Analyzing protein network robustness using graph serum
advertisement
ANALYZING PROTEIN NETWORK ROBUSTNESS USING GRAPH SPECTRUM
Jingchun Chen {jchen@systemsbiology.org}
The Ohio State University, Columbus, Ohio
Institute for Systems Biology, Seattle, Washington
ABSTRACT
Understanding the robustness of protein-protein interaction networks can shed
lights on the molecular mechanisms of many human diseases. Until very
recently, studies of network robustness have been mainly through network
breakdown simulation upon large-scale removal of nodes, which rarely happens
in biological networks. Here we present a method where a network is
represented by its Laplacian graph spectrum and topological change is
represented by spectral distance. The invariability of the spectrum
representation and the sensitivity of spectrum distance make it suitable for
studying network robustness where only very few nodes are removed. Applying
this framework to the protein-protein interaction network of yeast, we show that
graph spectral change not only correlates with number of pairwise interactions of
individual proteins, but more importantly, it also correlates well with the number
of connected functional modules. Therefore, this method may be used to study
network robustness at higher level. As one example of its potential applications,
we show that for yeast double mutants, removal of essential genes causes
bigger changes in the graph spectrum of the proteome network than nonessential genes. This suggests that this method may be used as an in silico “first
pass” in the screening of genetic interactions at genomic scale, which is
impossible to achieve directly in wet laboratories. Overall, our study shows that
graph spectrum is a new method to study network robustness with potential
applications in drug target identification.
INTRODUCTION
The robustness of a network is its capacity to maintain its structural and
functional integrity in response to internal failure or external attack.
Understanding how cellular networks behave at the time of internal failure (such
as gene mutation) or external attack (pathogen invasion) is essential to drug
development. Through network breakdown simulation, Barabasi and his
colleagues showed that, comparing to random networks, scale-free networks
are more robust against internal failure (random removal of edges) but are less
robust against targeted attacks (hub removal) (Albert et al., 2000). Application
of this principle on the protein network of yeast satisfactorily explains gene
essentiality (Jeong et al., 2001). One drawback of this simulation is that
network robustness is examined under large-scale removal of nodes. In reality,
cellular network perturbations usually involve very few components. Therefore,
new methods are desired to study network robustness when one or only a few
nodes are removed.
Graph spectrum is one of the network representations that may be used to
address this issue. The Laplacian spectrum of a graph is the set of eigenvalues
of its Laplacian matrix. The Laplacian spectrum of a network not only is closely
related to networks dynamics, it is also an invariant characterization of the
network (Cvetkovic et al., 1995). Therefore, graph spectral distance may be a
good measurement for network topological change upon node removal and may
be used to study network robustness.
Bo Yuan {yuanbo@cs.sjtu.edu.cn}
The Ohio State University, Columbus, Ohio
Shanghai Jiaotong University, Shanghai, China
RESULTS
CONCLUSIONS
Graph spectrum distance can be used to measure network
change at both individual and global levels, and can be used
to simulate network robustness under minor disturbance.
Correlation analysis suggests that graph spectrum can serve
as an in silico tool for pre-screening genetic interactions and
identification of potential drug targets.
FUTURE DIRECTION
Build predictive models that explain gene essentiality
through connectivity, modularity and graph spectral change
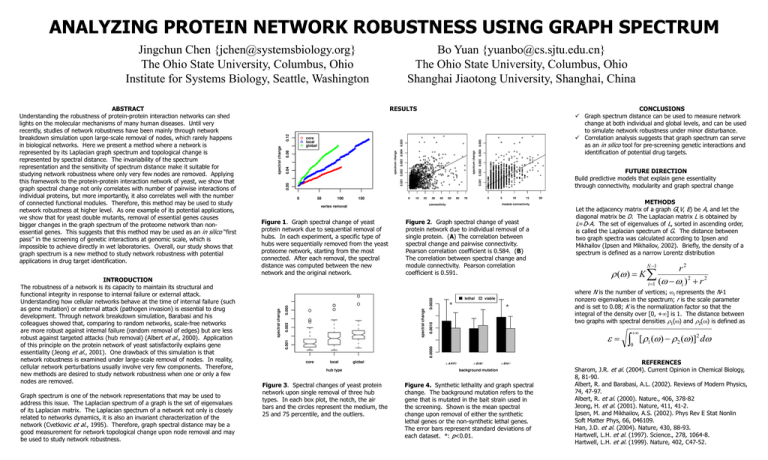
Figure 1. Graph spectral change of yeast
protein network due to sequential removal of
hubs. In each experiment, a specific type of
hubs were sequentially removed from the yeast
proteome network, starting from the most
connected. After each removal, the spectral
distance was computed between the new
network and the original network.
Figure 2. Graph spectral change of yeast
protein network due to individual removal of a
single protein. (A) The correlation between
spectral change and pairwise connectivity.
Pearson correlation coefficient is 0.584. (B)
The correlation between spectral change and
module connectivity. Pearson correlation
coefficient is 0.591.
*
*
METHODS
Let the adjacency matrix of a graph G(V, E) be A, and let the
diagonal matrix be D. The Laplacian matrix L is obtained by
L=D-A. The set of eigenvalues of L, sorted in ascending order,
is called the Laplacian spectrum of G. The distance between
two graph spectra was calculated according to Ipsen and
Mikhailov (Ipsen and Mikhailov, 2002). Briefly, the density of a
spectrum is defined as a narrow Lorentz distribution
N 1
r2
( ) K
2
2
(
)
r
i 1
i
where N is the number of vertices; i represents the N-1
nonzero eigenvalues in the spectrum; r is the scale parameter
and is set to 0.08; K is the normalization factor so that the
integral of the density over [0, +] is 1. The distance between
two graphs with spectral densities 1() and 2() is defined as
Figure 3. Spectral changes of yeast protein
network upon single removal of three hub
types. In each box plot, the notch, the air
bars and the circles represent the medium, the
25 and 75 percentile, and the outliers.
Figure 4. Synthetic lethality and graph spectral
change. The background mutation refers to the
gene that is mutated in the bait strain used in
the screening. Shown is the mean spectral
change upon removal of either the synthetic
lethal genes or the non-synthetic lethal genes.
The error bars represent standard deviations of
each dataset. *: p<0.01.
0
[ 1 ( ) 2 ( )]2 d
REFERENCES
Sharom, J.R. et al. (2004). Current Opinion in Chemical Biology,
8, 81-90.
Albert, R. and Barabasi, A.L. (2002). Reviews of Modern Physics,
74, 47-97.
Albert, R. et al. (2000). Nature., 406, 378-82
Jeong, H. et al. (2001). Nature, 411, 41-2.
Ipsen, M. and Mikhailov, A.S. (2002). Phys Rev E Stat Nonlin
Soft Matter Phys, 66, 046109.
Han, J.D. et al. (2004). Nature, 430, 88-93.
Hartwell, L.H. et al. (1997). Science., 278, 1064-8.
Hartwell, L.H. et al. (1999). Nature, 402, C47-52.