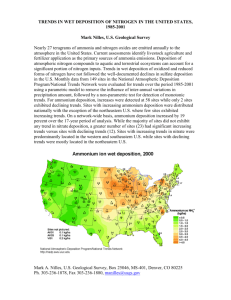
Acid Deposition
advertisement
Title: Lesson 13: Acid Deposition Learning Objectives: – Balancing the equations that describe the combustion of sulphur and nitrogen to their oxides and formation of their acids – Distinction between pre-combustion and post combustion methods of reducing sulphur oxide emissions. – Deduction of acid deposition equations for reactions with metals and carbonates Causes of acid deposition All rain water is naturally acidic (carbon dioxide dissolves in water forming weak carbonic acid) Carbonic acid ionizes to form the equilibrium: This gives a minimum pH of 5.6. Acid rain < pH 5.6 Oxides of Sulphur and Nitrogen (primary pollutants) are the cause of acid rain (secondary pollutant) Acid deposition is a broader term than acid rain and includes all process by which acidic components as precipitates or gases leave the atmosphere. Main Menu Types of acid deposition Wet acid deposition: rain, sleet, snow, fog, mist, dew fall to the ground as aqueous precipitates Dry acid deposition: acidifying particles, gases fall to the ground as dust and smoke later dissolves in water Richard Thornley - Acid Deposition Video Main Menu Sulphur dioxides Produced from burning fossil fuels (coal/heavy oil to generate electricity) Released in industrial processes such as metal extraction from ores (smelting) 50% global emissions from coal SO2 is a colourless gas with sharp smell and it dissolves in water to form sulfurous acid SO2 can be oxidized to Sulphur trioxide, which then dissolves in water to form Sulphuric acid Simple oxidation by atmospheric Oxygen is not involved. Complex interactions with hydroxyl radicals, ozone or hydrogen peroxide are involved. (Don’t worry too much about the detail of this) Main Menu Nitrogen oxides NO is produced mainly from internal combustion engines, where burning fuel releases heat energy causing nitrogen and oxygen to combine: A similar reaction gives rise directly to a brown gas, nitrogen dioxide: Nitrogen dioxide also forms from the oxidation of nitrogen monoxide: Nitrogen dioxide dissolves in water to form a mixture of nitrous acid (HNO2) and nitric acid (HNO3): Alternatively, nitrogen dioxide can be oxidized to form nitric acid: Main Menu The formation of acid deposition Simple oxidation by atmospheric Oxygen is not involved. Complex interactions with hydroxyl radicals, ozone or hydrogen peroxide are involved. Below if an example of how hydroxyl free radicals (.HO) contribute to the production of nitrous and nitric acid. Main active components of acid rain are : Main Menu Effects of acid deposition Acid deposition has a wide range of impacts: Materials Plant life Water Human health TASK: Using the information sheet and your student textbooks, write a summary of the effects of Acid Deposition. Main Menu Methods of dealing with acid deposition Improving design of vehicle engines Using catalytic converters Removing Sulphur before burning fossil fuels Using renewable power supplies Making greater and more efficient use of public transport Designing more efficient power stations ‘Liming’ of lakes – calcium oxide or hydroxide neutralizes acidity: CaO(s) + H2SO4(aq) CaSO4(aq) + H2O(l) Ca(OH)2(s) + H2SO4 CaSO4(aq) + 2H2O(l) Main Menu Reductions of S02 Emissions Pre-combustion methods Remove Sulphur in coal or oil before combustion. This is done by crushing coal and washing with water. High density metal sulfide sinks to bottom and separates from clean coal. Hydrodesulfurization (HDS) is a catalytic process that removes sulfur by reacting it with hydrogen to form hydrogen sulfide, H2S. This is toxic so is captured and converted into elemental sulfur for use in manufacturing sulfuric acid. Main Menu Reduction of SO2 emissions Post-combustion methods Flue-gas desulfurization removes up to 90% of SO2 from flue gas in the smoke stacks of coal-fired power stations before it is released into the atmosphere. A wet slurry of CaO and CaCO3 reacts with SO2 to form the neutral product calcium sulfate, CaSO4: Calcium sulfate (gypsum) is used in industry in the manufacture of plasterboard Main Menu Reductions of NOx Emissions Catalytic converters in vehicles Hot gases are mixed with air and passed over a platinum or palladium based catalyst. This converts toxic emissions into harmless products: Lower temperature combustion The formation of NO is reduced at lower temperatures. Recirculating exhaust gases back into the engine lowers the temperature to reduced NO emissions. Main Menu Main Menu Solutions Main Menu Main Menu Main Menu