File - V. Fonte Blog
advertisement

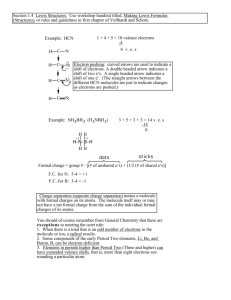
Lewis Structures The most difficult part of drawing Lewis structures is writing the skeleton structure of the molecules. As a general rule, the less electronegative element is at the center of the molecule. Example 1: the formulas of thionyl chloride (SOCl2) and sulfuryl chloride (SO2Cl2) can be transferred into the following skeleton structures. It is also useful to recognize that the formulas for complex molecules are often written in a way that hints at the skeleton structure of the molecule. Example 2: Dimethyl ether is often written as CH3OCH3, which translates to the following skeleton structure. Finally, it is useful to recognize that many compounds that are acids contain O-H bonds. Example 3: The formula for acetic acid is often written as CH3COOH, because this molecule contains the following skeleton structure. A step-by-step approach to drawing Lewis structures 1. 2. 3. 4. Determine the total number of valence electrons Write the skeleton structure of the molecule Use two valence electrons to form each bond in the skeleton structure Try to satisfy the octets of the terminal atoms by distributing the remaining valence electrons as non-bonding electrons 5. Try to satisfy the octets of the central atom by distributing the remaining electrons as non-bonding electrons. Example 4: chlorate ion ClO3- Molecule that contain too many or not enough electrons Too few electrons Occasionally we encounter a molecule that doesn’t seem to have enough valence electrons. If we can’t get a satisfactory Lewis structure by sharing a single pair of electrons, it may be possible to achieve this goal by sharing two or even three pairs of electrons. The elements that form strong double or triple bonds are C, N, O, P and S. Example 5: Consider Formaldehyde (H2CO) Every once and awhile, we encounter a molecule for which it is impossible to write a satisfactory Lewis structure Example 6: consider boron trifluoride (BF3), which contains 24 valence electrons BF3: 3+3(7)=24 Because neither boron nor fluoride falls in this category, we have to stop with what appears to be an unsatisfactory lewis structure. Too many electrons It is also possible to encounter a molecule that seems to have too many valence electrons. When this happens, we expand the valence shell of the central atom Example 7: Consider the Lewis structure for sulfur tetrafluoride (SF4)