Percent Composition - Chemistry
advertisement

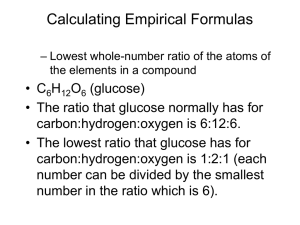
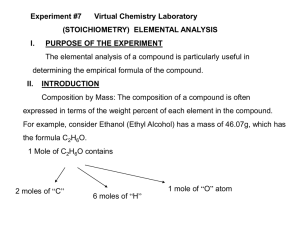
and Empirical Formula Determine the mass percentage of each element in the compound. mass _ of _ element 100 mass _ of _ compound 2 Fe 2 x 55.8 = 111.6 g 3 O 3 x 16.0 = 48.0 g Formula mass = 159.6 g % Fe = 111.6 159.6 70.0 % Fe %O= 48.0 159.6 30.0% O Gives the lowest whole # ratio of elements in a compound. The empirical formula for C6H12O6 is The empirical formula for C2H6 is * most basic ratio of elements in the compound CH2O CH3 X (empirical formula) = molecular formula So. . . . molecular _ formula _ mass X= empirical _ formula _ mass And. . . emp _ formula X mol _ formula _ mass What is the molecular formula of the molecule that has an empirical formula of CH2O and a molar mass of 120.12 g/mol? ◦ We need to find the molar mass of the empirical formula first. Easy level ◦ C4H8O4 What is the empirical formula? Medium Difficulty ◦ The previous problem Hardest Difficulty ◦ Next problem Find the empirical formula of a compound that contains 53.7% iron and 46.3% sulfur. Steps: ◦ % composition mass of 100g sample moles mole ratio 1 mol 53.7 g 55.8 g 0.962 mol Fe 46.3 g 1mol 1.44 mol S 32.1g Take smaller # and divide everything by it 0.962 Fe 1.0 0.962 1.44 S 1 .5 0.962 1:1.5 2 2:3 Fe2S3















![사본 Kyungtae Park - [Q4 Group Project] Final Report](http://s2.studylib.net/store/data/025972592_1-d587c54d0599377dbda1cdf1cad5cedb-300x300.png)
