Resonance Octet Rule Exceptions Expanded Octets More Exceptions
advertisement

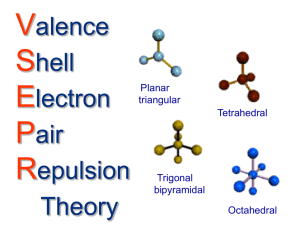
Resonance O3 CO32- Octet Rule Exceptions BF3 BeH2 ¯ Expanded Octets More Exceptions PCl5 ClF4 SiF5¯ BrF5 SF6 NO2 1 Molecular Shape Most molecules are not flat as they appear in twodimensional Lewis structures Molecules have three-dimensional shapes Shape can be predicted using Valence Shell Electron Pair Repulsion (VSEPR) theory Shape via VSEPR First obtain the Lewis structure Then apply the VSEPR rules: All valence shell electron pairs (electron charge clouds) are treated equally whether they are bonding or nonbonding Stereochemically significant electron pairs Double and triple bonds are treated as single bonds for predicting molecular shapes Electron pairs orient in space to get as far away from neighbors as possible Shape via VSEPR Electron pairs orient in space to get as far away from neighbors as possible Two pairs 180o CO2 linear O Three pairs BF3 C 120o B Four pairs CH4 Central atoms surrounded only by bonding pairs NH4+ PF5 O F F Shape via VSEPR trigonal planar F H 109.5o tetrahedral C H H H 2 Molecular Shape E A E Linear (Angle = 180) E E A E E E A Shape via VSEPR E E E Stereochemically significant pairs? Trigonal bipyramidal Trigonal planar (angles = 120) Molecular shape is determined by the positions of atoms Therefore NH3 will be pyramidal with a triangular base Trigonal pyramid N E E E A A E E E E Central atoms surrounded by bonding and lone pairs Example, NH3 Lewis dot structure prediction? E E E H octahedral Tetrahedral (angles = 109.5) H H Molecular Shape X A X A X Shape via VSEPR X H2O X trigonal planar bent Structure is ______________ X A X X X tetrahedral A X X X trigonal pyramidal A bent ClF2+ X X Structure is ______________ 3 Molecular Shape X X X A X X A X Shape via VSEPR X X A A X X X X X X trigonal bipyramidal see-saw t-shaped linear X X X A X X X X X A X X X X A Central atoms with expanded octets and lone pairs ClF4+ ICl4¯ BrF5 X X X square pyramidal octahedral square planar Effects of Lone Pairs X A X A X Effects of Lone Pairs X X trigonal planar X X X A X X A X A A X X bent X X X X X X trigonal bipyramidal see-saw t-shaped linear X X X A X X X tetrahedral A X X X trigonal pyramidal A bent X X X A X X X X X A X X X X A X X X octahedral square pyramidal square planar 4