Section 2
advertisement

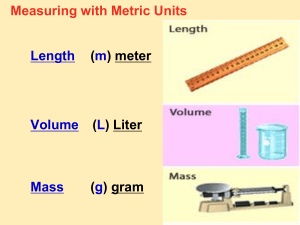
Chapter 2 Section 2 Section 2 Objectives • Be able to define: quantity, measurement, standard, length, mass, weight, derived unit, volume, density, conversion factor. • Be able to state the units of mass, length, temperature, and time in the SI system • Be able to explain the difference between mass and weight. Section 2 Objectives • Be able to state the meaning of common prefixes used in the SI system (Deka-, Hecto-, Kilo-, Mega-, Giga-, deci-, centi-, milli-, micro-, nano-. • Be able to convert units within the SI system. Section 2: Units of Measure • Measurements are quantitative information. • Measurements _______________ quantities. • A quantity is something that has __________________, __________, or ________________. • A quantity is not the same as a measurement. • Example: A teaspoon is a unit of measurement for volume (a quantity) • Nearly every measurement is a number plus a ___________. Section 2: Units of Measure • Measurements are quantitative information. • Measurements represent quantities. • A quantity is something that has __________________, __________, or ________________. • A quantity is not the same as a measurement. • Example: A teaspoon is a unit of measurement for volume (a quantity) • Nearly every measurement is a number plus a ___________. Section 2: Units of Measure • Measurements are quantitative information. • Measurements represent quantities. • A quantity is something that has magnitude, size, or amount. • A quantity is NOT the same as a measurement. • Example: A teaspoon is a unit of measurement for volume (a quantity) • Nearly every measurement is a number plus a ___________. Section 2: Units of Measure • Measurements are quantitative information. • Measurements represent quantities. • A quantity is something that has magnitude, size, or amount. • A quantity is NOT the same as a measurement. • Example: A teaspoon is a unit of measurement for volume (a quantity) • Nearly every measurement is a number plus a unit. Section 2: Units of Measure • Scientists all over the world have agreed on a single measurement system, ____________. • These units are defined in terms of standards of ______________________________. • International organizations monitor the defining process, such as the ____________________ ________________ ___ __________ ____ ____________________ in the United States. Section 2: Units of Measure • Scientists all over the world have agreed on a single measurement system, SI. • These units are defined in terms of standards of ______________________________. • International organizations monitor the defining process, such as the ____________________ ________________ ___ __________ ____ ____________________ in the United States. Section 2: Units of Measure • Scientists all over the world have agreed on a single measurement system, SI. • These units are defined in terms of standards of measurement. • International organizations monitor the defining process, such as the ____________________ ________________ ___ __________ ____ ____________________ in the United States. Section 2: Units of Measure • Scientists all over the world have agreed on a single measurement system, SI. • These units are defined in terms of standards of measurement. • International organizations monitor the defining process, such as the National Institute of Standards and Technology (NIST) in the United States. Section 2: Units of Measure For example, the number seventy five thousand is written ___________________ instead of ____________________________ because the comma is used in other countries to represent a decimal point Section 2: Units of Measure For example, the number seventy five thousand is written 75 000 instead of 75,000 because the comma is used in other countries to represent a decimal point SI System • The SI system defines 7 base units for 1. length, 2. mass, 3. time, 4. temperature, 5. amount of a substance SI Base Units Quantity Quantity Symbol Unit name Unit abbreviation 1. Length l Meter m 2. Mass m Kilogram kg 3. Time t Second s 4. Temperature T Kelvin K 5. Amt of Subst. n Mole mol SI Base Units: Mass • Mass is the measure of the ______________ ____ ________________. • The ___________, g, is 1/1000 of a kilogram and is more useful for measuring masses of small objects such as flasks and beakers. • For even smaller objects, such as tiny quantities of chemicals (think: medicines or vitamins!), the _____________ or ____ is used. SI Base Units: Mass • Mass is the measure of the quantity of matter. • The gram, g, is 1/1000 of a kilogram and is more useful for measuring masses of small objects such as flasks and beakers. • For even smaller objects, such as tiny quantities of chemicals (think: medicines or vitamins!), the milligram or mg is used. • 1 milligram = 1/1000 of a gram SI Base Units: Mass • The measure of the gravitational pull on matter (gravity) is _______________. • Mass does not depend on ____________. • As the force of Earths’ gravity on an object increases, the object’s weight _____________________. • The weight of an object on the moon is about ___________ of its weight on Earth. SI Base Units: Mass • The measure of the gravitational pull on matter (gravity) is weight. • Mass does not depend on gravity. • As the force of Earths’ gravity on an object increases, the object’s weight _____________________. • The weight of an object on the moon is about ___________ of its weight on Earth. SI Base Units: Mass • The measure of the gravitational pull on matter (gravity) is weight. • Mass does not depend on gravity. • As the force of Earths’ gravity on an object increases, the object’s weight increases. • The weight of an object on the moon is about one-sixth (1/6) of its weight on Earth. SI Base Units: Length • The SI standard unit for length is the ______________. • To express longer distances, the __________________, ___ is used. • To express short distances, the _____________, _____ is used. (add to notes) • One _____________ is 1000 meters. SI Base Units: Length • The SI standard unit for length is the meter. • To express longer distances, the kilometer, km is used. • To express short distances, the _____________, _____ is used. (add to notes) • One _____________ is 1000 meters. SI Base Units: Length •The SI standard unit for length is the meter. •To express longer distances, the kilometer, km is used. •To express short distances, the centimeter, cm is used. (add to notes) •One kilometer is 1000 meters. Derived SI Units •Combination of SI base units form ________ ______. •For example, area, is ________ x ________. m m2 m Derived SI Units •Combination of SI base units form derived units. •For example, area, is ________ x ________. m m2 m Derived SI Units •Combination of SI base units form derived units. •For example, area, is length x width. m Area = L x W Area = m x m Area = m2 m2 m Derived SI Units Quantity Symbol Unit name Unit abbrev. 1. Area A Square Meter m2 2. Volume V Cubic Meter m3 3. Density D 4. Molar Mass M 5. Molar Volume Vm 6. Energy E Kilograms per cubic meter Kilograms per mole cubic meters per mole Joule Derivation length x width l x w x height kg/ m3 kg/mol m3/mol J mass/volume m/amt. of sub. volume/n force x length Derived SI Units - Volume • The amount of space occupied by an object is ____________, and the derived SI unit is ___________ _________. • This amount is equal to the volumne of a cube whose edges are each ____ ___ long. • But in a chemistry laboratory, we need a smaller unit, so we often use _________________ ______________, ______. Derived SI Units - Volume • The amount of space occupied by an object is volume, and the derived SI unit is cubic meters, m3. • This amount is equal to the volume of a cube whose edges are each ____ ___ long. • But in a chemistry laboratory, we need a smaller unit, so we often use _________________ ______________, ______. Derived SI Units - Volume •The amount of space occupied by an object is volume, and the derived SI unit is cubic meters, m3. •This amount is equal to the volume of a cube whose edges are each 1 m long. •But in a chemistry laboratory, we need a smaller unit, so we often use cubic centimeter, cm3. Derived SI Units - Volume (1 m3) x (100 cm/1m) x (100 cm/1 m) x (100 cm/1 m) = 1 000 000 cm3 Derived SI Units - Volume • When chemists measure the volumes of liquid and gases, they often use a non-SI unit called the ________. • **Another non-SI unit, the ________________, or ___, is used for smaller volumes. There are _____________ mL in 1 L. • Because there are also __________ cm3 in a liter, the 2 units, ____________ and __________ _______________ are interchangeable. • View this in a equation: 1 L = 1 dm3 = ___________ cm3 = _________ mL Derived SI Units - Volume • When chemists measure the volumes of liquid and gases, they often use a non-SI unit called the liter, L. • **Another non-SI unit, the ________________, or ___, is used for smaller volumes. There are _____________ mL in 1 L. • Because there are also __________ cm3 in a liter, the 2 units, ____________ and __________ _______________ are interchangeable. • View this in a equation: 1 L = 1 dm3 = ___________ cm3 = _________ mL Derived SI Units - Volume • When chemists measure the volumes of liquid and gases, they often use a non-SI unit called the liter, L. • **Another non-SI unit, the milliliter, or mL is used for smaller volumes. There are 1000 mL in 1 L. • Because there are also __________ cm3 in a liter, the 2 units, ____________ and __________ _______________ are interchangeable. • View this in a equation: 1 L = 1 dm3 = ___________ cm3 = _________ mL Derived SI Units - Volume • When chemists measure the volumes of liquid and gases, they often use a non-SI unit called the liter, L. • **Another non-SI unit, the milliliter, or mL is used for smaller volumes. There are 1000 mL in 1 L. • Because there are also 1000 cm3 in a liter, the 2 units, milliliter and cubic centimeter are interchangeable. • View this in a equation: 1 L = 1 dm3 = ___________ cm3 = _________ mL Derived SI Units - Volume • When chemists measure the volumes of liquid and gases, they often use a non-SI unit called the liter, L. • **Another non-SI unit, the milliliter, or mL is used for smaller volumes. There are 1000 mL in 1 L. • Because there are also 1000 cm3 in a liter, the 2 units, milliliter and cubic centimeter are interchangeable. • View this in a equation: 1 L = 1 dm3 = 1000 cm3 = 1000 mL Derived SI Units - Density • Ever heard the riddle: Which is heavier, a pound of feathers or a pound of lead? • Answer: Neither is heavier, a pound is a pound no matter what the object….but when you want to answer “lead” you are thinking about the object’s density. • For another example, an object made of cork feels lighter than a lead object of the same size. • What you are comparing in such cases is how massive objects are compared with their size. Derived SI Units - Density • This property is called __________________, which is the ratio of __________ to ______________, or ____________ divided by _______________________. • Mathematically, the relationship for density can be written: Density = mass/volume or D = MV • By the SI base units of measurement, density is expressed as kg/m3. Again, for a chemistry laboratory, we make the units smaller, ___/____ or _______/ ________. Derived SI Units - Density • This property is called Density, which is the ratio of mass to volume, or mass divided by volume. • Mathematically, the relationship for density can be written: Density = mass/volume or D = M/V • By the SI base units of measurement, density is expressed as kg/m3. Again, for a chemistry laboratory, we make the units smaller, ___/____ or _______/ ________. Derived SI Units - Density • This property is called Density, which is the ratio of mass to volume, or mass divided by volume. • Mathematically, the relationship for density can be written: Density = mass/volume or D = MV • By the SI base units of measurement, density is expressed as kg/m3. Again, for a chemistry laboratory, we make the units smaller, g/cm3 or g/mL. Derived SI Units - Density • Densities of some familiar materials (Table 4): • Solids Density at 20oC (g/cm3) Liquids Density at 200C (g/mL) • Cork .24 Milk 1.031 • Ice .92 Water .998 • Sucrose (table sugar) 1.59 Sea Water • Diamond 3.26 Gasoline .67 • Lead 11.35 Mercury 13.6 1.025 Derived SI Units - Density • Sample Problem A: • A sample of aluminum metal has a mass of 8.4g. The volume of the sample is 3.1 cm3. Calculate the density of aluminum. -Given: mass (m) = 8.4g & volume (v) = 3.1 cm3 - Unknown: Density (D) Density = mass/volume = 8.4 g/3.1 cm3 = 2.7 g/cm3 Conversion Factors • A ratio derived from the equality between two different units that can be used to convert from one unit to the other is a _______________________ ___________________. • For example, suppose you want to know how many quarters there are in a certain number of dollars. • To figure out this answer, you need to know how _______________ and _________________ are related. • There are ____________ quarters in __________ dollar. Conversion Factors • A ratio derived from the equality between two different units that can be used to convert from one unit to the other is a conversion factor. • For example, suppose you want to know how many quarters there are in a certain number of dollars. • To figure out this answer, you need to know how _______________ and _________________ are related. • There are ____________ quarters in __________ dollar. Conversion Factors • A ratio derived from the equality between two different units that can be used to convert from one unit to the other is a conversion factor. • For example, suppose you want to know how many quarters there are in a certain number of dollars. • To figure out this answer, you need to know how quarters and dollars are related. • There are 4 quarters in 1 dollar. Conversion Factors • There are 4 ways to express this: 1. 4 quarters/1 dollar = 1 2. 1 dollar/4 quarters = 1 3. 0.25 dollar/1 quarter = 1 4. 1 quarter/0.25 dollar = 1 • Notice that each conversion factor equals _________. • That is because the top and bottom quantities divided in any conversion factor and ____________ to each other. In this case 4 quarters = 1 dollar. Conversion Factors • There are 4 ways to express this: 1. 4 quarters/1 dollar = 1 2. 1 dollar/4 quarters = 1 3. 0.25 dollar/1 quarter = 1 4. 1 quarter/0.25 dollar = 1 • Notice that each conversion factor equals ONE. • That is because the top and bottom quantities divided in any conversion factor and ____________ to each other. In this case 4 quarters = 1 dollar. Conversion Factors • There are 4 ways to express this: 1. 4 quarters/1 dollar = 1 2. 1 dollar/4 quarters = 1 3. 0.25 dollar/1 quarter = 1 4. 1 quarter/0.25 dollar = 1 • Notice that each conversion factor equals ONE. • That is because the top and bottom quantities divided in any conversion factor and equivalent to each other. In this case 4 quarters = 1 dollar. Conversion Factors • You can use conversion factors to solve problems through __________________ ____________________; which is a mathematical technique that allows you to use __________ to solve problems involving ________________. • For example, to determine the number of quarters in 12 dollars, you would use a unit conversion that allows you to change from dollars to quarters: • Number of quarters = 12 dollars x conversion factor Conversion Factors • You can use conversion factors to solve problems through dimensional analysis; which is a mathematical technique that allows you to use units to solve problems involving measurements. • For example, to determine the number of quarters in 12 dollars, you would use a unit conversion that allows you to change from dollars to quarters: • Number of quarters = 12 dollars x conversion factor Conversion Factors • Then you have to decide which conversion factor gives you an answer in the desired unit. • In this case, you have _____________ and you want __________________, to eliminate dollars, you must divide the quantity by ____________________. • That factor would be __________________ / ___________________ Conversion Factors • Then you have to decide which conversion factor gives you an answer in the desired unit. • In this case, you have dollars and you want quarters, so to eliminate dollars, you must divide the quantity by dollars. • That factor would be __________________ / ___________________ Conversion Factors • Then you have to decide which conversion factor gives you an answer in the desired unit. • In this case, you have dollars and you want quarters, so to eliminate dollars, you must divide the quantity by dollars. • That factor would be: 4 quarters/1 dollar Conversion Factors • And the calculation would be set up as follows: ? quarters = 12 dollars x conversion factor 12 dollars x 4 quarters/1 dollar = 48 quarters • Notice that the dollars have divided out, leaving the answer in the desired unit, quarters. Conversion Factors **For review of this section, it is imperative to be familiar with the SI Prefixes Table on page 35.** GREEK LATIN 10 Deka- 10 x deci- 1/10 100 Hecto- 100 x centi- 1/100 1000 Kilo- 100 x milli- 1/1000 Million Mega- Million x micro- millionth Billion Giga- Billion x nano- billionth Deriving Conversion Factors • You can derive conversion factors if you know the relationship between the unit you HAVE and the unit you WANT. • For example, from the fact that deci- means “1/10”, you know that there is a 1/10 of a meter per decimeter and that each meter must have 10 decimeters. (1m = 10dm). • You can write the following conversion factor relating meters and decimeters: (1 meter/10 decimeter) and (.1 meter/1 decimeter) and (10 decimeter/1 meter) Deriving Conversion Factors • Sample Problem B: Express a mass of 5.712 grams in milligrams and in kilograms. • Given: 5.712 grams • Unknown: mass in mg and kg • 1 g = 1000 mg • Possible conversion factors: • 1000 mg/1 g and 1 g/1000 mg • To derive an answer in mg, you’ll need to multiply 5.712 g by 1000mg/g: • 5.712 g × 1000 mg/1 g =5712 mg • Answer in kg: • 5.712 g × 1 kg/1000 g= .005712 kg