Polar Bonds & Molecules
advertisement

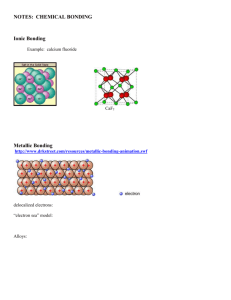
8.4 Describe ionic bonding in 1 complete sentence. Bond Polarity Bonding Ionic Describe covalent bonding in 1 complete sentence. Covalent Polar Nonpolar nonpolar covalent bond: nonpolar covalent bond: atoms with equal pull (similar electronegativity) so bonding electrons are shared equally. H2, N2, O2, F2, Cl2, Br2, I2 + H + H polar covalent bond: bonding electrons are shared unequally. less EN + more EN H Cl “partial” dipole: molecule with 2 poles (partial – end and partial + end) Which of these bonds is the most polar? greatest H-Cl H-F O-H C-N diff. EN F is a 4.0 (most EN element) Electronegativity Differences (& Bond Types) C–H is a NONpolar bond (2.5 – 2.1 = 0.4) Bonding Summary Ionic (e– transferred) Polar Covalent (e– unequally shared) (ΔEN) Nonpolar Covalent (e– equally shared) SHAPE polar molecule: •has polar bonds matters •arranged asymmetrically (unbalanced pull in different directions) (polar bonds do not cancel out) net dipole nonpolar molecule: •has no polar bonds, or… •has polar bonds… arranged symmetrically (balanced pull in every direction) (polar bonds do cancel out) Cl Cl Quick Quiz! 1. In a nonpolar covalent bond, electrons are A. gained by one atom. B. shared unequally between atoms. C. shared equally between atoms. D. lost by both atoms. Give 2 example of nonpolar molecules: Br2, CO2, CCl4 Quick Quiz. 2. In a molecule, the most electronegative atom A. repels electrons more strongly and acquires a partial negative charge. B. repels electrons more strongly and acquires a partial positive charge. C. attracts electrons more strongly and acquires a partial positive charge. + D. attracts electrons more strongly and acquires a partial negative charge. Quick Quiz. 3. Which of the following bonds is the most polar? A. H-N B. O-N C. H-F D. Cl-Cl greatest difference in EN Quick Quiz. 4. Why is carbon tetrachloride, CCl4,a nonpolar molecule even though it contains 4 polar C–Cl bonds? A. The polar bonds are too weak. B. The polar bonds cancel in opposite directions. C. Carbon is always nonpolar. D. Carbon tetrachloride has single bonds. Quick Quiz. 5. Which of the following is a polar molecule? A. H2S B. CO2 C. NH3 D. I2 S Cl Cl Polar? or Nonpolar? AB4 AB2 AB3 AB4 AB3E AB2E2