Thermal Physics
advertisement
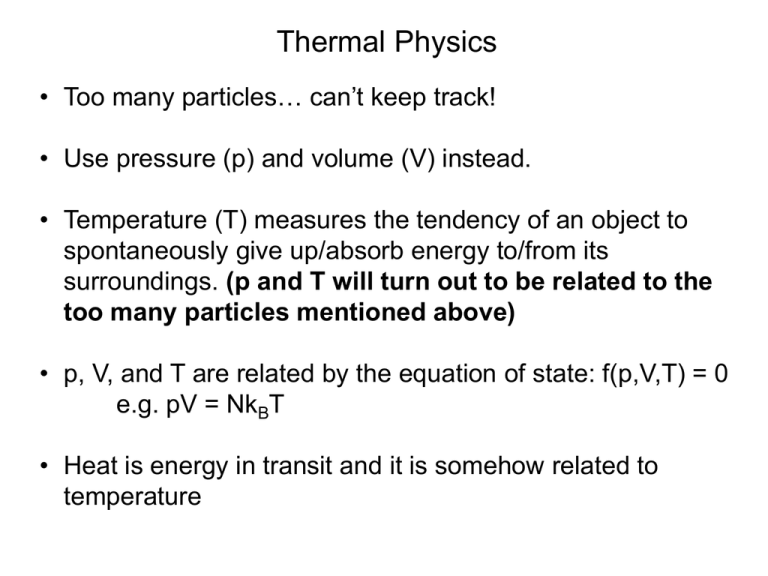
Thermal Physics • Too many particles… can’t keep track! • Use pressure (p) and volume (V) instead. • Temperature (T) measures the tendency of an object to spontaneously give up/absorb energy to/from its surroundings. (p and T will turn out to be related to the too many particles mentioned above) • p, V, and T are related by the equation of state: f(p,V,T) = 0 e.g. pV = NkBT • Heat is energy in transit and it is somehow related to temperature Zeroth law of thermodynamics A C If two systems are separately in thermal equilibrium with a third system, they are in thermal equilibrium with each other. Diathermal wall B C C can be considered the thermometer. If C is at a certain temperature then A and B are also at the same temperature. • Temperature is related to heat and somehow related to the motion of particles • Need an absolute definition of temperature based on fundamental physics • A purely thermal physics definition is based on the Carnot engine • Can also be defined by statistical arguments 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 Microstates and Macrostates 1 Microstates and Macrostates 1 Microstates and Macrostates 1 All these microstates belong to the macrostate of 1 head in 100 coins Most likely macrostate the system will find itself in is the one with the maximum number of microstates. Number of Microstates () 1.2e+029 1e+029 8e+028 6e+028 4e+028 2e+028 0 0 20 40 x Macrostate 60 80 100 n = 170; x = 0:1:n; y = factorial(n)./(factorial(x).*fac torial(n-x)); figure; plot(x,y); 1.Each microstate is equally likely 2.The microstate of a system is continually changing How is all this @#$%^& 3.Given enough to time, the system related thermal will explore all possible physics? microstates and spend equal time in each of them (ergodic hypothesis). Big question: How do we relate the number of microstates for a particular macrostate to temperature? E1 + E T1 < T2 E2 - E But no particular relation for E1 and E2 At thermal equilibrium the temperature (whatever it is) will be the same for both systems. Total energy E = E1 + E2 is conserved. clear all; n1 = 4; n2 = 8; e = 6; i = 0; for x = 0:1:n1 y1 =(factorial(n1)./(factorial(x).*factorial(n1-x))); y2 = (factorial(n2)./(factorial(e-x).*factorial(n2-(e-x)))); i=i+1; y(i)=y1*y2 x1(i)=x; end figure; plot(x1,y); 450 400 350 300 250 200 150 100 50 0 0 0.5 1 1.5 2 2.5 3 3.5 4 Most likely macrostate the system will find itself in is the one with the maximum number of microstates. E1 E2 1(E1) (E) 2(E2) d ln 1 d ln 2 1 dE1 dE2 k BT Ensemble: All the parts of a thing taken together, so that each part is considered only in relation to the whole. E (E) Microcanonical ensemble: An ensemble of snapshots of a system with the same N, V, and E Microcanonical ensemble: An ensemble of snapshots of a system with the same N, V, and E Canonical ensemble: An ensemble of snapshots of a system with the same N, V, and T E1 1(E1) E2 2(E2)