurized spherical cavity, arr and uee,
I compute the transient response to a step-function pressurization, P = 15 GPa, of a spherical
cavity of radius a=4 nm, at the radial distance of r-7 nm (3 nm from the cavity surface).
108
From the symmetry of the stress fields, the von Mises equivalent stress reduces to
v
= Iqrr - aToO . The transient response of am (t, r = 7 nm) is plotted in Fig. 7.3 (d) with the
aYM measured with MD in the material adjacent (average distance of 3.2 nm from adjacent voxel
centers to thermal spike surface) to the thermal spike.
The timescales for the rapid rise in stress at the material point adjacent to the thermal
spike is in quantitative agreement between the MD data and the analytical model. However, the
magnitude of the stress pulse, as well as its long-time behavior, are qualitatively different. The
origin of this discrepancy is the assumption that the thermal spike pressure can be modeled with
a step-function. Clearly, pressurization of the thermal spike is much closer to a "pulse" (e.g. a
boxcar function) than a step-function. Therefore, I next explore
approximations of the pressurization of the thermal spike.
more sophisticated
Model evaluation with multiple step function approximation to cavity pressurization
Because the transient solution was developed for a linear elastic material, it is possible to
superimpose the stress response to different step-function pressure inputs, shifted in time, to
build-up more complex initial loading states. Approximating the pressurization of the thermal
spike with two step functions, I analyze the stress response resulting from superimposition of
two different initial loading conditions: A positive step-function pressurization of P=12.1 GPa at
t=0.38 ps, followed by a step-function contraction of P=-l 1.1 GPa at t=0.79 ps (see Table 7.1).
This combined loading state is equivalent to a short-duration, high intensity pulse of amplitude
ab
X
x
Fig. 7.2: Comparison of thermal spike data from molecular dynamics simulation of 475 keV Nb
irradiation of Cu5 oNb5o and transient linear elastic model. (a) Thermal spike zone, with red cubes
indicating voxels with Tmax>1500 K and black cubes indicating EPM >0.01. (b) Spherical cavity
approximation of thermal spike in (a) with a radius r-4 nm.
109
12.1 GPa, followed by a long-duration pulse of 1.1 GPa [see Fig. 7.3 (b)]. The stress amplitude
and width of these two step functions are chosen such that the integral of the combined functions
is equal to the integral of the entire P(t) response measured in the irradiated material. The stress
fields (qrr and qr0 ) are evaluated independently for the two loading states at each material point
for all times. The superposition of these stress fields at the appropriate times and radial positions
yields the total stress due to the two step cavity pressurization. The von Mises stress is computed
from the superimposed values of a, and e,.
As can be seen in Fig. 7.3 (e), the agreement between the stress response from the model
and from the MD data is remarkably good at short times. The average stress at the peak
amplitude of the stress pulse, as well as its time of arrival to the material point, is identical to the
MD data. While the long-time stress response is incorrect, this is hardly surprising in light of the
ellipsoidal geometry of the thermal spike, as well as neglect of interactions with other adjacent
thermal spikes.
ab
15
c-
15
-------
15
12
9
10
6
C-
6
a_50
a.a
O
C
.
3
0
j
.5t25
12
4
d
6
8
Time (ps)
10
12
0.5
0
-- -- - --- - --- -- ------------
2
-- - -----
0
e
2
4
--
3
0.75
--
1
1.25
&25
0
oH 2
Data, Adjacent TS
-Step
Model
0.5
0.75
1
0_
10
12
1.2
__ _
0
f
2
4
Data, Adjacent TS
Two Step Model
6
8
Time (ps)
10
12
---Data, Adja
-
4
0IV
5-
a.51.
-- -
6
8
Time (ps)
--
4
9
.10
a. 5
.5
0
12
9 ,
-10
Multistep Model
4
-43
9o3
---
4
6
8
Time [psi
10
12
0
2
4
6
8
Time [ps]
10
12
00
2
4
6
Time
8
[ps]
10
12
Fig. 7.3: Application of transient elastic model to model stress response of material adjacent to
thermal spikes. The pressure input is modeled with a single step function (a), two step functions
(b), and multiple step functions (c). The dashed blue line (a-c) is the pressure measured in the
thermal spike shown in Fig. 7.2 (a). The approximation for P(t) is shown in the solid black line
(a-c). Using the P(t) approximation shown in (a-c), the stress response of a material point at r = 7
nm is plotted with a solid black line. The actual stress data measured at this material point
adjacent to the thermal spike is plotted in the dashed black line.
110
Inputs
P(t) Input
Step
Pulse
to
#1
0.5
tf
--
Total Output
P
15
a
4
dw- <(0.9<t<1.3)>
3
Data
Model
3.00
2.46
<(5<t<12.5)>
Data
Model
1.04
4.24
Two Step
#1
0.38
0.79
12.2 4 3
3.00
2.95
1.04
0.30
Two__Step
#2
0.79
12.5 -11.1 4
3
3.0_
29_.4
.3
Multistep
41 Step Functions
4
3
3.00
3.30
1.04
0.27
Table 7.1: Model inputs for Fig. 7.3. The average stress predicted at various times is indicated,
as well as the actual stress measured in the material in the irradiated material.
To demonstrate that the agreement in Fig. 7.3 (e), due to the two step function input Fig.
7.3 (b), is not due to arbitrary tuning of the width and amplitude of the two step functions, I
repeat the process using an integrated approximation of the entire P(t) profile, with an
approximately constant time increment of dt=0.I ps [see Fig. 7.3 (c)]. As show in Fig. 7.3 (f), the
agreement is likewise excellent. For a comparison of the results of the model predictions for the
different P(t) input approximations, refer to Table 7.1.
7.2.3
Model-based predictions of maximum stress adjacent to thermal spikes
Above, I demonstrated that superposition of step-function approximations for the
pressurization of a spherical cavity is able to reproduce the transient stress response of material
adjacent to thermal spikes. Now, I turn my attention to predicting the maximum von Mises
40
Plasticity,
30
20
S 10
U
0
5
10
=3GPa
15
r [nm]
Fig. 7.4: Model-based prediction of maximum von Mises stress (um) as a function of distance
from the surface of the thermal spike.
111
stress, ul"LX, in material adjacent to thermal spikes. Using the two-step function approximation
of thermal spike pressurization, presented above in Fig. 7.3(b) and Fig. 7.3(e), I compute the
transient stress response am (r,t) at every material point. Using this transient stress response, I
subsequently evaluate the maximum in the von Mises stress. The result is plotted in Fig. 7.4. In
excellent agreement with the results from Chapter 6, plasticity is only predicted within the first 4
nm away from the surface of the thermal spike. This result provides another demonstration that
the physical mechanism, and its micro-mechanical analog, are in good agreement.
7.3
Modeling onset of collision-induced plasticity
Having demonstrated that the simple transient linear elastic model is able to capture the
stress response adjacent to thermal spikes remarkably well, without the use of any free,
adjustable parameters, I next extend the model to formulate a parameter able to predict the
susceptibility of irradiated metallic glasses to collision-induced plasticity. The derivation of the
parameter proceeds in three steps. First, I show that the thermal spike is a dynamic stress
concentrator, inducing a maximum stress:
1- V
Second, I require that yield occurs when:
avM
> cY
Third, I develop a thermodynamic model for predicting the pressure in thermal spikes, which is
used to predict the maximum stress adjacent to thermal spikes.
7.3.1
Maximum von Mises stress adjacent to thermal spikes
As illustrated above in Fig. 7.4, the maximum stress adjacent to the thermal spike is at the
interface of the thermal spike and adjacent, unmelted material. While predicting the stress
response in adjacent material requires the use of at least a two-step function approximation for
the pressurization of the thermal spike, the maximum stress at the interface is dictated solely the
magnitude of the first step pulse. Thus, I evaluate the maximum stress at the thermal spike
interface, in reponse to a single step-function.
As given above, the von Mises stress is am = arr - aoe 1. Evaluating, I obtain the timedependent von Mises stress at a distance r from the cavity surface:
112
,n(r-r)
3a3p
2r3 +exp (-;)
((3ac, - 2r)
-r;+C2)) inwwr
cosaw + (2r;(-2c,+ r;)+ a (3c,(-r+)sin
The stress is maximum at the interface, r -> a, at the initial pressurization, r -+ 0.
Evaluating aV (a, 0), the equation for the transient von Mises equivalent stress reduces to:
2max
-V
max
The Poisson ratio v in a typical metal is v = 0.33, yielding Umax ~ 2.5P1 Pmax For comparison, I evaluate uvm (a, oo) to obtain the static solution of the maximum stress,
finding:
3
Csmtatic =31p.
=2
The average maximum pressure inside thermal spikes in irradiated Cu50Nb5O is
Pmax = 13 GPa, while the average residual pressure (i.e. at times t > 10 ps) is P" = 1 GPa.
Evaluating the ratio of the dynamic and static stress concentration factors, aMa
atic = 22, it
is evident that only by considering the transient pressurization of the thermal spike can stresses
sufficiently large for yielding be obtained. The material will yield if Cmatx > Cy, where Uy is the
material yield strength. Thus, all that remains is to develop an expression for Pmax7.3.2 Maximum pressure inside thermal spikes
Using thermodynamics, I develop a simple expression to predict the pressure inside
thermal spikes. The prediction of the pressure is based on a hypothetical three-step process. First,
energy is added to the thermal spike and its temperature increases rapidly. Second, the increase
in temperature leads to an increase in the stress-free volume through thermal expansion. Third,
the unmelted material adjacent to the thermal spike compresses the liquid back to its initial
volume, pressurizing the thermal spike. Below, I develop expressions for each of these three
steps. Evaluation of each step is illustrated below in Table 7.2.
First, I assume that the temperature rise inside the thermal spike is given by
CP =
AQ
A
yielding
TF
= TO + -Cp
113
To is the initial temperature, AQ is the average increase in energy per atom in the thermal spike,
and C, is the heat capacity at the glass transition temperature. As a first approximation, I
evaluate for TF assuming the value of the heat capacity at the glass transition temperature.
Second, I assume that the increase in temperature leads to a uniform thermal expansion,
AV
--
V0
G(GL(
= 3a(TG
- TO) + 3aL (TF
TG)
To is the initial temperature, TG is the glass transition temperature, and TF is the final temperature
of the thermal spike (predicted above). I assume that the thermal expansion in the glass (ac)
is
constant up to the glass transition and that the thermal expansion in the liquid (at) is also a
constant.
Finally, I assume that unmelted surrounding material is rigid, leading to a compression of
the liquid according to the isothermal compressibility. Recalling the expression for adiabatic
compressibility (Ps),
1 (aV
V aPIs
I assume that the 1/V term is a constant and write the differentials as:
1 dV
dP =-
fls VO
Integrating and noting that the initial pressure is zero,
1 AV
Because I assume that the surrounding material exerts a negative strain (compression from stress
free to pressurized), the negative sign is dropped:
Pf =
1 AV
fls V0
I assume that fls is evaluated at the glass transition temperature.
Putting everything together, I obtain:
Pf = 1
3ai(TG
-
T) + 34 (T0
-
which simplifies to:
3
Pf=flS
_aG)
- (TT)(aL
tLAQ
LJ
GOL
Lc
114
G+
7.3.3
Collision-induced plasticity susceptibility parameter X
The material will yield when am" > cy. Therefore, I define the ratio of the dynamic
stress concentration (a0 m") to the material yield stress as,
X =y
A
Y
or
X
=Pmax
/y
1
Because the maximum pressure inside the thermal spike is hydrostatic, and all metals have a
positive Poisson ratio, I drop the absolute value signs and simply write:
S2 - v
X
=
Pmax (
1
_)
/y
The collision-induced susceptibility parameter X has a value X < 1 if the thermal spike induced
stress pulse does not exceed the material yield stress. Such materials should be resistant to
collision-induced plasticity. For X > 1, X indicates that the stress pulse exceeds the yield stress,
and predicts that the material will exhibit collision-induced plasticity. Since X > 1 indicates the
onset of plastic flow, I predict larger values of X will correlate with more plastic strain.
Combining the expressions for the maximum von Mises stress with the pressure due to
Values of constants from MD
simulation of 475 keV Nb ion in
Expression
value
Value/source
Cu5 oNb 5 o
Thermal
spike
temperature
AQ
TF = T0 +
TO=300 K,
TG=1500 K
2119
+305K
AQ = 1.27 eV/atom
'
3795 K
C,(TG
= 3.63x10- 4 eV/(atom K)
Va
.
ChangeimOa
Volume
TO)
3ct(TG
+ 3a (TF
Thermal
spike
pressure
(TO)
1 AV
Pf
=
= 9x10- 6 1/K
6
cf (TO) = 17x10To=300 K,
o
G
Thermal
spikes
1/K
0.1517
Quenched
liquid
TG=1500 K
TF = 3795 K (expression above)
fls(TG) = 1X10- 1 1/GPa
41A= 0.1517 (expression above)
VO
Table 7.2: Predicting the maximum stress in thermal spikes.
115
13.3 GPa
15.2 GPa
Thermal
spikes
the thermal spike energy, I finally obtain the following expression:
S{LQ
fls
2_V
L
CP
-(TG
- T)(aL aL)
1
1- _V UY
This expression can be evaluated for various materials to identify those with high or low
susceptibility to collision-induced plasticity.
7.4 Validation of micro-mechanical model with irradiated Cu-Nb alloys
7.4.1 Testing damage resistance parameter X with simulation data
To test the reliability of the collision-induced plasticity resistance parameter X, I compute
X for all six alloys in two ways. First, I compute the average maximum pressure in thermal
spikes and directly evaluate
X
=
Pmax
(2 - v
( _)
/y
Second, I compute X,
(TG - T)(aL
X= 'aLQ
s
where the material constants
-
Is aL, TG,
)
1-
Lc
C,
V cy
Cp, cY, and v are all obtained from molecular dynamics
simulations.
EvaluatingX using thermal spike pressurefrom MD simulations
As can be seen in Fig. 7.5 (a), the pressure does tend to increase with glass transition
temperature. Similarly, Fig. 7.5 (b), X increases with glass transition temperature in the materials.
15.5
17
-As
Quenched
- - - Relaxed
16-
1515
14.51
14
2 14-
?13
X
13.5 -
12
101
12.5
Cu25Nb75
Cu50Nb5O
Cu75Nb25
9
Cu25Nb75
Cu50Nb5O
Cu75Nb25
Fig. 7.5: (a) Variation of thermal spike pressure with material. (b) variation of X with material
type, evaluated using pressure measured in thermal spikes.
116
It is evident that X varies by a factor of approximately 2 between the three different alloys. This
suggests that metallic glasses may have widely varying resistance to collision-induced plasticity
and encourages characterization of the radiation damage resistance in other metallic glass alloy
systems.
EvaluatingX using thermal spike pressures computedfrom materialproperties
The material properties used to evaluate X are computed at 300 K, with the exception of
the Poisson ratio, which was evaluated from the 0 K elastic constants (Properties summarized in
Chapter 5). The average energy deposited per atom in thermal spikes, AQ, was previously shown
in Chapter 6 to be independent of thermal spike energy and size in the irradiated Cu 5 oNb5 o asquenched system. The energy flow into thermal spikes, AQ, is approximated by dividing the total
PKA energy by the total thermal spike volume. The compressibility is the inverse of the bulk
modulus (K), so I take f3s = 1/K. The bulk modulus in quenched Cu 5oNb5 o at the glass transition
temperature is K(1500 K) = 100 GPa, yielding fls(TG) = 1x10-" 1/GPa. As a first
approximation, I assume that /3s (TG) is equal for the different systems.
Evaluating X using the individual system material properties, in Fig. 7.6 I plot the X
computed from the actual thermal spike pressures (averaged over the thermal spikes in each
system) versus the X computed from material properties alone. The agreement between the two
0
20
2*
10
-
Cu25Nb75
L Cu5ONb5O
, '
KCu75Nb25
5
10
15
20
x (Thermal Spikes)
25
30
Fig. 7.6: Collision-induced susceptibility parameter X computed from material properties versus
X evaluated directly from thermal spike properties. Open symbols correspond to as-quenched
systems while filled symbols indicate relaxed systems.
117
approaches is remarkably good for the Cu 25Nb75 and Cu 5oNb5o systems, suggesting that X can in
fact be computed from material properties alone. The X computed from material properties in
Cu 7 5Nb 2 5 shows poor agreement, but this may be a consequence of assuming a constant value of
fls(TG) for the three systems. This discrepancy is identified as a good opportunity for future
research.
118
8 Conclusions
In this Thesis, I have demonstrated that the atomic-level radiation damage mechanisms in
irradiated metallic glasses are qualitatively different from the mechanisms in irradiated
crystalline alloys. While the thermal spikes in crystalline alloys quench down to a defective
crystalline, thermal spikes in metallic glasses quench to form amorphous low-density zones
(SQZs), with the SQZ properties determined by the quench rate of the thermal spike liquid.
Because of the extremely high (~1014 K/s) quench rates in thermal spikes, SQZs have a lower
density than the parent, un-irradiated material. Under continuous radiation, SQZs will eventually
overlap and the bulk properties of the material will converge to that of the liquid quenched at the
thermal spike quench rate.
Additionally, I have shown that the dynamics of the collision cascade process directly
affect the damage left by irradiation. Constrained melting in the thermal spike leads to a highly
pressurized liquid that emits a stress pulse that permanently deforms the unmelted material
adjacent liquid zones. This dynamic mechanism reflects the unique deformation mechanisms of
metallic glasses and emphasizes the unique role that the amorphous atomic structure plays in
radiation response.
The insights obtained through my analysis of the irradiated Cu-Nb amorphous alloys
demonstrate the necessity of analyzing the spatiotemporal variations of nanoscale properties and
no just aggregate measures of structure, such as the fraction of all atoms in icosahedral order.
These insights required the use of parallelized post-processing analysis, a technique that will
likely find useful application in the simulation of other materials at the atomic scale.
The onset of collision-induced plasticity can be quantitatively described with a simple
micro-mechanical model. A future research opportunity would be to screen bulk metallic glasses
for radiation damage resistance, based on the predictions of the theory. This may enable glasses
with optimized radiation resistance to be identified.
The quenching processes in thermal spikes presented an opportunity to probe the glass
transition physics in Cu 5 oNb5o. My identification that the glass transition occurs by gelation may
open a new research opportunity for synthesis of more conventional bulk metallic glasses.
119
120
9
1.
References
Averback, R. & de la Rubia, T. Displacement damage in irradiated metals and
semiconductors. Solid State Physics 51, 281-402 (1998).
2. Calder, A. F., Bacon, D. J., Barashev, A. V., & Osetsky, Y. N. On the origin of large
interstitial clusters in displacement cascades. Philos. Mag. 90, 863-884 (2010).
3. Cawthorne, C. & Fulton, E. J. Voids in irradiated stainless steel. Nature 216, 575-576 (1967).
4. Mansur, L. K. Theory and experimental background on dimensional changes in irradiated
alloys. J.Nucl. Mater. 216, 97-123 (1994).
5. Odette, G. R., Wirth, B. D., Bacon, D. J., & Ghoniem, N. M. Multiscale-multiphysics
modeling of radiation-damaged materials: Embrittlement of pressure-vessel steels. MRS Bull.
26, 176-181 (2001).
6. Odette, G. R. & Lucas, G. E. Embrittlement of nuclear reactor pressure vessels. JOM 53, 1822 (2001).
7. Demkowicz, M. J., Bellon, P., & Wirth, B. D. Atomic-scale design of radiation-tolerant
nanocomposites. MRS Bull. 35, 992-998 (2010).
8. Demkowicz, M. J. & Hoagland, R. G. Simulations of collision cascades in Cu-Nb layered
composites using an EAM interatomic potential. IJAM 1, 421-442 (2009).
9. Was, G. S. Fundamentals of Radiation Materials Science. Springer, Berlin, (2007).
10. Race, C. P., Mason, D. R., Finnis, M. W., Foulkes, W. M. C., Horsfield, A. P., & Sutton, A.
P. The treatment of electronic excitations in atomistic models of radiation damage in metals.
Rep. Prog.Phys. 73, 116501 (2010).
11. de la Rubia, T. D., Averback, R. S., Hsieh, H., & Benedek, R. Molecular dynamics
simulation of displacement cascades in Cu and Ni: Thermal spike behavior. J.Mater. Res. 4,
579-586 (1989).
12. Zhu, H., Averback, R. S., & Nastasi, M. Molecular dynamics simulations of a 1 OkeV cascade
in beta-NiAl. Philos. Mag. A 71, 735-758 (1995).
121
13. Zinkle, S. J. Fusion materials science: Overview of challenges and recent progress. Phys.
Plasmas 12, 058101 (2005).
14. Bacon, D. J., Gao, F., & Osetsky, Y. N. The primary damage state in fcc, bcc and hcp metals
as seen in molecular dynamics simulations. J.Nucl. Mater. 276, 1 - 12 (2000).
15. Gerling, R. & Wagner, R. Density of neutron irradiated and annealed amorphous
Fe40Ni40B20. Scripta Metall. 16, 963-967 (1982).
16. Gerling, R. & Wagner, R. Properties of in-core reactor-irradiated amorphous Fe40Ni4OB20.
J.Nucl. Mater. 107, 311-317 (1982).
17. Gerling, R., Schimansky, F. P., & Wagner, R. Restoration of the ductility of thermally
embrittled amorphous-alloys under neutron-irradiation. Acta Metall. 35, 1001-1006 (1987).
18. Hou, M.-d., KlaumUnzer, S., & Schumacher, G. Dimensional changes of metallic glasses
during bombardment with fast heavy ions. Phys. Rev. B 41, 1144-1157 (1990).
19. Debenedetti, P. G. & Stillinger, F. H. Supercooled liquids and the glass transition. Nature
410, 259-267 (2001).
20. Angell, C. A. Formation of Glasses from Liquids and Biopolymers. Science 267, 1924-1935
(1995).
21. Schuh, C. A., Hufnagel, T. C., & Ramamurty, U. Mechanical behavior of amorphous alloys.
Acta. Mater. 55, 4067-4109 (2007).
22. Blink, J., Farmer, J., Choi, J., & Saw, C. Applications in the nuclear industry for thermal
spray amorphous metal and ceramic coatings. Metall. Mater. Trans. A 40A, 1344-1354
(2009).
23. Kumar, G., Tang, H. X., & Schroers, J. Nanomoulding with amorphous metals. Nature 457,
868-872 (2009).
24. Johnson, W. L. Bulk glass-forming metallic alloys: science and technology. MRS Bull. 24,
42-56 (1999).
25. Michaelsen, C., Gente, C., & Bormann, R. The thermodynamics of amorphous phases in
immiscible systems: The example of sputter-deposited Nb--Cu alloys. J.Appl. Phys 81, 6030
(1997).
122
26. Zhang, R., Li, J., & Liu, B. Dual-phase metallic glass and its two-dimensional fractal
morphology. Journalof the Physical Society ofJapan 74, 2937-2940 (2005).
27. Klement, W., Willens, R. H., & Duwez, P. Non-crystalline Structure in Solidified GoldSilicon Alloys. Nature 187, 869-870 (1960).
28. Duwez, P. & Willens, R. H. Rapid quenching of liquid alloys. Trans. Metall. Soc. AIME 227,
362 (1963).
29. Kittel, C. Introduction to Solid State Physics. Wilely, New York (1975).
30. BERNAL, J. D. A Geometrical Approach to the Structure Of Liquids. Nature 183, 141-147
(1959).
31. Bernal, J. D. The Bakerian Lecture, 1962. The Structure of Liquids. Proc.R. Soc. A 280,
299-322 (1964).
32. Miracle, D. B. A structural model for metallic glasses. Nat. Mater. 3, 697 - 702 (2004).
33. Sheng, H. W., Luo, W. K., Alamgir, F. M., Bai, J. M., & Ma, E. Atomic packing and shortto-medium-range order in metallic glasses. Nature 439, 419-425 (2006).
34. Hirata, A., Guan, P., Fujita, T., Hirotsu, Y., Inoue, A., Yavari, A. R., Sakurai, T., & Chen, M.
Direct observation of local atomic order in a metallic glass. Nat. Mater. 10, 28-33 (2011).
35. Hwang, J., Melgarejo, Z. H., Kalay, Y. E., Kalay, I., Kramer, M. J., Stone, D. S., & Voyles,
P. M. Nanoscale Structure and Structural Relaxation in Zr5oCu 4 5 AI5 Bulk Metallic Glass.
Phys. Rev. Lett. 108, 195505 (2012).
36. Banerjee, R., Puthucode, A., Bose, S., & Ayyub, P. Nanoscale phase separation in
amorphous immiscible copper-niobium alloy thin films. Appl. Phys. Lett. 90, 021904 (2007).
37. He, J. H., Sheng, H. W., Schilling, P. J., Chien, C. L., & Ma, E. Amorphous structures in the
immiscible Ag-Ni system. Phys. Rev. Lett. 86, 2826-2829 (2001).
38. Luo, W. K., Sheng, H. W., Alamgir, F. M., Bai, J. M., He, J. H., & Ma, E. Icosahedral ShortRange Order in Amorphous Alloys. Phys. Rev. Lett. 92, 145502 (2004).
39. Baumer, R. E. & Demkowicz, M. J. Glass transition by gelation in a phase separating binary
alloy. Phys. Rev. Lett. 110, 145502 (2013).
123
40. Spaepen, F. A microscopic mechanism for steady state inhomogeneous flow in metallic
glasses. Acta Metall. 25, 407-415 (1977).
41. Argon, A. S. Plastic deformation in metallic glasses. Acta Metall. 27, 47 - 58 (1979).
42. Hofmann, D. C., Suh, J. Y., Wiest, A., Lind, M. L., Demetriou, M. D., & Johnson, W. L.
Development of tough, low-density titanium-based bulk metallic glass matrix composites
with tensile ductility. Proc.Nat. Acad Sci. USA 105, 20136-20140 (2008).
43. Demetriou, M. D., Launey, M. E., Garrett, G., Schramm, J. P., Hofmann, D. C., Johnson, W.
L., & Ritchie, R. 0. A damage-tolerant glass. Nat. Mater. 10, 123-128 (2011).
44. Wagner, H., Bedorf, D., Kuechemann, S., Schwabe, M., Zhang, B., Arnold, W., & Samwer,
K. Local elastic properties of a metallic glass. Nat. Mater. 10, 439-442 (2011).
45. Mayr, S. G. Relaxation kinetics and mechanical stability of metallic glasses and supercooled
melts. Phys. Rev. B 79, 060201 (2009).
46. Ye, J. C., Lu, J., Liu, C. T., Wang, Q., & Yang, Y. Atomistic free-volume zones and inelastic
deformation of metallic glasses. Nat. Mater. 9, 619-623 (2010).
47. Chen, H., He, Y., Shiflet, G. J., & Poon, S. J. Deformation-induced nanocrystal formation in
shear bands of amorphous alloys. Nature 367, 541--543 (1994).
48. Kerrache, A., Mousseau, N., & Lewis, L. J. Crystallization of amorphous silicon induced by
mechanical shear deformations. Phys. Rev. B 84, 014110 (2011).
49. Magagnosc, D. J., Ehrbar, R., Kumar, G., He, M. R., Schroers, J., & Gianola, D. S.
Tunable tensile ductility in metallic glasses. Sci. Rep. 3, 1096 (2013).
50. Chaki, T. K. & Li, J. C. M. Heavy-ion damage of an amorphous metallic alloy. J.Nucl.
Mater. 140, 180-184 (1986).
51. Chang, B. T.-A. & Li, J. C. M. Irradiation swelling of amorphous Fe 40Ni 4OP
Scripta Metall. 11, 933-936 (1977).
4 B6
alloy.
52. Morris, D. G. Voids in an Amorphous Material.Scripta Metall. 14, 879-880 (1980).
53. Morris, D. G. Voids in an Amorphous Material - Reply. ScriptaMetall. 15, 813-814 (1981).
124
54. Shibayama, T., Hashimoto, T., Kayano, H., Yamaguchi, S., & Inoue, A. Effects of heavy ion
irradiation in low activation ferritic amorphous steels. Sci. Rep. Res. Inst. Tokohu Univ. A,
Phys. Chem. Metall. (Japan)40, 69 - 76 (1994/07/).
55. Tiwari, R. S. & Von Heimendahl, M. Voids in an Amorphous Material - Comment. Scripta
Metall. 15, 809-811 (1981).
56. Raghavan, R., Boopathy, K., Ghisleni, R., Pouchon, M. A., Ramamurty, U., & Michler, J.
Ion irradiation enhances the mechanical performance of metallic glasses. Scripta Mater. 62,
462-465 (2010).
57. Raghavan, R., Kombaiah, B., Doebeli, M., Erni, R., Ramamurty, U., & Michler, J.
Nanoindentation response of an ion irradiated Zr-based bulk metallic glass. Mat. Sci. Eng. AStruct 532, 407-413 (2012).
58. Averback, R. S. & Hahn, H. Radiation-Enhanced Diffusion in Amorphous Ni-Zr Alloys.
Phys. Rev. B 37, 10383-10386 (1988).
59. Frank, W., Hamlescher, U., Kronmuller, H., Scharwaechter, P., & Schuler, T. Diffusion in
amorphous metallic alloys - Experiments, molecular-dynamics simulations, interpretation.
Physica Scripta T66, 201-206 (1996).
60. Faupel, F., Frank, W., Macht, M. P., Mehrer, H., Naundorf, V., Ratzke, K., Schober, H. R.,
Sharma, S. K., & Teichler, H. Diffusion in metallic glasses and supercooled melts. Rev. Mod.
Phys. 75, 237-280 (2003).
61. Klauminzer, S., Li, C., Loffler, S., Rammensee, M., Schumacher, G., & Neitzert, H. C. Ionbeam-induced plastic deformation: a universal behavior of amorphous solids. Radiation
Effects and Defects in Solids 108, 131-135 (1989).
62. Trinkaus, H. & Ryazanov, A. I. Viscoelastic model for the plastic flow of amorphous solids
under energetic ion bombardment. Phys. Rev. Lett. 74, 5072-5075 (1995).
63. Trautmann, C., Klaumunzer, S., & Trinkaus, H. Effect of stress on track formation in
amorphous iron boron alloy: Ion tracks as elastic inclusions. Phys. Rev. Lett. 85, 3648-3651
(2000).
64. Mayr, S. G., Ashkenazy, Y., Albe, K., & Averback, R. S. Mechanisms of radiation-induced
viscous flow: role of point defects. Phys. Rev. Lett. 90, 055505 (2003).
65. F. Spaepen, in Defects in Amorphous Metals, Les Houches Lectures XXXV on Physics of
Defects edited by R. Balian et al. 133. (North-Holland, Amsterdam, 1981),
125
66. Carter, J., Fu, E. G., Martin, M., Xie, G., Zhang, X., Wang, Y. Q., Wijesundera, D., Wang,
X. M., Chu, W.-K., & Shao, L. Effects of Cu ion irradiation in Cu5 oZr 45Ti5 metallic glass.
Scripta Mater. 61, 265 - 268 (2009).
67. Nagase, T. & Umakoshi, Y. Thermal stability and electron irradiation effect on Zr-based
amorphous alloys. J.App. Phys. 93, 912-918 (2003).
68. Rechtin, M. D., Sande, J. V., & Baldo, P. M. Ion-implantation damage in amorphous and
crystalline Nb 4oNi6 o. Scripta Metallurgica12, 639 - 643 (1978).
69. Brimhall, J. L., Charlot, L. A., & Wang, R. Irradiation effects in amorphous and crystalline,
sputter-deposited Mo-Ni. Scripta Metallurgica13, 217 - 220 (1979).
70. Dunlop, A., Jaskierowicz, G., Rizza, G., & Kopcewicz, M. Partial crystallization of an
amorphous alloy by electronic energy deposition. Phys. Rev. Lett. 90, 015503 (2003).
71. Rizza, G., Dunlop, A., & Kopcewicz, M. Deformation bands in metallic glasses induced by
swift heavy ions. Nucl. Instrum. Meth. B 245, 130 - 132 (2006).
72. Yamamoto, R., Shibuta, H., & Doyama, M. Computer simulation of radiation damage in
amorphous metals. J.Nucl. Mater. 85-86, 603 - 606 (1979).
73. Chaki, T. K. & Li, J. C. M. Radiation damage in an amorphous Lennard-Jones solid. A
computer simulation. Philos. Mag. B 51, 557-565 (1985).
74. Laakkonen, J. & Nieminen, R. M. Computer simulations of radiation damage in amorphous
solids. Phys. Rev. B 41, 3978-3997 (1990).
75. Mattila, T., Nieminen, R. M., & Dzugutov, M. Simulation of radiation-induced structural
transformation in amorphous metals. Phys. Rev. B 53, 192-200 (1996).
76. Dzugutov, M. Monatomic model of icosahedrally ordered metallic glass formers. J. NonCryst. Solids 156-158, 173-176 (1993).
77. Mayr, S. G. Impact of ion irradiation on the thermal, structural, and mechanical properties of
metallic glasses. Phys. Rev. B 71, 144109 (2005).
126
78. Xiao, Q., Huang, L., & Shi, Y. Suppression of shear banding in amorphous ZrCuAl
nanopillars by irradiation. J.Appl. Phys. 113, 083514 (2013).
79. Avchaciov, K. A., Ritter, Y., Djurabekova, F., Nordlund, K., & Albe, K. Controlled softening
of Cu64Zr36 metallic glass by ion irradiation. Appl. Phys. Lett. 102, 181910 (2013).
80. Chaudhari, P., Spaepen, F., & Steinhardt, P. J. Defects and atomic transport in metallic
glasses. Topics in Applied Physics 53, 127-168 (1983).
81. Daw, M. S., Foiles, S. M., & Baskes, M. I. The embedded-atom method: a review of theory
and applications. MaterialsScience Reports 9, 251 - 310 (1993).
82. Bertseka, D. P. Nonliner Programming,Athena Scientific, (1995).
83. Allen, M. P. & Tildesley, D. J. Computer Simulations of Liquids. (Oxford University Press,
Oxford, 1987).
84. Honeycutt, J. D. & Andersen, H. C. Molecular-dynamics study of melting and freezing of
small Lennard-Jones clusters. J Phys. Chem. 91, 4950-4963 (1987).
85. Faken, D. & Jonsson, H. Systematic analysis of local atomic structure combined with 3D
computer graphics. ComputationalMaterials Science 2, 279 - 286 (1994).
86. Tsuzuki, H., Branicio, P. S., & Rino, J. P. Structural characterization of deformed crystals by
analysis of common atomic neighborhood. Comput. Phys. Commun. (Netherlands)177, 518 23 (15 Sept. 2007).
87. Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO--the
Open Visualization Tool. Model. Simul. Mater. Sci. 18, 015012 (2010).
88. Childs, H., Brugger, E. S., Bonnell, K. S., Meredith, J. S., Miller, M., Whitlock, B. J., &
Max, N. A contract-based system for large data visualization. IEEE Visualization 2005, 190198 (2005).
89. Tsamados, M., Tanguy, A., Goldenberg, C., & Barrat, J.-L. Local elasticity map and
plasticity in a model Lennard-Jones glass. Phys. Rev. E 80, 026112 (2009).
90. Nomura, K.-i., Chen, Y.-C., Kalia, R. K., Nakano, A., & Vashishta, P. Defect migration and
recombination in nanoindentation of silica glass. Appl. Phys. Lett. 99, 111906-111906-3
(2011).
127
91. Branicio, P. S., Kalia, R. K., Nakano, A., Vashishta, P., Shimojo, F., & Rino, J. P. Atomistic
damage mechanisms during hypervelocity projectile impact on AIN: A large-scale parallel
molecular dynamics simulation study. J.Mech. Phys. Solids 56, 1955-1988 (2008).
92. McQuarrie, D. A. Statistical Mechanics. University Science Books, Sausalito, CA, (2000).
93. Hsieh, H., Diaz de la Rubia, T., Averback, R. S., & Benedek, R. Effect of temperature on the
dynamics of energetic displacement cascades: A molecular dynamics study. Phys. Rev. B 40,
9986-9988 (1989).
94. Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J.Comp. Phys.
117, 1-19 (1995).
95. Bower, A. F. Applied Mechanics of Solids. CRC Press, Boca Raton, (2009).
96. Falk, M. L. & Langer, J. S. Dynamics of viscoplastic deformation in amorphous solids. Phys.
Rev. E 57, 7192-7205 (1998).
97. Shimizu, F., Ogata, S., & Li, J. Theory of shear banding in metallic glasses and molecular
dynamics calculations. Mater. Trans. 48, 2923-2927 (2007).
98. Cheng, Y. Q., Ma, E., & Sheng, H. W. Atomic Level Structure in Multicomponent Bulk
Metallic Glass. Phys. Rev. Lett. 102, 245501 (2009).
99. Wang, T. L., Li, J. H., Tai, K. P., & Liu, B. X. Formation of amorphous phases in an
immiscible Cu-Nb system studied by molecular dynamics simulation and ion beam mixing.
Scripta Mater. 57, 157 - 160 (2007).
100. Tai, K. P., Wang, T. L., Li, J. H., & Liu, B. X. Observations of distinct atomic packings in
Cu--Nb metallic glasses synthesized by ion beam mixing. J.Phys.: Condens. Matter 18,
L459-L464 (2006).
101. Banerjee, R., Bose, S., Genc, A., & Ayyub, P. The microstructure and electrical transport
properties of immiscible copper-niobium alloy thin films. J.Appl. Phys. 103, 7 (2008).
102. Puthucode, A., Kaufman, M. J., & Banerjee, R. Early Stages of Crystallization in PhaseSeparated Amorphous Copper-Niobium Alloy Thin Films. Metallurgicaland Materials
TransactionsA 39, 1578-1584 (2008).
103. Bose, S., Puthucode, A., Banerjee, R., & Ayyub, P. The influence of nanoscale phase
separation and devitrification on the electrical transport properties of amorphous Cu--Nb
128
alloy thin films. J.Phys.: Condens. Matter 21, 285305 (2009).
104. Ma, E. Alloys created between immiscible elements. Progressin MaterialsScience 50, 413
- 509 (2005).
105. Nastasi, M., Mayer, J. W., & Hirvonen, J. K. Ion-Solid Interactions,Cambridge University
Press, (1996).
106. Zhang, Y., Mattern, N., & Eckert, J. Atomic structure and transport properties of
Cu 5oZr 4 5Al5 metallic liquids and glasses: Molecular dynamics simulations. J Appl. Phys.
110, 093506 (2011).
107. Moine, P., Naudon, A., Kim, J. J., Marshall, A. F., & Stevenson, D. A. Characterization of
Ni-Ti alloys synthesized by vapor quenching. J.Phys. Colloques 46, C8-223-C8-227 (1985).
108. Bhatia, A. B. & Thornton, D. E. Structural Aspects of the Electrical Resistivity of Binary
Alloys. Phys. Rev. B 2, 3004--3012 (1970).
109. Zaccarelli, E. Colloidal gels: equilibrium and non-equilibrium routes. J Phys.: Condens.
Matte 19, (2007).
110. Patrick Royall, C., Williams, S. R., Ohtsuka, T., & Tanaka, H. Direct observation of a local
structural mechanism for dynamic arrest. Nat Mater. 7, 556-561 (2008).
111. Zallen, R. The Physics ofAmorphous Solids. John Wiley and Sons, New York, (1998).
112. Cao, A. J., Cheng, Y. Q., & Ma, E. Structural processes that initiate shear localization in
metallic glass. Acta Mater. 57, 5146 - 5155 (2009).
113. Delogu, F. Irreversible atomic rearrangements in elastically deformed metallic glasses.
Intermetallics 19, 86 - 92 (2011).
114. Stauffer, D. Introduction to Percolation Theory. Taylor and Francis, London (1995).
115. Stauffer, D., Coniglio, A., & Adam, M. Gelation and critical phenomena. in Polymer
Networks, Advances in Polymer Science, edited by K. Dusek (Springer, New York), 44,
103-158 (1982).
116. Miracle, D. B., Egami, T., Flores, K. M., & Kelton, K. F. Structural aspects of metallic
glasses. MRS Bull. 32, 629 (2007).
129
117. Cheng, Y. Q., Ma, E., & Sheng, H. W. Alloying strongly influences the structure, dynamics,
and glass forming ability of metallic supercooled liquids. Appl. Phys. Lett. 93, 111913
(2008).
118. Fujita, T., Guan, P. F., Sheng, H. W., Inoue, A., Sakurai, T., & Chen, M. W. Coupling
between chemical and dynamic heterogeneities in a multicomponent bulk metallic glass.
Phys. Rev. B 81, 140204 (2010).
119. Cohen, M. H. & Grest, G. S. Liquid-glass transition, a free-volume approach. Phys. Rev. B
20, 1077-1098 (1979).
120. Busch, R., Schneider, S., Peker, A., & Johnson, W. L. Decomposition and primary
crystallization in undercooled Zr 4 .2 Til 3 .8Cui 2 .5NiioBe22. 5 melts. AppL. Phys. Lett. 67, 1544-
1546 (1995).
121. Schneider, S., Thiyagarajan, P., & Johnson, W. L. Formation of nanocrystals based on
decomposition in the amorphous Zr 4 1,2 Ti1 3.8Cu 2 .NijoBe 22 .5 alloy. AppL. Phys. Lett. 68, 493495 (1996).
122. Park, B. J., Chang, H. J., Kim, D. H., & Kim, W. T. In situ formation of two amorphous
phases by liquid phase separation in Y--Ti--Al--Co alloy. AppL. Phys. Lett.85, 6353-6355
(2004).
123. Borchers, C., Schroers, J., & Busch, R. Prediction of spinodal wavelength in continuously
cooled metallic liquid. Ann. Phys. (Berlin) 18,4 (2009).
124. Park, B. J., Chang, H. J., Kim, D. H., Kim, W. T., Chattopadhyay, K., Abinandanan, T. A.,
& Bhattacharyya, S. Phase Separating Bulk Metallic Glass: A Hierarchical Composite. Phys.
Rev. Lett. 96, 245503 (2006).
125. Stoller, R. E. The role of cascade energy and temperature in primary defect formation in
iron. J.NucL. Mater. 276, 22-32 (2000).
126. de la Rubia, T. D. & Phythian, W. J. Molecular dynamics studies of defect production and
clustering in energetic displacement cascades in copper. J.NucL. Mater. 191-194,108-115
(1992).
127. Luneville, L., Simeone, D., & Weber, W. J. Study of the fragmentation of a displacement
cascade into subcascades within the Binary Collision Approximation framework. J.Nucl. Mater.
415, 55 - 60 (2011).
130
128. Ziegler, J. F., Ziegler, M. D., & Biersack, J. P. SRIM-The stopping and range of ions in
matter (2010). NucL. Instrum. Methods Phys. Res., Sect. B 268, 1818-1823 (2010).
129. Ziegler, J. F., Biersack, J. P., and Ziegler, M. D. SRIM - The Stopping and Range ofIons in
Matter, LuLu Press, 2008. The authors used SRIM-2008.3, available from http://www.srim.org,
which was modified for our use by the authors.
130. Skirlo, S. A. & Demkowicz, M. J. The role of thermal spike compactness in radiationinduced disordering and Frenkel pair production in Ni3Al. Scripta Mater. 67, 724-727 (2012).
131. Alurralde, M., Caro, A., & Victoria, M. Radiation damage cascades: Liquid droplet
treatment of subcascade interactions. J.NucL. Mater. 183, 33-45 (1991).
132. Myers, M., Fu, E. G., Myers, M., Wang, H., Xie, G., Wang, X., Chu, W.-K., & Shao, L. An
experimental and modeling study on the role of damage cascade formation in nanocrystalization
of ion-irradiated Nis 2.5NbjoZr 15Ti 15 Pt7 .5 metallic glass. Scripta Mater. 63, 1045-1048 (2010).
133. van Dillen, T., Polman, A., Onck, P. R., & van der Giessen, E. Anisotropic plastic
deformation by viscous flow in ion tracks. Phys. Rev. B 71, 024103 (2005).
134. Correa, A. A., Kohanoff, J., Artacho, E., Sainchez-Portal, D., & Caro, A. Nonadiabatic
Forces in Ion-Solid Interactions: The Initial Stages of Radiation Damage. Phys. Rev. Lett. 108,
213201 (2012).
135. Kaufman, L., Perepezko, J., & Hildal, K. Synthesis and performance of Fe-based
amorphous alloys for nuclear repository applications. ProceedingsofM&C+SNA 2007 (2007).
136. Graff, K. F. Wave Motion in Elastic Solids. Dover Publications, New York, (1975).
131