What to know for midterm exam 1
advertisement

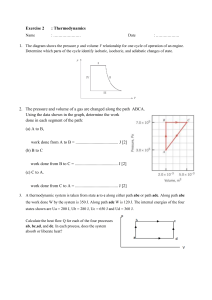
What to know for midterm exam 1 • Concept of thermal equilibrium • First law of thermodynamics, definition of heat and work • Ideal gas law, classical equipartition of energy, degrees of freedom • Thermal energy, rms speed of molecules in ideal gas • Compression work, quasistatic processes, isothermal and adiabatic compression • Heat capacity and latent heat • Basic combinatorics, binomial distribution • Concepts of macrostate, microstate, multiplicity, statistical ensemble • Macrostates and multiplicities of two-state systems, Einstein solid • Second law of thermodynamics • Reversible vs. irreversible processes • Stirling approximation, law of large numbers • Systems in thermal equilibrium • Definitions of entropy, temperature • Relationship between entropy and heat You may bring one 8 1/2 x 11 crib sheet. Please remember to bring a calculator.