THE HISTORY OF THE ATOMIC MODEL
advertisement

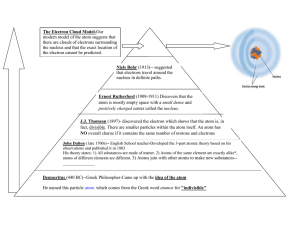
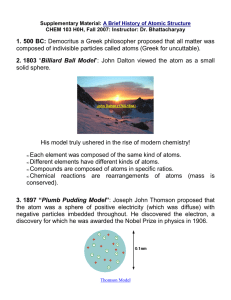
Atomic Model Cut and Paste Note page THE HISTORY OF THE ATOMIC MODEL John Dalton – Early 19th century (1808) James Chadwick – 1932 Niels Bohr – Early 20th Century (1913) J.J. Thomson – 1897 Ernest Rutherford -1906/1911 Electron Cloud Model – 1932 to present He was a scientist/school teacher. He said atoms were the smallest particles of matter. He said atoms could not be destroyed or divided. He thought atoms were hard spheres that were the same throughout. His model was similar to a marble. He discovered the nucleus of the atom. He also determined that the nucleus was small and dense. It made up most of the mass of an atom. He proposed that most of the atom was made up of empty space. (He experimented with alpha particles and gold foil). This 20th century scientist worked on a theory to explain how electrons are arranged in the atom. He proposed the idea that energy levels or shells housed the electrons which he thought moved around the nucleus much the way a planet orbits the sun. He worked with Ernest Rutherford and was credited for discovering the neutron. The neutron was proposed when Rutherford’s model could not explain why the nucleus was 2 times the mass of the number of protons. This scientist discovered the electron. His model of the atom was a sphere of positive charge with negatively charged particles spread evenly throughout. His model was likened to a “raisin bun”. This is the most current model of the atom. It states that electrons are in constant motion around the nucleus. This motion is unpredictable and can’t be described by an orbit. The new model simply defines a region (the electron cloud) where the electrons are most likely to be found.