Medical Education for Undergraduate MD Students

http://www.google.com/imgres?q=reproduction+in+protozoa&hl=en&sa=G&gbv=2&tbm=isch&tbnid=c
ZefxN3IOlQIzM:&imgrefurl=http://www.tulane.edu/~wiser/protozoology/notes/morph.html&docid=12 egOZFNyd8uzM&w=608&h=705&ei=3bFYTtqxOsn0gaJuLGTDA&zoom=1&iact=hc&vpx=348&vpy=62&dur=566&hovh=242&hovw=208&tx=107&ty=144&pa ge=1&tbnh=123&tbnw=109&start=0&ndsp=29&ved=1t:429,r:1,s:0&biw=1440&bih=707
http://mdmedicine.wordpress.com/2011/04/22/protozoa-basic-facts/
Medical Education for Undergraduate MD Students
All Day I Dream About Studies…
Skip to content
Home
About
Clinical Info.
Para-Clinical Info.
Pre-Clinical Info.
← Fungi
Types of Sleep →
Protozoa-Basic Facts
Posted on April 22, 2011 by mdmedicine
Protozoa
, from the Greek meaning ‘first animal’, refers to simple, eukaryotic organisms composed of a microscopic single cell. Reproduction is through simple asexual cell division, or binary fission, in which two daughter cells are formed or, if many daughter cells are formed, multiple fission. Certain protozoa have complex life cycles involving both asexual (schizogony) and sexual reproduction. Some protozoa form resistant cysts that can survive in the environment.
There are over 65 000 known species of protozoa, of which approximately 10 000 are parasites, deriving nourishment and environmental protection from inhabiting a living animal host.
However, the majority of parasites are non-pathogenic, living as harmless commensals within the host. Some animals can harbour parasites and serve as reservoirs for human disease. Infections that are naturally transmitted between animals and humans are termed zoonoses. These may be acquired either by direct contact with an animals or indirectly through the ingestion of contaminated water and food. Some zoonoses are spread by the bite of an insect, termed a vector in which part of the organism’s life cycle is completed.
Only a small number of protozoa cause human disease but those that do affect millions of people world-wide, causing considerable suffering, mortality and economic hardship. Protozoal diseases are largely confined to countries with poor economic and social structure. However, trichomoniasis, crytosporidiosis, and toxoplasmosis are common in developed countries.
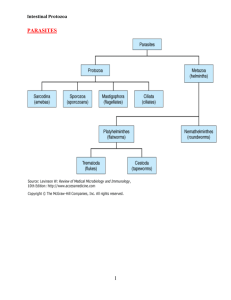
The pathogenic protozoa are part of the subkingdom Protozoa. Those of medical importance are placed in the phyla Sarcomastigophora, Apicomplexa and Ciliophora. Within these phyla the protozoa are divided into four major classes based on their locomotive form: the amoebae
(Sarcodina), the flagellates (Mastigophora), the sporozoa, and the ciliates
(Kinetofragminophorea).
Amoebae : These are the simplest of the protozoa and are characterised by a feeding and dividing trophozoite stage that moves by temporary extensions of the cell called pseudopodia (‘false feet’). In some species the trophozoite can form a resistant cyst stage able to survive in the environment. Those that infect the gut are true parasites being unable to reproduce except in a
living host. Others occur naturally in soil and water and are not true parasites. They are termed
‘free-living’ and infect humans as opportunistic pathogens.
Flagellates : These organisms have a trophozoite form but also possess flagella for locomotion and food gathering. All pathogenic species are true parasites, being unable to reproduce outside the host.
Ciliates : These possess rows of hair-like cilia around the outside of the body for motility and also to direct food into a primitive mouth termed a cytostome. All ciliates possess two nuclei: a large polyploid micronucleus and a small micronucleus active only during sexual reproduction.
Some species form cysts.
Apicomplexa : This is a unique group lacking any visible means of locomotion. They are all parasitic and most are intracellular, having a life cycle involving both sexual and asexual reproduction. The common feature of all members is the presence of an apical complex (visible only by electron microscopy) at the anterior pole in one or more stages of the life cycle. The exact components of the apical complex vary among members. Its function is thought to enable cell penetration.
Diagnosis and treatment The relatively large size of the protozoa enables most human pathogens to be easily identified by microscopic examination of clinical material. Those of the gut are observed in freshly taken faecal samples. Blood and tissue protozoa are visualised after staining. Detection of elevated antibodies to the infecting organism may be diagnostic in some instances (e.g. toxoplasmosis). Culture methods are not routinely used, as they are technically demanding and time consuming: an exception is the diagnosis of trichomoniasis.
Antiprotozoal therapy is generally unsatisfactory. Treatment is hampered by lack of effective agents for many diseases, their potential toxicity to humans and inability to destroy all forms of the organism. The emergence of drug resistance has also limited the therapeutic potential of many agents.
Featured on EcoPressed Standing Eco World Content From Across The Internet.
Against Oil Sands—and Standing for the Climate
Like this:
Like
One blogger likes this post.
About mdmedicine
Medical student. Interested in learning and promoting medical field.
View all posts by mdmedicine →
This entry was posted in Microbiology and tagged amoebae , apicomplexa , ciliates , Diagnosis and treatment , flagellates , Microbiology , protozoa . Bookmark the permalink .
← Fungi
Types of Sleep →
Leave a Reply
guest
Enter your comment here...
Guest
Log In
Log In
Log In
Email (required) (Not published)
Name (required)
Website
Please log in to WordPress.com to post a comment to your blog.
You are commenting using your Twitter account. ( Log Out )
You are commenting using your Facebook account. ( Log Out )
Connecting to %s
Notify me of follow-up comments via email.
Notify me of new posts via email.
Post Comment 396 0
1314436145
Search for:
Search
Email Subscription
Enter your email address to subscribe to this blog and receive notifications of new posts by email. subscribe 21409821 http://mdmedicine w idget 7a0d8eaf43 /2011/04/22/proto
Sign me up!
Recent Posts
o o
Mixed Connective Tissue Disease (MCTD)
Pilonidal Sinus o o o
Migraine
Congestive Heart Failure
Cerebrospinal Fluid (CSF)
Archives
o o
August 2011
July 2011 o o o o
June 2011
May 2011
April 2011
March 2011
Total Visits
o 15,104 hits
Categories
o o o o o o o o o o o o o
Anatomy
Clinical Cases
General Surviving Tips
Genetics
Medical Ethics
Microbiology
Neurology
Pharmacology
Physiology
Presentation Slides
Psychology
Surgery
Uncategorized
Meta
o o o o o
Register
Log in
Entries RSS
Comments RSS
WordPress.com
Medical Education for Undergraduate MD Students
Theme: Twenty Ten Blog at WordPress.com
. http://mdmedicine.wordpress.com/2011/04/22/protozoa-basic-facts/
http://www.google.com/imgres?q=reproduction+in+protozoa&hl=en&sa=G&gbv=2&tbm=isch&tbnid=w aDhbk8sJRBL8M:&imgrefurl=http://www.tulane.edu/~wiser/protozoology/notes/intes.html&docid=Tkz vM8jfJ6tPCM&w=669&h=348&ei=3bFYTtqxOsn0gaJuLGTDA&zoom=1&iact=hc&vpx=516&vpy=243&dur=1790&hovh=162&hovw=311&tx=165&ty=94&p age=1&tbnh=92&tbnw=176&start=0&ndsp=29&ved=1t:429,r:9,s:0&biw=1440&bih=707
INTESTINAL PROTOZOA
Lumen-Dwelling Protozoa
Flagellates:
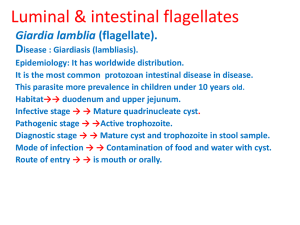
Giardia lamblia
Dientamoeba fragilis
Chilomastix mesnili
Enteromonas hominis
Retortamonas intestinalis
Trichomonas hominis
Trichomonas tenax (oral)
Trichomonas vaginalis
(urogenital)
Ameba:
Entamoeba histolytica
Entamoeba dispar
Entamoeba coli
Entamoeba hartmanni
Entamoeba polecki
Entamoeba gingivalis (oral)
Endolimax nana
Iodamoeba bütschlii
Apicomplexa:
Cryptosporidium parvum
Cryptosporidium hominis
Cyclospora cayetanensis
Other:
Isospora belli
Microsporidia:
Enterocytozoon bieneusi
Encephalitozoon intestinalis
Numerous protozoa inhabit the gastrointestinal tract of humans (see Box). This list includes representatives from many diverse protozoan groups. The majority of these protozoa are non-pathogenic commensals, or only result in mild disease. Some of these organisms can cause severe disease under certain circumstances. For example, Giardia
Blastocystis hominis
Balantidium coli lamblia can cause severe acute diarrhea which may lead to a chronic diarrhea and nutritional disorders; Entamoeba histolytica can become a highly virulent and invasive organism that causes a potentially lethal systemic disease.
Apicomplexa and microsporidia species
(discussed elsewhere), which normally do not evoke severe disease, can cause severe and life-threatening diarrhea in AIDS patients and other immunocompromised individuals. Trichomonas vaginalis does not reside within the gastro-intestinal tract, but is often discussed with the intestinal flagellates. It infects the urogenital tract and and causes a sexuallytransmitted disease.
Intestinal protozoa are transmitted by the fecal-oral route and tend to exhibit similar life cycles consisting of a cyst stage and a trophozoite stage
(Figure). Fecal-oral transmission involves the ingestion of food or water contaminated with cysts. After ingestion by an appropriate host, the cysts transform into trophozoites which exhibit an active metabolism and are usually motile. The parasite takes up nutrients and undergoes asexual replication during the trophic phase. Some of the trophozoites will develop into cysts instead of undergoing replication. Cysts are characterized by a resistant wall and are excreted with the feces. The cyst wall functions to
protect the organism from desiccation in the external environment as the parasite undergoes a relatively dormant period waiting to be ingested by the next host. Factors which increase the likelyhood of ingesting material contaminated with fecal material play a role in the transmission of this intestinal protozoa (see Box). In general, situations involving close humanhuman contact and unhygenic conditions promote transmission.
TOPICS:
Fecal-Oral Transmission Factors
Giardiasis o
Life Cycle and Morphology
Trophozoite
o o
Cyst
The Adhesive Disk
Symptoms and Pathogenesis
Diagnosis o o
Treatment and Control
Trichomoniasis
Balantidosis
Amebiasis
Non-Pathogenic Commensals poor personal hygiene
children (eg, day-care centers)
institutions (eg, prisons, mental
hospitals, orphanages) food handlers developing countries
poor sanitation
lack of indoor plumbing
endemic
travelers' diarrhea
GIARDIASIS water-borne epidemics
Giardia lamblia (also known as G. duodenalis, see comments on taxonomy ) is a protozoan parasite that colonizes the upper portions of the small intestine. It has a worldwide distribution and is the most common protozoan isolated from human stools. The incidence is estimated at 200 million clinical cases per year. In fact, it was probably the
water treatment failures
male homosexuality
oral-anal contact zoonosis?
first symbiotic protozoan ever observed. It is quite likely that Van Leeuwenhoek, the inventor of the microscope, first described
Giardia in 1681in his own stools based upon
Entamoeba = no
Cryptosporidium = yes
Giardia = controversial his description of its characteristic movement. However, van Leeuwenhoek never submitted drawings of the organisms and Lambl is usually given credit for the identification of Giardia in the stools of pediatric patients in Praque in 1859.
Typically Giardia is non-invasive and often results in asymptomatic infections. Symptomatic giardiasis is characterized by acute or chronic diarrhea and/or other gastro-intestinal manifestations.
LIFE CYCLE AND MORPHOLOGY
Giardia exhibits a typical fecal-oral transmission cycle ( see above ). The infection is acquired through the ingestion of cysts. Factors leading to contamination of food or water with fecal material are correlated with transmission (Box). For example, giardiasis is especially prevalent in children and particularly those children in institutions or day-care centers. In developing countries, poor sanitation contributes to the higher levels of giardiasis, and water-borne outbreaks due to inadequate water treatment have also been documented. Backpackers in areas of no human habitation are believed to acquire from drinking from streams and some data suggest that beavers are the reservoir. However, the zoonotic transmission of
Giardia is controversial and has not been unambiguously demonstrated. It is not clear whether Giardia lamblia represents a single species capable of infecting a wide range of animals, or whether each host has their own 'pet'
Giardia. Evidence indicating that Giardia transmission between dogs and humans is quite rare favors the latter. Molecular evidence suggests that some isolates exhibit narrow host ranges whereas others exhibit wide host ranges (see notes on taxonomy ). Regardless of whether zoonotic transmission is possible, person-to-person transmission is the most prevalent mode of transmission and the risk factors are close human contact combined with unhygienic conditions.
The ingested cyst passes through the stomach and excystation takes place in the duodenum. Excystation can be induced in vitro by a brief exposure of the cysts to acidic pH (~2) or other sources of hydrogen ions. This exposure to the acidic pH mimics the conditions of the stomach and probably functions as an environmental cue for the parasite. Flagellar activity begins within 5-
10 minutes following the acid treatment and the trophozoite emerges through a break in the cyst wall. The breakdown of the cyst wall is believed to be mediated by proteases. The trophozoite will undergo cytokinesis (cell division without nuclear replication) within 30 minutes after emerging from the cyst resulting in two binucleated trophozoites.
The Giardia trophozoite exhibits a characteristic pear, or tear-drop, shape with bilateral symmetry when viewed from the top (Figure). It is typically 1215 µm long, 510 µm wide, and 2 4 µm thick.
Characteristic features of the stained trophozoite include: two nuclei (Nu) with central karyosomes (k), fibrils running the length of the parasite, and median bodies
(MB). The large karyosome and lack of peripheral chromatin gives the nuclei a halo appearance. The fibrils are called axonemes (Ax) and are formed from the proximal regions of the flagella (Fg) within the body of the trophozoite.
The median bodies are a pair of curved rod-shaped structures which lie posterior to the nuclei. At the ultrastructural level the median bodies contain an array of microtubules. The function of the median bodies is not known, but most believe they are somehow involved with the adhesive disk and its formation. An adhesive disk (AD), not always visible by light microscopy, occupies the ventral side of the anterior end.
Giardia trophozoites possess four pairs of flagella and are motile. Three pairs of flagella emerge from the dorsal surface (anterior, posterior-lateral, caudal) and one pair emerges from the ventral surface. Trophozoites exhibit a distinctive erratic twisting motion, sometimes compared to that of a falling leaf. However, the trophozoites are predominantly found attached to epithelial cells of the small intestine (especially the duodenum and jejunum) and are rarely found in stools, except in the cases of severe diarrhea. This attachment to the intestinal epithelium is mediated by an organelle on the ventral side of the parasite referred to as the adhesive disk ( see below ). The trophozoite absorbs nutrients from the intestinal lumen via pinocytosis and no specialized feeding organelles have been described.
The trophic stage is also characterized by an asexual replication. Both nuclei divide at about the same time and cytokinesis restores the binucleated state.
Each daughter cell receives one copy of each nuclei. Both nuclei appear equal in regards to gene expression and other properties.
As an alternative to replication the trophozoite can encyst. During encystment the parasite rounds up, detaches from the intestinal epithelium, and secretes a cyst wall. Encystation can also be carried out in vitro. Optimal induction of encystment is obtained by depriving the trophozoites of bile at pH 7 followed by an exposure to high concentrations of bile at pH
7.8. The lack of bile at neutral pH mimics the conditions under the mucus blanket adjacent to the intestinal epithelial cells, whereas exposure to high concentrations of bile at more alkaline pH is analogous to the intestinal lumen. These studies highlight the extent to which Giardia has adapted to life within the gastrointestinal tract.
Molecular and ultrastructural studies reveal the synthesis of cyst wall proteins and the appearance of large secretory vesicles in the parasite cytoplasm follow the induction of encystment. After cyst wall formation the parasite undergoes one round of nuclear division without cytokinesis resulting in four nuclei. These four nuclei (Nu) are usually located at the anterior end of the cyst (Figure). The flagella and adhesive disk are lost as the cyst matures, but the axonemes (Ax) and median bodies (MB) persist.
The distinctive fibrils (ie, axonemes), which extend across the length of the cyst, result in Giardia being relatively easy to unambiguously identify. The cysts are oval shaped and typical measure 1114 µm in length and 6 10 µm wide. Other characteristics of Giardia cysts include a well-defined wall (CW) which is often set apart from the cytoplasm of the parasite. The cysts are passed in the feces and can survive for up to three months under appropriate temperature and moisture conditions. Mature cysts are infective to the next host that happens to ingest them, thus completing the life cycle.
THE ADHESIVE DISK
A unique ultrastructural feature of Giardia is the adhesive disk (also called ventral disk, sucking disk, sucker, or striated disk). The adhesive disk is a concave structure which occupies approximately two-thirds of the anterior end of the ventral surface (Figure, left panel). As the names imply, this structure plays a role in the attachment of the trophozoite to the intestinal epithelium and ultrastructural studies reveal close associations between the adhesive disk and the intestinal brush border (Figure, upper right panel).
( Click here for larger image .)
The adhesive disk appears to be a relatively rigid structure and striations are evident by transmission electron microscopy. These striations are the result of microtubules (mT) and a unique cytoskeletal element called microribbons
(mR). Microribbons are long flattened structures and each microribbon is associated with a microtubule (Figure, middle right panel). The combined microtubule-microribbon structure are arranged in concentric rows that form a flatten spiral with minimal overlap. The outer rim of the adhesive disk, called the lateral crest, contains components of the actin-myosin cytoskeleton.
A major component of microribbons are proteins called giardins (aka beta-giardins).
These giardins play primarily a structural role in the formation of the microribbons.
Interestingly, the giardins show a limited homology to a protein called 'striated fibre assemblin' from Chlamydomonas (a free-living, bi-flagellated unicellular algae). In
Chlamydomonas this protein forms filamentous structures at the base of the flagella. The giardins have evolved to play a different functional role in Giardia, but are still associated with microtubule based cytoskeletal elements.
This association of proteins involved in the generation of contractile force and other cytoskeletal elements in the adhesive disk suggests that attachment is mediated by mechanical forces generated by the parasite. The observation that imprints and circular dome-shaped lesions remain in the intestinal brush border (ie, microvilli) following detachment of trophozoites
(Figure, lower right panel) is consistent with contractile forces playing a role in attachment. Other proposed mechanisms for the attachment of Giardia to the intestinal epithelium include hydrodynamic forces generated by the ventral flagella and receptor-mediated binding via lectins on the trophozoite surface. However, flagellar movement is poorly correlated with attachment and the surface lectins cover the entire trophozoite and are not specifically localized to the adhesive disk.
SYMPTOMS AND PATHOGENESIS
The clinical features associated with Giardia infection range from total latency (ie, asymptomatic), to acute self-resolving diarrhea, to chronic syndromes associated with nutritional disorders, weight loss and failure to thrive. Children exhibit clinical symptoms more frequently that adults and subsequent infections tend to be less severe than initial infections. The incubation period is generally 1-2 weeks, but ranges of 1-75 days have been reported.
The first signs of acute giardiasis include nausea, loss of appetite and an upper gastro-intestinal uneasiness. These signs are often followed or accompanied by a sudden onset of explosive, watery, foul-smelling diarrhea.
Stools associated with Giardia infection are generally described as loose, bulky, frothy and/or greasy with the absence of blood or mucus, which may help distinguish giardiasis from other acute diarrheas. Other gastro-intestinal disturbances associated with giardiasis include: flatulence, bloating, anorexia, cramps, and foul sulfuric belching (sometimes called 'purple burbs'). The acute stage usually resolves spontaneously in 3-4 days and is often not recognized as being giardiasis. Occasionally, though, an acute infection will persist and lead to malabsorption, steatorrhea (excessive loss of fat in the feces), debility (loss of strength) and weight loss. Some of the
individuals who resolve the acute symptoms do not clear the infection, but become asymptomatic cyst passers without clinical manifestations, whereas others may have a few sporadic recurrences of the acute symptoms.
Acute infections can also develop into long-standing subacute or chronic infections which in rare cases last for years. The typical chronic stage patient presents with recurrent brief episodes of loose foul stools which may be yellowish, frothy and float, accompanied by intestinal gurgling, abdominal distention and flatulence. Between episodes the stools are usually mushy, but normal stools or constipation can also occur. Cramps are uncommon during chronic infections, but sulfuric belching is frequent. Anorexia, nausea, and epigastric uneasiness are additional frequent complaints during chronic infections. In the majority of chronic cases the parasites and symptoms spontaneously disappear.
The specific mechanisms of Giardia pathogenesis leading to diarrhea and intestinal malabsorption are not completely understood and no specific virulence factors have been identified. Attachment of trophozoites to the brush border could produce a mechanical irritation or mucosal injury. In addition, normal villus structure is affected in some patients. For example, villus blunting (atrophy) and crypt cell hypertrophy and an increase in crypt depth have been observed to varying degrees. The increase in crypt cells will lead to a repopulation of the intestinal epithelium by relatively immature enterocytes with reduced absorptive capacities. An increased
Click for larger image inflammatory cell infiltration in the lamina propria has also been observed and this inflammation may be associated with the pathology. Giardia infection can also lead to lactase deficiency (see lactose intolerance below) as well as other enzyme deficiencies in the microvilli. This reduced digestion and absorption of solutes may lead to an osmotic diarrhea and could also explain the malabsorption syndromes. Thus far, no single virulence factor or unifying mechanism explains the pathogenesis of giardiasis. [See also
Pathophysiology of Diarrhea for a general discussion of diarrhea.]
Post-Giardia Lactose Intolerance. Some patients may present with a lactose intolerence during active Giardia infections which can persist after parasite clearance. This clinical manifestation is due to the parasite-induced lactase deficiency and is most common in ethnic groups with a predisposition
for lactase deficiency. Lactase is an enzyme that breaks down lactose, a sugar found in milk, to monosaccharides which can be absorbed. This lactose intolerence syndrome should be considered in persons who still present mushy stools and excessive gas following treatment, but have no detectable parasites. http://www.tulane.edu/~wiser/protozoology/notes/intes.html