13.4 The Integrated Rate Law The Dependence of Concentration on
advertisement
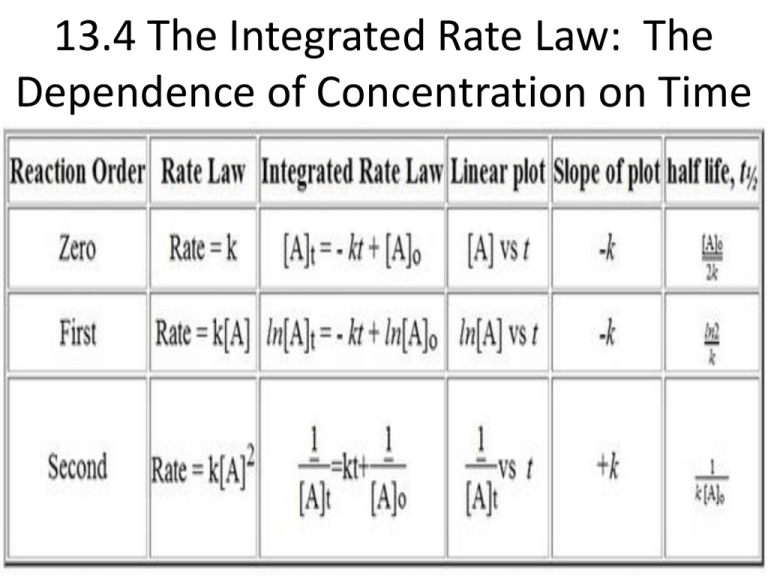
13.4 The Integrated Rate Law: The Dependence of Concentration on Time The Integrated Rate Law • Integrated rate law for a chemical reaction is a relationship between the concentration of the reactant(s) and time. The following equations are on your reference table! The first order integrated rate law is: ln[A]t = -kt + ln[A]0 OR [A]t ln ------ = -kt [A]0 Where [A]t is the concentration at any time t, k is the rate constant, and [A]0 is the initial concentration of A. The rate law above has the form of an equation of a straight line with decreasing slope: y = mx + b. This is because a plot for a first order reaction is a straight line, and even though the slope is negative the rate constant is always positive. Let’s Try a Practice Problem! Cyclopropane rearranges to form propene in the gas phase. The reaction is first order in cyclopropene and has a measured rate constant of 3.36X10-5s-1 at 720 K. If the initial cyclopropane concentration is 0.0445 M, what will the cyclopropane concentration be after 235.0 min? ln[A]t = -kt + ln[A]0 ln[cyclopropane]t = -(3.36X10-5s-1)((235 min X (60 s / 1 min)) + ln(0.0445 M) ln[cyclopropane]t = -3.586, so [cyclopropane]t = e-3.586 = 0.0277 M Second Order Integrated Rate Law 1 1 ---- = kt + ---[A]t [A]0 This equation is also in the form of a straight line, but has an increasing slope because the plot is of the inverse of concentration of the reactant with respect to time. Zero-Order Integrated Rate Law [A]t = -kt + [A]0 Again here, the equation is in the form of a straight line. These straight lines that can be produced by these integrated rate law equations can be used to aid in interpreting data, and extrapolating points on the graph. Half-Life of a Reaction • The half-life (t½) of a reaction – the time required for the concentration of a reactant to fall to one-half of its initial value. First order reaction half-life: (here t½ is independent of the initial concentration. This causes the half-life to be constant, making the concept of half-life particularly useful for first-order reactions). 0.693 t½ = -------k This formula is on your reference table From the College Board: Let’s Try a Practice Problem! Molecular iodine dissociates at 625 K with a first-order rate constant of 0.271 s-1. What is the half-life of this reaction? 0.693 t½ = ---------k 0.693 k = -------------- = 2.56 s 0.271s-1 Let’s Try Another! A first-order reaction has a half-life of 26.4 s. How long does it take for the concentration of the reactant in a reaction to fall to one-eighth of its initial value? Half-life Time Amount 0 0 Initial value 1 26.4 s ½ initial value 2 52.8 s ¼ initial value 3 79.2 s 1/8 initial value If you wanted to use an equation instead of a table here, you would need to use: Time = half-life X log2(1/y) where y= fraction of material remaining… so the table may seem easier. Summarizing Basic Kinetic Relationships • The reaction order and the rate law must be determined experimentally. • The rate law relates the rate of the reaction to the concentration of the reactants. • The integrated rate law (which is mathematically derived from the rate law) relates the concentration of the reactant(s) to time. • The half-life is the time it takes for the concentration of the reactant to fall to one-half of its initial value. • The half-life of a first order reaction is independent of the initial concentration. (We will only worry about half-lives of first order reactions in this course) 13.4 pgs. 640-641 #’s 45, 48, 53 & 56 Read 13.5 pgs. 615-622