Uncertainty in Measurements
advertisement

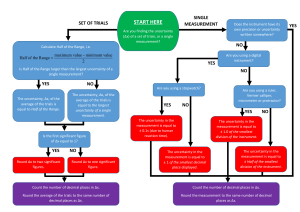
Uncertainty in Measurements • There is uncertainty in every measurement, this uncertainty carries over through the calculations – Need a technique to account for this uncertainty • We will use rules for significant figures to approximate the uncertainty in results of calculations Section 1.4 Significant Figures • A significant figure is a reliably known digit • All non-zero digits are significant • Leading zeros are not significant • Trailing zeros are significant unless they are just used to locate a decimal point – Using scientific notion to indicate the number of significant figures removes ambiguity when the possibility of misinterpretation of trailing 0’s is present Section 1.4 Number Significant Figures 3.35 3 2 1 2.10 3 .00456 3 5.00 3 1200 Ambiguous 1.2 x 103 2 Operations with Significant Figures • When multiplying or dividing two or more quantities, the number of significant figures in the final result is the same as the number of significant figures in the least accurate of the factors being combined – Least accurate means having the lowest number of significant figures – e.g. 2 x 3.1 = 6, 2 x 3.5 = 7, 2.0 x 3.1 = 6.2, 2.01 x 3.1 = 6.2 • When adding or subtracting, round the result to the smallest number of decimal places of any term in the sum (or difference) – e.g. 2 + 3.1 = 5, 2.0 + 3.1 = 5.1, 2.01 + 3.1 = 5.1 Section 1.4 Rounding • Calculators will generally report many more digits than are significant – Be sure to properly round your results • Slight discrepancies may be introduced by both the rounding process and the algebraic order in which the steps are carried out – Minor discrepancies are to be expected and are not a problem in the problem-solving process • In experimental work, more rigorous methods would be needed Section 1.4 Conversions • When units are not consistent, you may need to convert to appropriate ones • See the inside of the front cover for an extensive list of conversion factors • Units can be treated like algebraic quantities that can “cancel” each other • Example: Section 1.5 Unit Must be Consistent • Solving equations requires a consistent choice of units • Let v = 5 m/s and t = 5 s, then if x = vt x vt 5 m 5 s 5m s • Suppose v = 50 km/hr and t = 0.5 hr km x vt 50 0.5hr 25km hr • But is v = 50 km/hr and t = 600 s, we get x vt 48 km km 1 600s 48 600s 8km s hr hr 3600 hr Estimates • Can yield useful approximate answers – An exact answer may be difficult or impossible • Mathematical reasons • Limited information available • Can serve as a partial check for exact calculations Section 1.6 Order of Magnitude • Approximation based on a number of assumptions – May need to modify assumptions if more precise results are needed • Order of magnitude is the power of 10 that applies Section 1.6