Expt7a
advertisement
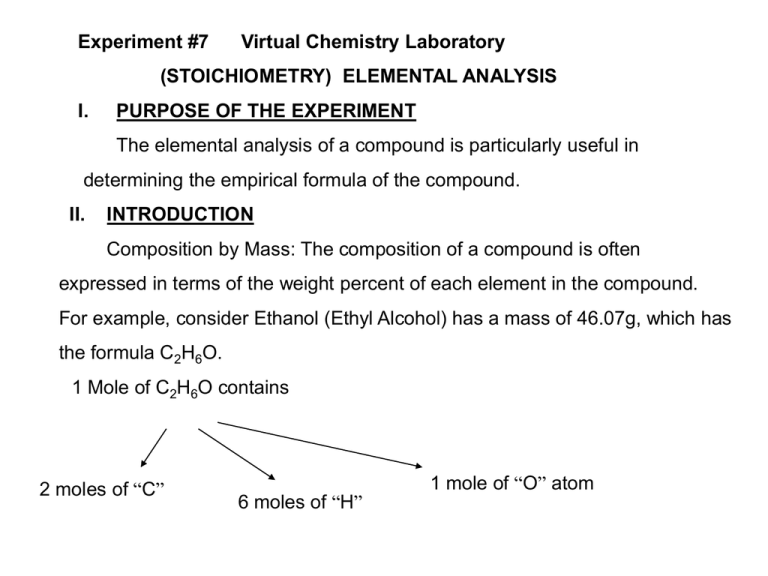
Experiment #7 Virtual Chemistry Laboratory (STOICHIOMETRY) ELEMENTAL ANALYSIS I. PURPOSE OF THE EXPERIMENT The elemental analysis of a compound is particularly useful in determining the empirical formula of the compound. II. INTRODUCTION Composition by Mass: The composition of a compound is often expressed in terms of the weight percent of each element in the compound. For example, consider Ethanol (Ethyl Alcohol) has a mass of 46.07g, which has the formula C2H6O. 1 Mole of C2H6O contains 2 moles of “C” 6 moles of “H” 1 mole of “O” atom %of" C" 2moles" C" (12.01g / mol" C" ) x100% 52.14% 46.07g..Ethanol %of" H" 6moles" H" (1.008g / mol" H" ) x100% 13.13% 46.07g..Ethanol %of" O" 1moles" O" (16.01g / mol" O" ) x100% 34.73% 46.07g..Ethanol Notice: that the sum of the weight percents of all the elements in a compound must equal 100%. Empirical Formula: The formula that contains the smallest set integer ratios for the elements in the compound that gives the correct elemental composition by mass. Example: Octane , whose molecular formula is C8H18. 8: 18 Divided by 8 The smallest ratio of integers is Therefore Empirical Formula is 1: 2.25 x 2(or)3(or)4 4: 9 (1:2.25 x 4) [C4 H9] Note: Molecular formula is an integer (n) multiple of the Empirical formula. For Octane E.F = C4H9 M.F = C4nH9n, In this case n = 2, which produces C8H18. Be aware that the elemental analysis is not perfectly accurate. The experimental error will generally produces atom ratios that are not perfect integers but are close to integers. A separate measurement of the molecular mass is required to determine the molecular formula .(Mass Spectrometry) III. EXPERIMENTAL 3.1 Chemicals: 3.2 Equipment: 3.3 Procedure: Refer (http://www.chm.davidson.edu/chemistryapplets/stoichiometry/CH.html) IV. RESULTS and DISCUSSIONS V. CONCLUSION








