ppt file for this lecture (click to file)
advertisement
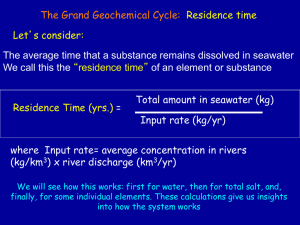
Lectures 5 Geosc 040 Chemical Properties of Water --The Wonder Substance! Inputs, Outputs and Residence Time Ocean Salinity Map A new planet? • Lecture Review Questions: • TA Office Hours Tue. 10-11, Wed. 2-3, Room 313 Deike Bld. • Homework 1 due on Thurs. (28th) by 11pm • Sign up to take Quiz 1 on Feb 1 • Cell Phone Recycling • Lecture Review Questions: • TA Office Hours Tue. 10-11, Wed. 2-3, Room 313 Deike Bld. • Homework 1 due on Thurs. (28th) by 11pm • Sign up to take Quiz 1 on Feb 1 • Cell Phone Recycling Jonas! Jonas! Jonas: Read about storms like it on the course web site! Hurricane Tracks Sea Surface Temperature & Latent heat are key factors in Hurricane development and sustainability. Sea Surface Temperature & Latent heat are key factors in Hurricane development and sustainability. Warm water is the energy source for hurricanes and storms like Jonas energy Warm water is the energy source 150 Vapor Latent heat of evaporation 540cal/gm 100 Liquid water 50 0 -50 Ice -100 energy 0 200 400 600 Heat input (cal/gram) 800 General Classification of Water Masses Why is ocean water stratified into these layers? What are the characteristics of each of these regions? Why do we care? Density of Seawater Salinity (S) - Temperature (T) - Density Diagram •note dual effects of S & T •typical range of seawater S & T Define Sigma t : t = (Density-1.0) x 1000 For example: seawater with a density of 1.026 would have t = 26 We'll use this diagram to differentiate water masses t = (Density-1.0) x 1000 t = 21 t = 24 t = 29 More complete Salinity- Temperature- Density Diagram for the normal range of salinity and temperature in the oceans. You should become familiar with this diagram. Let’s look at this in a bit more detail. Example 1: What is the density of 1 vs. 2? Water masses 1 & 2 have same S (35 o/oo) But 2 (at 4°C) is colder (T is lower ) than 1 (20°C); Therefore: Water mass 2 (t = 27.7) is more dense than water mass 1 (t = 24.8) 1 2 Dissolved Gases in Seawater Depth profiles of dissolved oxygen and dissolved carbon dioxide Indicates: Photosynthesis Oxygen production in surface waters Consumption of oxygen below surface waters Respiration Indicates: CO2 consumption in surface waters Production of CO2 in deep waters Chemical Properties of Water --The Wonder Substance! Inputs, Outputs and Residence Time Seawater is essentially an NaCl solution (saltwater) Cl-, Na+, S04-2, Mg+2, Ca+2, K+ >99% of salt in sea water HC03-2, Br-, Sr-2, B+2, F- (with these, 99.99%) http://www.webelements.com/ Rivers vs. Other Sources •Difference in chemical compositions between rivers and ocean --reflects sedimentation (precipitation) processes --other inputs/exchanges, such as basalt-seawater reactions at midocean ridges For example, exchange of Magnesium (Mg) in seawater for Ca in ocean crust supplies excess Calcium ocean chemistry: A balance of inputs and outputs Evaporation plays a big role in Surface Water Salinity 30 ppt Surface water salinity 37 ppt ocean chemistry: A balance of inputs and outputs Evaporation plays a big role in Surface Water Salinity What is this stuff? Can we explain ocean chemistry using the inputs of rivers alone? atmosphere rock ocean weathering rivers We’ve already examined why water is a powerful Solvent, now let’s look at the whole picture Ocean Chemistry and the Geochemical Cycle The Ocean has Both Inputs and Outputs Outputs include: 1--sea salt aerosols 2--biogenic sediments (biological processes); deposited on ocean floor (CaCO3, SiO2) 3--inorganic sediments (precipitates, evaporites; adsorption) 4--interaction of seawater with hot basalt (Mg and SO4 "sink”) Outputs compete with Inputs to shape the chemistry of seawater Outputs: Seawater Evaporation in isolated basins. These sediments (“evaporites”) provide a record of seawater chemistry Mediterranean Sea Suez Canal Sinai Peninsula Nile River Egypt Gulf of Suez Red Sea Dead Sea Rift Salt from the Sea Dead Sea Salt, Evaporation! At work Salt from the Sea Dead Sea Salt, Evaporation! At work Pay attention to the sequence, from the open sea, first calcite … and eventually Halite! The Grand Geochemical Cycle: Residence time Let’s consider: The average time that a substance remains dissolved in seawater We call this the “residence time” of an element or substance Residence Time (yrs.) = Total amount in seawater (kg) Input rate (kg/yr) where Input rate= average concentration in rivers (kg/km3) x river discharge (km3/yr) We will see how this works: first for water, then for total salt, and, finally, for some individual elements. These calculations give us insights into how the system works Residence time of water in the ocean Volume = 1.4 x 109 km3 River Influx = 3.7 x 105 km3 /yr t = Volume / Influx How long does it take to cycle ocean water through rivers and back again? 1.4 x 109 km3 t= 3.7 x 105 km3 t ≈ 4000 years Residence Time: total amount divided by input rate For Coconuts on the beach: • 10 coconuts fall on the beach each day • There are generally 20 coconuts on the beach The residence time for coconuts on this beach is: A) 1 day B) 2 days C) 0.5 days D) 20 days The Grand Geochemical Cycle •How much time to make the ocean salty? • about 5 x 1022 grams of dissolved solids in ocean • rivers bring in about 2.5 x 1015 gm dissolved solids per year --think about it! •Should only take about 2 x 107 years (20 million yrs.) to bring oceans to present salinity Assuming: •rivers have kept approx. same input through time •oceans have kept approx. same composition through time --but we know oceans are 3.8 billion yrs. old •This confirms that there must be output of material from ocean!! The Grand Geochemical Cycle Typical Element Residence Times Cl 80 million yrs. Na 60 million yrs. Mg 10 million yrs. SO4 9 million yrs. Ca 1 million yrs. PO4 100 thousand yrs. Don’t worry too much about absolute numbers, but be able to explain why Cl residence time is so much longer than, say, that of phosphate The Grand Geochemical Cycle Residence time is inversely related to extent of involvement in chemical reactions in the ocean •Na and Cl primarily precipitate as evaporite deposits (infrequent events over geologic history). Bio-inert •Ca used by organisms to make CaCO3 (calcium carbonate) skeletons •PO4 used in biological cycle (organic matter production)--this is a nutrient element. Biolimiting Was the Chemistry of the Ancient Oceans the Same as Today? 35 0/00 Ocean Salinity We can use ancient evaporite deposits to tell us how ocean chemistry changed through time (different sequence of minerals precipitated) We also can use the chemistry of “brine” inclusions in the evaporites to constrain elemental ratios of major elements in seawater through time. New data suggest that ocean chemistry has changed a bit through time. Perhaps this reflects changes in ocean spreading rates and cycling of seawater through hydrothermal systems! Time (billions of yrs) Ocean’s Chemistry and The hydrologic and geochemical cycles Outputs compete with Inputs to shape the chemistry of seawater Gases in Seawater % in atmosphere •Nitrogen (N2) 78.08 •Oxygen (O2) 20.95 •Carbon dioxide (CO2) 0.03 (365 ppm) •Argon, Helium, Neon (Ar, He, Ne) 0.95 Gases Dissolved in Seawater • Gases are soluble in seawater in proportion to their atmospheric concentration --Gas Exchange Gas exchange is enhanced by mixing and biologic processes For Gases Dissolved in Seawater Solubility (amount that can be dissolved) 1) Depends on temperature and salinity of seawater • Decreases with increasing Temperature • Decreases with increasing Salinity 2) Helped by wind-mixing of surface layer • Important for oxygenation of seawater and CO2 uptake from the atmosphere • Important for fishes and other aerobic critters. Gasp! They need Oxygen to Breathe! Solubility of gases in seawater is controlled by temperature (and also by salinity) These curves reflect maximum amount that can be held in solution under these conditions Cold water holds more gas in solution Pepsi Solubility of Gases in Seawater A) Gases dissolve more readily in warmer water B) Cold freshwater can dissolve more gas than warm saltwater C) Fishes need oxygen to live and they get that by breaking O molecules from H20 D) A and B E) B and C What controls variations in oxygen and carbon dioxide with depth in the ocean? What controls variations in oxygen and carbon dioxide with depth in the ocean? Dissolved Gases in Seawater Depth profiles of dissolved oxygen and dissolved carbon dioxide •more oxygen in surface waters • less oxygen in deep waters Indicates consumption of dissolved oxygen below surface waters •less carbon dioxide in surface waters •more carbon dioxide in deep waters Indicates a process that creates carbon dioxide in deep waters