UNIT 5 THERMOCHEMISTRY 1
advertisement

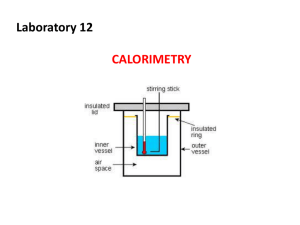
UNIT 5 THERMOCHEMISTRY 1 A study of the energy produced by Chemical Reactions HEAT Energy: The ability to do work HEAT: form of energy transferred from a body at high temperature to one at lower temperature TEMPERATURE: average kinetic energy of the molecules of a substance; measures heat flow JOULES (J) or (kJ): unit of measure of heat (or any form of energy) PHASE: state of matter and depends on temperature and pressure Heat System: part of the universe on which attention is focused Surrounding- exchanges energy with the system, make up the rest of the universe Example: a beaker with water sitting on a hot plate warming. System: water Surroundings: beaker, hot plate, air surrounding the beaker HEAT is represented by q ENDOTHERMIC: heat flows from surroundings into system; heat absorbed; +q 50 g of water is being warmed on the hot plate EXOTHERMIC: heat flows from system into surroundings; heat escapes; -q The hot plate is turned off and the water starts heating its surroundings. The temp of water decreases and the temp of the surroundings increase Measuring Heat HEAT CAPACITY: amount of heat to raise the temperature 1oC; C = J/ D oC (will sometimes be given in KJ SPECIFIC HEAT: amount of heat to raise 1 g of a substance 1oC; c = J/g D oC Specific heat is an intensive property. ***Note that Heat Capacity is a Big C and Specific Heat is a little c Calorimeter A device to measure heat flow. Walls are insulated do no exchange with outside air. Two-types Coffee-cup calorimeter Bomb calorimeter (used for gases) q reaction = - q calorimeter Calorimeter q = m c Dt q = -C Dt (q in J, m in g, t in Celsius) Exothermic when q reaction is < 0, q calorimeter is + Endothermic when q reaction is > 0, q calorimeter is - Example: Coffee-Cup Calorimeter When 1 gram of calcium chloride is added to 50 grams of water in a coffee-cup calorimeter, it dissolves. The temperature rises from 25oC to 28.51oC. Assuming that all the heat given off by the reaction is transferred to the water, calculate q for the reaction system. Is this exothermic or endothermic? Example: Bomb Calorimeter The reaction between hydrogen and chlorine can be studied in a bomb calorimeter. It is found that when a 1.00 gram of hydrogen completely reacts, the temperature rises from 20oC to 29.82oC. Taking the heat capacity of the calorimeter to be 9.33 kJ/oC, calculate the amount of heat evolved in the reaction. Enthalpy The is the measure of heat from at constant pressure between reactants and products. DH rxn = DHproducts - DH reactants q= DH rxn Exothermic vs Endothermic Energy Diagram Thermochemical equations An equation will be given and it will tell you the overall DH rxn The sign of DH rxn indicates whether the reaction is endothermic and exothermic. The coefficients represent the number of moles The phase symbols must be used Again, this is at constant pressure and at 25oC Rules of Thermochemistry 1. the magnitude of DH is directly proportional to the amount of reactant and product Heat of fusion: solid to liquid Heat of vaporization: liquid to gas If going from in opposite direction (liquid to solid) Heat of fusion is same but opposite Calorimetry A 2.200 g sample of quinone, C6H4O2, is burned in a bomb calorimeter whose heat capacity is 7.854 kJ/oDC. The temperature increases from 23.44oC to 30.57oC. What is the heat of combustion per gram? Per mole? Calorimetry The heat of combustion of glucose, C6H12O6, is 15.57 kJ/g. A 2.500 g sample burned in a bomb calorimeter raises the temperature from 20.55oC to 23.25oC. What is the heat capacity of the calorimeter? Calorimetry A 1.200 g sample of benzoic acid, HC7H5O2, is burned in a calorimeter with a heat capacity of 2.423 kJ/oDC. When the calorimeter contains 1.500 kg of water, the temperature rises from 22.45oC to 26.10oC. What is the heat of combustion of benzoic acid in kJ/mol? Phase Changes The phase of a material changes based on temperature and pressure. The phase is dependent on the motion of the molecules of the substance. When a substance melts(freezes) or boils(condenses), there is no temperature change, but there is a change of heat involved. Phase Diagrams PHASE DIAGRAM Normal melting (freezing) and boiling (condensing) points at standard pressure Triple point: 3 phases exist at same T&P Critical temperature: above this T, only exists as a gas Critical pressure: the pressure to cause condensation at critical temperature Sublimation: from solid to vapor directly Example How much heat is needed to melt 25 g of ice? Example How much heat is evolved when one mole of water cools from 100oC to 5oC? Rules of Thermochemistry 2. DH for a reaction is equal in magnitude by opposite in sign to DH for the reverse reaction. 3. The value of DH for a reaction is the same whether it occurs in one step or in a series AKA: Hess’s Law DHtotal = DH1 + DH2, etc.. How much heat? Calculate DH for the decomposition of liquid sulfuric acid to steam, oxygen and sulfur dioxide gas. How much heat will be generated when 25 g of sulfuric acid decomposes? What is the Hf? Chlorine trifluoride reacts with ammonia to form nitrogen, chlorine and hydrogen fluoride gases. When two moles of chlorine trifluoride reacts, 1196 kJ of heat is evolved. Find Hf for chlorine trifluoride. Enthalpy Change and Hf Acetylene, C2H2, and benzene, C6H6, have the same empirical formula. Benzene can be made from acetylene: 3C2H2C6H6. The heats of combustion for C2H2 and C6H6 are 1299.4 kJ/mol and -3267.4 kJ/mol, respectively. Calculate the heat of formation for each and the heat of reaction of the formation of benzene from acetylene.