Measurements and Calculations 2010
Measurements and Calculations
W H E R E M A T H B E C O M E S R E A L I T Y !
Measurement standards
Quantities such as:
Time
Distance or length
“weight”
Light brightness
MANY standards of measure have been used over the years.
Do you recognize any of these units?
Millennium
Slug
Bushel
Kilogram
Calorie
Cubit
Foot-pound
Fahrenheit
Only 7 quantities can be measured directly!
Quantity
Time
Mass
Distance or length
Temperature
Amount of substance
Amount of electricity
Light brightness
Base Unit
Second
Kilogram
Meter
Kelvin
Mole
Ampere
Candela
...everything else is calculated!
Speed
Current
Energy
Volume
Weight
Force
…Which we call “derived” units…
What do you think “modified” units might be?
“metric” system
Actually, called “SI” for systeme international a worldwide agreement among scientists to adopt this method of measurement.
Also called, “kg-m-s” system for
Kilogram
Meter
Second
Should US officially adopt?
Refresher……
Accuracy vs. Precision
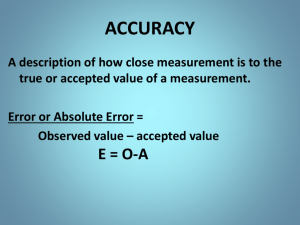
Accuracy – how close a measured value is to an accepted value
Precision – how close a series of measurements compare to one another
Sucrose density – 1.59 g/mL
Trial 1
Trial 2
Trial 3
Average
Student A Student B Student C
1.54 g/mL
1.60 g/mL
1.40 g/mL
1.68 g/mL
1.70 g/mL
1.69 g/mL
1.57 g/mL
1.57 g/mL
1.45 g/mL
1.51 g/mL
1.71 g/mL
1.70 g/mL
Precision
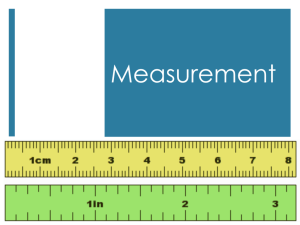
Measurements are as only as specific as the instrument being used.
Consider a ruler marked in whole inches OR a ruler marked in tenths of inches.
This is called the “precision” of the instrument and is indicated by the number of places used in writing the measurement.
For example….
That ruler marked in whole inches can only be written down to the tenths place.
10.5
1.7
8.3
Matter of fact, since the “tenth” was estimated, anyway, it is called a “guess digit”.
How about the ruler marked in tenths?
Well, you could estimate in the hundredths place.
10.58
1.46
0.58
Consider the measurement 11.20 inches using that ruler……why write the “zero”?
Scientific Notation Refresher….
The Arabic number system is based on 10!
10 1 is one decimal place, right?
What about 10 -3 ?
Scientific Notation
Two factors:
1.
A number between 1 and 10
2.
10 raised to a power (exponent)
Tells how many times the first factor must be multiplied by
10
Positive exponent – larger than 1 (move decimal to right)
Negative exponent – smaller than 1 (move decimal to left)
Examples:
1392000 – 1.392 x 10 6
0.000000028 – 2.8 x 10 -8
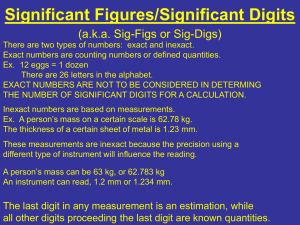
Which numbers are significant?
All non-zeroes.
72.3
Zeroes between non-zeroes.
60.5
All zeroes to the right of a non-zero if the number contains a decimal.
6.20, 620
NEVER leading zeroes!
0.0253, .00054
Counting numbers and constants do not count as sig figs.
Significant Figures
When adding and subtracting: Answer must have the same # of digits to the right of the decimal point as the value with the fewest digits to the right of the decimal point
Example: 28.0
23.538
+25.68
77.218 = 77.2
Significant Figures
Multiplication and Division: Answer must have the same # of significant figures as the measurement with the fewest sig figs.
Example: Volume of an object with dimensions
L = 3.65 cm, W= 3.20 cm, H= 2.05 cm
3.65 x 3.20 x 2.05= 23.944 cm 3
How m any sig figs does it need?
Whew! Let’s summarize…
Measured quantities are used to calculate other quantities of interest.
Those measurements come in a variety of scales and definitions, SO we all have to agree on a system.
Measurements are written in such a way as to indicate the precision of the instrument used.
Next….
How does that precision get indicated when we calculate with the number?
In other words, if I’m calculating with two numbers: one is made to the tenths….another is measured to the thousandths, where should I round my answer?
How precise can my calculation be?