Gas Laws Problems
advertisement

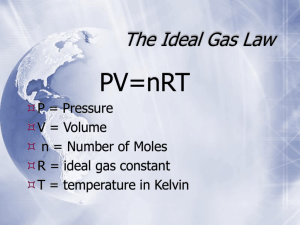
Please answer these questions. Kinetic Theory and Pressure conversions: For questions 1-5, please answer in full sentences. 1. If you inflate a raft, what will happen to the pressure of the raft as you add more gas?Why does this occur? When more gas is added to the raft, the pressure inside the raft will increase because according to the kinetic theory, the increase in particles will cause the volume to increase, thus increasing the pressure. And if the pressure exceeds the strength of the raft, then it will burst open. 2. If you heat up an aerosol can, what will happen to the can?Why does this occur? When the can is heated, the gas particles inside it will start to gain more kinetic energy and move faster. And when the gas particles move faster they strike the walls of the can with more energy. And eventually with the can would burst because with the increase in temperature, the pressure increases too. 3. When you sit on an exercise ball, what will happen to the pressure inside the ball? Why does this occur? When you sit on an exercise ball, you increase the pressure inside the ball because when you sit, you decrease the volume of the ball. And the decrease in volume will cause an increase in pressure. 4. 5. 6. Use kinetic molecular theory to explain a. Why evaporation causes a decrease in a liquid’s temperature b. Why gases are more easily compressed than solids and liquids Evaporation is caused when a particle gains enough energy to escape the intermolecular forces of attraction. When this happens the particle takes this energy along with itself and this energy is gained from the liquid itself. So every time a particle evaporates it take some energy from the liquid with it and this decreases the liquid’s average kinetic energy and thus the temperature. b. The intermolecular forces are too weak in gases and there is a lot more space between gas particles than solids and liquids. This means that when gases are compressed, the space between particles can be reduced easily as the particles can easily relocate (unlike solids and liquids whose particles cannot move around easily and don’t have space between them) when the space between particles is reduced. A small sample of gas is released in a corner of the room and starts to diffuse to the other side. If the room pressure is increased, will the gas diffuse faster, slower, or at the same speed? Explain. If the room pressure is increased there will more collisions between particles and they will gain more kinetic energy so their velocity will increase and thus they will diffuse faster. For each of the following, convert from the units given to the desired units using dimensional analysis. SHOW YOUR WORK if you wish to receive credit. Include all units and box or highlight your answers. a. 202.6 kPa = ? atm Pressure=202.6 kPa Pressure=?atm For converting kPa atm, the appropriate conversion factor is 1 atm/ 101.3 kPa pressure= 202.6 kPa*1atm/101.3kPa=2.0 atm Therefore 202.6 kPa is equal to 2.0 atm The answer 2 atm makes sense because at 1 atm the pressure is 101.3 kPa and since 202.6 is exactly double 101.3 therefore the pressure in atm would be double as well b. 560 kPa = ? mmHg Pressure=560 kPa Pressure=?mmHg For converting kPa mm Hg, the appropriate conversion factor is 760 mm Hg/ 101.3 kPa pressure= 560 kPa*760mmHg/101.3kPa=4201.38 mm Hg Therefore 560 kPa is equal to 4210.38 mm Hg Because at 101.3 kPa the pressure is 760 mm Hg and since 560 is six times 101.3 it would make sense that the pressure in mm Hg would be almost 6 times 760 c. 5 atm = ? kPa d. Pressure=5 atm Pressure=?kPa For converting atm kPa, the appropriate conversion factor is 101.3 kPa/1 atm pressure= 5atm*101.3kPa/1atm= 506.5 kPa Therefore 5 atm is equal to 121.56 kPa Because at 1 atm the pressure is equal to 101.3 and 5 atm is exactly 5 times 1 atm, the answer would be 5 times 101.3 kPa which is 506.5 kPa 3 atm = ? mmHg Pressure=202.6 kPa Pressure=?atm For converting atm mmHg, the appropriate conversion factor is 760mmHg/1atm pressure= 3 *760mmHg/1atm= 2280 mm Hg Therefore 3 atm is equal to 2280 mm Hg e. f. Because at 1 atm the pressure is equal to 760 mm Hg and 3 atm is exactly 3 times 1 atm, the answer would be 3 times 760 mm Hg which is 2280 mm Hg 830 mmHg = ? kPa Pressure=830 mmHg Pressure=?kPa For converting mm Hg kPa, the appropriate conversion factor is 760 mm Hg/101.3 kPa pressure= 830 mm Hg*101.3kPa/760 mm Hg= 110.63 kPa Therefore 830 mm Hg is equal to 110.63 kPa 760 mm Hg is equivalent to 101.3 kPa, and since 830 is only a little greater In value than 760mm, it makes sense that the pressure in kPa will only be a little greater than 101.3 kPa and thus the answer 110.63 kPa seems reasonable 43 mmHg = ? atm Pressure=43 mmHg Pressure=?atm For converting mm Hg atm, the appropriate conversion factor is 1 atm/ 760 mmHg pressure= 43mmHg*1atm/760mmHg= 0.057 atm Therefore 43 mm Hg is equal to 0.057 atm Because the conversion factor is smaller than 1, and 43 is a lot smaller than the denominator 760 as well, it makes sense that the answer is a lot smaller than 1 and 0.057 atm seems reasonable. Boyle’s Law 1. What is the new volume when a 125 mL container at 120.0 kPa is expanded until the pressure is 60.0 kPa? (assume the temperature is constant) Given: V1 = 125mL P1 = 120kPa P2 = 60kPa Required: V2 Analyze: I will need to use Boyle’s law to solve the problem and find V2. The formula needed is P1 x V1 = P2 x V2. Solve: V2 = (P1 x V1)/ P2 V2 = (120kPa x 125mL)/ 60kPa (The kPa will cancel out) V2 = 250mL Paraphrase: Therefore the volume of the container will be at 250mL, if the temperature is constant. Justify: I know the answer I got is correct, due to the fact if the pressure decreases, then the volume will have to increase to maintain the ratio of 1 : 1 between the starting gas and the gas in the container. This also is correct because due to Boyle’s law, when one goes up, the other must go down, which occurs here. 2. What is the new pressure if a 100.0 L container at standard pressure (101.3 kPa) is compressed until the volume is 50.0 L? (assume the temperature is constant) Given: V1 = 100L P1 = 101.3kPa V2 = 50L Required: P2 Analyze: I will need to use Boyle’s law to solve the problem and find P2. The formula needed is P1 x V1 = P2 x V2. Solve: P2 = (P1 x V1)/ V2 P2 = (101.3kPa x 100L)/ 50L (The L will cancel out) P2 = 202.6kPa Paraphrase: Therefore the pressure of the container will be at 202.6kPa, if the temperature is constant. Justify: I know the answer I got is correct, due to the fact if the volume decreases, then the pressure will have to increase to maintain the ratio of 1 : 1 between the starting gas and the gas in the container. This also is correct because due to Boyle’s law, when one goes up, the other must go down, which occurs here. Charles’s Law 3. What is the new volume of a 10.0 mL container at 0.00 K when the temperature is adjusted to 283 K? (Assume pressure is constant) Given: V1 = 10mL T1 = 0K T2 = 283 K Required: V2 Analyze: I will need to use Charles’s law to solve the problem and find V2. The formula needed is V1/ T1 = V2/ T2. Solve: V2 = (V1 x T2)/ T1. V2 = (10mL x 283K)/ (0K) V2 = undefined Paraphrase: Therefore there is no new volume, if the pressure is constant. Justify: I know the answer I got is correct, due to the fact the temperature is at absolute zero, as such there won’t be any volume. 4. A 50.0 mL container is at 273 K. After the volume is adjusted to 100.0 mL, what is the new temperature? (Assume pressure is constant) Given: V1 = 50mL T1 = 273K V2 = 100 ml Required: T2 Analyze: I will need to use Charles’s law to solve the problem and find T2. The formula needed is V1/ T1 = V2/ T2. Solve: T2 = (T1 x V2)/ V1. T2 = (273K x 100mL)/ (50mL) T2 = 546K Paraphrase: Therefore the new temperature is 546K, if the pressure is constant. Justify: I know the answer I got is correct, V2 is double that of V1, as such T2 should be double T1 to maintain the same ratio which it is. Gay-Lussac’s Law 5. What is the new pressure of a set volume of gas at 101 kPa when it is heated from 295 K to 400K? (assume that volume is constant) Given: T1 = 295K P1 = 101kPa T2 = 400K Required: P2 Analyze: I will need to use Gay-Lussac’s law to solve the problem and find P2. The formula needed is P1/ T1 = P2/ T2. Solve: P2 = (P1 x T2)/T1 P2 = (101kPa x 400K)/(295K) (The K will cancel out) P2 = 136.95kPa Paraphrase: Therefore pressure of the new pressure is 136.95kPa, if the volume is constant. Justify: I know the answer I got is correct, due to the fact if the temperature increases, then the pressure will have to increases to maintain the ratio of 1 : 1 between the starting gas and the gas in the container. This also is correct because due to Gay-Lussac’s law, when one goes up, the other must up, which occurs here. 6. A container of gas at 325K and 500 kPa decreases in pressure to 50 kPa. What is the new temperature of the gas? (assume that volume is constant) Given: T1 = 325K P1 = 500kPa P2 = 50kPa Required: T2 Analyze: I will need to use Gay-Lussac’s law to solve the problem and find T2. The formula needed is P1/ T1 = P2/ T2. Solve: T2 = (T1 x P2)/P1 T2 = (325K x 50kPa)/(500kPa) (The kPa will cancel out) T2 = 32.5K Paraphrase: The new temperature of the gas is 32.5K. Justify: I know the answer I got is correct, due to the fact the original pressure is 10 times as much as the end pressure, as such the temperature will be 10 times as small as the original temperature which it is. Combined Gas Law 7. What is the new pressure of a 10.5 L sample of gas at 350 K and 101 kPa when it is heated to 375 K and the volume increases to 11.5L? Given:T1 = 350K P1 = 101kPa V1 = 10.5L T2 = 375K V2 = 11.5L Required: P2 Analyze: I will need to use Combined Gas law to solve the problem and find P2. The formula needed is (P1 x V1)/ T1 = (P2 x V2)/ T2. Solve: P2 = (P1 x T2 x V1)/(T1 x V2) P2 = (101kPa x 375K x 10.5L)/(350K x 11.5L) (The L and K will cancel out) P2 = 98.8kPa Paraphrase: Therefore the new pressure will be at 98.8kPa. Justify: I know the answer I got is correct, as when I use the original formula and solve both sides of the equation I get the ration of 1:1 which means my answer is correct. 8. What is the new volume of a 15 L sample of gas at 365 K and 107 kPa when it is heated to 395 K and the pressure increases to 112 kPa? Given: T1 = 365K P1 = 107kPa V1 = 15L T2 = 395K P2 = 112kPa Required: V2 Analyze: I will need to use Combined Gas law to solve the problem and find V2. The formula needed is (P1 x V1)/ T1 = (P2 x V2)/ T2. Solve: V2 = (P1 x T2 x V1)/(T1 x P2) V2 = (107kPa x 395K x 15L)/(365K x 112kPa) (The kPa and K will cancel out) V2 = 15.51L Paraphrase: Therefore the new volume will be at 15.51L. Justify: I know the answer I got is correct, as when I use the original formula and solve both sides of the equation I get the ration of 1:1 which means my answer is correct. 9. What is the new temperature of a 4 L sample of gas at 375 K and 102 kPa when it is expanded to 9 L and has a pressure of 95 kPa? Given: T1 = 375K P1 = 102kPa V1 = 4L V2 = 9L P2 = 95kPa Required: T2 Analyze: I will need to use Combined Gas law to solve the problem and find T2. The formula needed is (P1 x V1)/ T1 = (P2 x V2)/ T2. Solve: T2 = (P2 x T1 x V2)/(V1 x P1) T2 = (95kPa x 375K x 9L)/(4L x 102kPa) (The kPa and K will cancel out) V2 = 785.85K Paraphrase: Therefore the new temperature will be at 785.85K. Justify: I know the answer I got is correct, as when I use the original formula and solve both sides of the equation I get the ration of 1:1 which means my answer is correct. More Problems: 1. What is the new volume of a 15 L sample of gas at 67oC and 107 kPa when it is heated to 95 oC and the pressure increases to 112 kPa? Given: T1 = 340K P1 = 107kPa V1 = 15L T2 = 368K P2 = 112kPa Required: V2 Analyze: I will need to use Combined Gas law to solve the problem and find V2. The formula needed is (P1 x V1)/ T1 = (P2 x V2)/ T2. Solve: V2 = (P1 x T2 x V1)/(T1 x P2) V2 = (107kPa x 368K x 15L)/(340K x 112kPa) (The kPa and K will cancel out) V2 = 15.51L Paraphrase: Therefore the new volume will be at 15.51L. Justify: I know the answer I got is correct, as when I use the original formula and solve both sides of the equation I get the ration of 1:1 which means my answer is correct. 2. What is the new volume of a 10.0 mL container at 42o C when the temperature is adjusted to 283 oC? (Assume pressure is constant) Given: V1 = 10mL T1 = 313K T2 = 556 K Required: V2 Analyze: I will need to use Charles’s law to solve the problem and find V2. The formula needed is V1/ T1 = V2/ T2. Solve: V2 = (V1 x T2)/ T1. V2 = (10mL x 556K)/ (313K) V2 = 17.76mL Paraphrase: Therefore the new volume is 17.76mL, if the pressure is constant. Justify: I know the answer I got is correct, due to the new temperature is almost double the old temperature and this is also see with the volume, showing that the ratio is 1: 1. 3. A container of gas at 25 oC and 101 kPa increases in pressure to 200 kPa. What is the new temperature of the gas? (assume that volume is constant) Given: T1 = 298K P1 = 101kPa P2 = 200kPa Required: T2 Analyze: I will need to use Gay-Lussac’s law to solve the problem and find T2. The formula needed is P1/ T1 = P2/ T2. Solve: T2 = (T1 x P2)/P1 T2 = (298K x 200kPa)/(101kPa) (The kPa will cancel out) T2 = 590.1K Paraphrase: The new temperature of the gas is 590.1K. Justify: I know the answer I got is correct, due to the fact the original pressure is 2 times less as the end pressure, as such the temperature will be 2 times as more as the original temperature which it is. 4. What is the new volume when a 50.0 mL container at standard pressure (101.3 kPa) is expanded until the new pressure is 2.5 atm? (assume the temperature is constant) Given: V1 = 50mL P1 = 101.3kPa P2 = 253.31kPa Required: V2 Analyze: I will need to use Boyle’s law to solve the problem and find V2. The formula needed is P1 x V1 = P2 x V2. Solve: V2 = (P1 x V1)/ P2 V2 = (101.3kPa x 50mL)/ 253.31kPa (The kPa will cancel out) V2 = 19.93mL Paraphrase: Therefore the volume of the container will be at 19.93mL, if the temperature is constant. Justify: I know the answer I got is correct, due to the fact if the pressure increases, then the volume will have to decrease to maintain the ratio of 1 : 1 between the starting gas and the gas in the container. This also is correct because due to Boyle’s law, when one goes up, the other must go down, which occurs here. 5. What is the new pressure of a 10.5 L sample of gas at 75oC and 101 kPa when it is heated to 125oC and the volume increases to 11.5L? Given:T1 = 348K P1 = 101kPa V1 = 10.5L T2 = 398K V2 = 11.5L Required: P2 Analyze: I will need to use Combined Gas law to solve the problem and find P2. The formula needed is (P1 x V1)/ T1 = (P2 x V2)/ T2. Solve: P2 = (P1 x T2 x V1)/(T1 x V2) P2 = (101kPa x 398K x 10.5L)/(348K x 11.5L) (The L and K will cancel out) P2 = 105.47kPa Paraphrase: Therefore the new pressure will be at 105.47kPa. Justify: I know the answer I got is correct, as when I use the original formula and solve both sides of the equation I get the ration of 1:1 which means my answer is correct. 6. Explain why gases at the same temperature and pressure have different densities (in g/L)? Gases at the same temperature and pressure have different densities because; all gases have different molar mass. Some gases like hydrogen gas only weight around 2 grams per mol, while other like oxygen gas weight around 32g per mol. This then translates into different densities. As such even though gases have the same temperature and pressure they might not have the same density. Remember to convert from oC to K whenever temperature is given in oC. 1) How many moles of gas does it take to occupy 120 liters at a pressure of 202.3 kPa and a temperature of 340 K? Given: P= 202.3kPa, V=120L, T =340K, R = 8.31LkPa/(Kmol), Required: n = ? Analyze: Use the formula n=P x V/ R x T to find n Solve: n=P x V/ R x T n= (202.3kPa) x (120L) / 8.31 LkPa/(Kmol) x 340K = 8.59 mol gas Paraphrase: It takes 8.59 mol of gas Justify: This makes sense because since the volume and pressure is high, and according to the formula we know that both the volume and pressure are directly proportional to each other, therefore we can expect the mol to be high. 2) If I have a 50 liter container that holds 45 moles of gas at a temperature of 2000 C, what is the pressure inside the container? Given: V=50L, T =200°C, R = 8.31LkPa/(Kmol), n = 45 of gas Required: P = ? Analyze: Use the formula PV=nRT Solve: 0°C = 273K K of 200°C= 273K + 200°C = 473K P = nRT/V P= (45 mol gas) x (8.31LkPa/(Kmol)) x (473K) / (50L) P= 3537.56kPa Paraphrase: The pressure inside the canister is 3537.56kPa. Justify: This makes sense because the volume being small and the temperature and moles being so high. And according to the formula we see that the pressure is directly proportional to the moles, temperature but indirectly proportional to the volume. 3) 4) 5) It is not safe to put aerosol canisters in a campfire, because the pressure inside the canisters gets very high and they can explode. If I have a 1.0 liter canister that holds 2 moles of gas, and the campfire temperature is 14000 C, what is the pressure inside the canister? Given: V=1L, T =1400°C, R = 8.31LkPa/(Kmol), n = 2 moles of gas Required: P = ? Analyze: Use the formula PV=nRT Solve: 0°C = 273K K of 1400°C= 273K + 1400°C = 1673K P = nRT/V P= (2 mol gas) x (8.31LkPa/(Kmol)) x (1673K) / (1L) P= 27805.26kPa Paraphrase: The pressure inside the canister is 27805.26kPa. Justify: This makes sense because a very high pressure will be needed to explode a canister, and I think that 27805.26kPa is a very high pressure considering the fact that the temperature is very high, and we know that as the temperature increases the pressure increases simultaneously. How many moles of gas are in a 30 liter scuba canister if the temperature of the canister is 300 K and the pressure is 2026 kPa? Given: P= 2026kPa, V=30L, T =300K, R = 8.31LkPa/(Kmol), Required: n = ? Analyze: Use the formula n=P x V/ R x T to find n Solve: n=P x V/ R x T n= (2026kPa) x (30L) / 8.31 LkPa/(Kmol) x 300K = 24.38 mol gas Paraphrase: It takes 24.38 mol of gas Justify: This makes sense because pressure is high, and the temperature is not as much high, and since the moles are directly proportional to the pressure and indirectly proportional to the temperature, we can expect a high amount moles of gas. I have a balloon that can hold 100 liters of air. If I blow up this balloon with 3 moles of oxygen gas at a pressure of 101.3 kPa, what is the temperature of the balloon? Given: P= 101.3kPa, V=100L, n = 3 mol O2, R = 8.31LkPa/(Kmol) Required: T = ? Analyze: Use the formula T=P x V/ R x n to find T Solve: T=P x V/ R x n T= (101.3kPa) x (100L) / 8.31 LkPa/(Kmol) x 3 mol O2 = 406.3K Paraphrase: the temperature of the balloon is 406.3K. Justify: This makes sense because the pressure is high, and with high pressure we know that the temperature is going to be high too. 6) If I have a 67 L container that holds 5 grams of nitrogen gas at a temperature of 25oC, what is the pressure inside the container? Given: V=67L, T =25°C, R = 8.31LkPa/(Kmol), n = 5g of nitrogen gas Required: P = ? Analyze: Use the formula PV=nRT Solve: 0°C = 273K K of 25°C= 273K + 25°C = 298K n N2 gas = 5g N2 x 1 mol N2 / 2(14g) N2 gas n N2 gas = 0.178 mol N2 gas P = nRT/V P= (0.178 mol N2) x (8.31LkPa/(Kmol)) x (298K) / (67L) P= 6.6kPa Paraphrase: The pressure inside the container is 6.6kPa. Justify: This makes sense because the temperature is low and so is the moles, and according to the formula we know that the pressure is directly proportional to the temperature and moles, and indirectly proportional to the volume. And since the temperature and moles are low, we can expect the pressure to be low as well. 7) If I have a 32 L container that holds 5.4 x 1023 molecules of oxygen gas at a pressure of 101.3 kPa, what is the temperature inside the container? Given: P= 101.3kPa, V=32L, 5.4 x 1023 O2 molecules, R = 8.31LkPa/(Kmol) Required: T = ? Analyze: Use the formula T=P x V/ R x n to find T Solve: n= 5.4 x 1023 molecules of O2 x 1 mol O2 / 6.02 x 1023 molecules of O2 n=0.89 mol O2 T=P x V/ R x n T= (101.3kPa) x (32L) / 8.31 LkPa/(Kmol) x 0.89 mol O2 = 438.29K Paraphrase: the temperature of the balloon is 438.29K. Justify: This makes sense because the pressure is high, and with a high pressure we know that the temperature is going to be high too. Partial Pressures and Graham’s Law Practice: 1. A metal tank contains three gases: oxygen, helium, and nitrogen. If the partial pressures of the three gases in the tank are 3 atm of O2, 5 atm of N2, and 7 atm of He, what is the total pressure inside of the tank? P 02 = 3 atm P N2=5 atm PHe=7 atm Ptotal=? atm Ptotal= P 02+ PHe+P N2 = 3atm+8atm+7atm = 18atm Therefore the total pressure inside the gas tank is 18 atm The answer seems reasonable as all the values are smaller than it and it is the sum 2. Blast furnaces give off many unpleasant and unhealthy gases. If the total air pressure is 1.45 atm, the partial pressure of carbon dioxide is 0.43 atm, and the partial pressure of hydrogen sulfide is 0.23 atm, what is the partial pressure of the remaining air? P 02 = 6.6 kPa and P N2=23.0 kPa, what is P CO2 PH2=0.23 Ptotal=1.45atm PCO2= 0.43atm Ptotal= PN2+ P02+ PCO2 Premaining air=?atm Premaining air = Ptotal -PCO2- PH2 = 1.45atm-0.43atm-0.23atm = 0.79atm The partial pressure of the remaining gas is 0.79 atm The partial pressure of the remaining gas should be greater than that of carbon dioxide and hydrogen because their values are really small but should be smaller than the sum and thus 0.79atm seems reasonable. 3. If the air from problem 2 contains 22% oxygen, what is the partial pressure of oxygen near a blast furnace? Ptotal=1.45atm Percent O2 = 0.22% P O2 = 22*1.45atm*1/100=0.319atm 4. Compare the effusion rates of O2 (molar mass, 32.0 g/mol) and N2 (molar mass, 28.0 g/mol). Molar mass O2=32g/mol Molar mass N2=28g/mol So from the same hole nitrogen will escape 1.069 times faster than oxygen. This makes sense because at the same temperature both gases possess the same kinetic energy but the mass of oxygen is higher, and thus the velocity of nitrogen will be higher as it has less mass. 5. If I place 3 moles of N2 and 4 moles of O2 in a 27 L container at a temperature of 298 K, what will the pressure of the resulting mixture of gases be? Moles N2 and O2 = 3 mol + 4 mol =7 mol Note we can add molN2 and molO2 as they are in a mixture and since there really are no forces of attraction between gases anyways their values can be safely combined and they can be looked at as one gas in our calculations. Volume = 27 L Temperature = 298 K R = 8.31 LkPa/(Kmol) P = ? kPa P=nRT/V = 7 mol* 8.31LkPa/ K mol *298 K /27L = 642.01 kPa The total pressure in the container is 642.02 kPa This makes sense because there are a lot of gas particles but the container is only 27 L so it makes sense that the pressure will be high as the particles won't have much space to move around freely and keep colliding with each other, thus increasing the pressure. Mixed Gas Problems: 1. If 690.0 mL of oxygen is collected over water at 26.0 ˚C and 725 mmHg, what is the dry volume of this oxygen sample at 52.0 ˚C and 106.6 kPa? Given: T1 = 299K P1 = 96.66kPa V1 = 690mL T2 = 325K P2 = 106.6kPa Required: V2 Analyze: I will need to use Combined Gas law to solve the problem and find V2. The formula needed is (P1 x V1)/ T1 = (P2 x V2)/ T2. Solve: V2 = (P1 x T2 x V1)/(T1 x P2) V2 = (96.66kPa x 325K x 690mL)/(299K x 106.6kPa) (The kPa and K will cancel out) V2 = 680.07mL Paraphrase: Therefore the new dry volume will be at 680.07mL. Justify: I know the answer I got is correct, as when I use the original formula and solve both sides of the equation I get the ration of 1:1 which means my answer is correct. 2. If 400.0 mL of hydrogen gas is collected over water at 18.0˚C and a total pressure of 98.6 kPa, what would be the dry volume of this hydrogen sample at STP? Given: T1 = 291K P1 = 98.6kPa V1 = 400mL T2 = 273.15K P2 = 101.33kPa Required: V2 Analyze: I will need to use Combined Gas law to solve the problem and find V2. The formula needed is (P1 x V1)/ T1 = (P2 x V2)/ T2. Solve: V2 = (P1 x T2 x V1)/(T1 x P2) V2 = (98.6kPa x 273.15K x 400mL)/(291K x 101.33kPa) (The kPa and K will cancel out) V2 = 365.35mL Paraphrase: Therefore the new dry volume will be at 365.35mL. Justify: I know the answer I got is correct, because if the temperature and pressure only deviate a little from the original, then the volume must also be only a bit different than the original volume, which it is. Also when I use the original formula and solve both sides of the equation I get the ration of 1:1 which means my answer is correct. 3. A 6.12 L sample of xenon gas was collected over water at 2.00 X 105 Pa and 80.0˚C. What would be the gas volume of pure xenon at SATP? Given: T1 = 353K P1 = 200kPa V1 = 6.12L( At SATP conditions Temperature is 298.15K, and pressure is at 101.325pKa.) T2 = 298.15K P2 = 101.33kPa Required: V2 Analyze: I will need to use Combined Gas law to solve the problem and find V2. The formula needed is (P1 x V1)/ T1 = (P2 x V2)/ T2. Solve: V2 = (P1 x T2 x V1)/(T1 x P2) V2 = (200kPa x 298.15K x 6.12L)/(353K x 101.33kPa) (The kPa and K will cancel out) V2 = 10.2L Paraphrase: Therefore the new dry volume will be at 10.2L. Justify: I know the answer I got is correct, as the temperature increases, while the pressure decreases, this tells us that the volume will have to increase which it does. Also when I use the original formula and solve both sides of the equation I get the ration of 1:1 which means my answer is correct. 4. How many moles of gas are contained in 890.0 mL at 21.0˚C and 750.0 mmHg? Given: P= 750 mmHg, V=890mL, T =21.0°C, R = 8.31LkPa/(Kmol) Required: n = ? Analyze: Use the formula n=P x V/ R x T to find n Solve: P=750 mm Hg*101.3kPa/760 mm Hg= 99.9kPa P=99.9kPa V= 890mL x (1L / 1000mL) V= 0.89L 0°C = 273K K of 21°C= 273K + 21°C = 294K n=P x V/ R x T n= (99.9kPa) x (0.89L) / 8.31 LkPa/(Kmol) x 294K = 0.036 mol gas Paraphrase: It takes 0.036 mol of gas Justify: This makes sense because pressure and the volume is low and we know according to the formula that the moles is directly proportional to the pressure and the volume, therefore it is not a surprise that the mole of gas is low. 5. What volume will 20.0 g of Argon gas occupy at STP? Given: mass = 20g at STP molar mass of Argon = 39.95g/mol Required: V Analyze: I will need to use a bit of Stoichiometry. Solve: V = 20g x ( 1 mol/ 39.95g) x (22.4L/1 mol) V = 11.21L Paraphrase: Therefore the volume of Argon will be at 11.21L. Justify: I know the answer I got is correct, as the mass of Argon gas given is around half the mass of 1 mol of Argon, as such the volume should be half the volume of a gas at normal STP, which it is. 6. What volume would 32.0 g NO2 gas occupy at 3.12 atm and 18.0˚C? Given: P= 3.12 atm, T =18.0°C, R = 8.31LkPa/(Kmol), 32g NO2 gas Analyze: Use the formula PV=nRT Solve: 0°C = 273K K of 18°C= 273K + 18°C = 291K P= 3.12atm*101.3kPa/1atm= 316.05kPa n= 32g NO2 x 1 mol NO2 / (14g) + 2(16g) n= 0.69 mol NO2 V = nRT/P V= (0.69mol NO2) x (8.31LkPa/(Kmol)) x (291K) / (316.05kPa) V= 5.29L Paraphrase: The volume is 5.29L. Justify: This makes sense because the according o the formula we know that the temperature and moles are directly proportional to the volume, and these factors being small cause the volume to be small as well. 7. Calculate the molar mass of a gas if 35.44 g of the gas stored in a 7.50 L tank exerts a pressure of 60.0 atm at 35.5˚C. Given: P= 60.0atm, V=7.5L, T =35.5.0°C, R = 8.31LkPa/(Kmol) , 35.44g Required: M= ? Analyze: Use the formula n=P x V/ R x T to find n Solve: P=60atm*101.3kPa/1atm= 6078kPa P=6078kPa 0°C = 273K K of351°C= 273K + 35.5°C = 308.5K n=P x V/ R x T n= (6078kPa) x (7.5L) / 8.31 LkPa/(Kmol) x 308.5K = 17.8 mol gas M=17.8 mol gas x (35.44g of gas / 1 mol gas) M=631.1g of gas Paraphrase: The molar mass is 631.1g of gas Justify: This does make sense because the moles are of a high value, therefore the molar mass too should be of a high value. 8. Determine the number of moles of Krypton contained in a 3.25 L tank at 5.88 X 105 Pa and 25.5˚C. If the gas was oxygen instead of krypton, will the answer be the same? Given: P= 5.88 x 105 Pa, V=3.25L, T =25.5°C, R = 8.31LkPa/(Kmol) , Required: n= ? Analyze: Use the formula n=P x V/ R x T to find n Solve: P=5.88 x 105 P x 1kPa / 1000Pa P=588kPa 0°C = 273K K of25.5°C= 273K + 25.5°C = 298.5K n=P x V/ R x T n= (588kPa) x (3.25L) / 8.31 LkPa/(Kmol) x 298.5K = 0.77 mol krypton n= 0.77 mol gas Paraphrase: Theerfore it is 0.77 of krypton. Even if the krypton was replaced by oxygen, the mol would be the same but the molar mass would be different. Justify: This does make sense because the moles doesn’t show the characteristics of an element but the molar mass does. 9. Determine the mass of carbon dioxide in a 450.6 mL flask at 1.80 atm and –50.5˚C. Determine the mass of oxygen that would be present in the same container under the same conditions. Given: P= 1.8atm, V=450.6mL, T =-50.5°C, R = 8.31LkPa/(Kmol) , Required: M= ? Analyze: Use the formula n=P x V/ R x T to find n Solve: P=1.8atm x 101.3kPa/1atm P=182.34kPa V= 450.6mL x (1L / 1000mL) V= 0.4506L 0°C = 273K K of-50.5°C= 273K + (-50.5°C) = 222.5K n=P x V/ R x T n= (182.34kPa) x (0.4506L) / 8.31 LkPa/(Kmol) x 222.5K = 0.044 mol CO2 n= 0.044 mol CO2 M of CO2=0.044 mol CO2 x (12g) + 2(16g) CO2 / 1 mol CO2 M of CO2=1.936 g of CO2 M of O2 = 0.044 mol CO2 x (2 mol O2 / 1 mol CO2) x (16g O2/ 1 mol O2) M of O2 = 1.408g O2 Paraphrase: The molar mass of carbon dioxide is 1.936g and the molar mass of oxygen is 1.408g Justify: This make sense because the mole ration is 1:2, therefore there are two oxygen atoms and oxygen element has a greater mass than that of carbon. 10. At what temperature will 0.654 mol neon gas occupy 12.30 L at 197.5 kPa? Given: P= 197.5kPa, V=12.30L, n = 0.654 mol neon, R = 8.31LkPa/(Kmol) Required: T = ? Analyze: Use the formula T=P x V/ R x n to find n Solve: T=P x V/ R x n T= (197.5kPa) x (12.3L) / 8.31 LkPa/(Kmol) x 0.654 mol neon = 446.9K Paraphrase: At the temperature of 446.9K. Justify: This makes sense because the pressure of the volume is high, and with high pressure we know that the temperature is going to be high too. 11. A container holds three gases: oxygen, carbon dioxide, and helium. The partial pressures of the three gases are 2.00 atm, 303.9 kPa, and 3040 mm Hg respectively. What is the total pressure of this sample in kPa, atm, and mmHg? Given: P1 = 2atm, P2 = 303.9kPa, P3 = 3040mmHg Required: Pt Analyze: I will need to use Dalton’s law Solve: PT = P 1 + P2 + P3 PT = 2atm + (303.9 kPa * (1 atm/101.325 kPa)) + ( 3040mmHg * (1 atm/760mmHg)) PT= 9atm PT= 9atm x (101.325 kPa /1 atm) PT= 911.93kPa PT= 9atm x (760mmHg /1 atm) PT= 6840mmHg Paraphrase: Therefore the total pressure can be expressed as 9atm, 911.93kPa, and 6840mmHg. Justify: I know the answer I got is correct, as when u subtract each value from the total, you can get back the original pressure values. 12. A tank contains 480.0 g of oxygen and 80.0 g of helium at a total pressure of 7.00 mmHg. What are the partial pressures of oxygen and helium? Mass O2=480 g Mass He=80 g nO2=480g O2*1 mol O2/16g O2 = 30 mol O2 nHe= 80g He*1 mol He/4 g He = 20 mol He Total gas particles = 20 mol + 30 mol = 50 mol % composition O2=30*100%/50=60% O2 % composition He=20*100%/50=40% He Partial pressure of O2=60/100*7mmHg=4.2mmHg Partial pressure of He=40/100*7mmHg=2.8mmHg Therefore the partial pressure of oxygen in the tank is 4.2 mmHg and the partial pressure of helium in the tank is 2.8 mmHg The answer makes sense as the partial pressure 4.2mmHg and 2.8mmHg, add up to 7mmHg Gas Stoichiometry: 1. Pentane, C5H12(l), burns completely to form carbon dioxide and water. a) Write the balanced chemical equation for this reaction. C5H12(l) + 8O2(g) → 5CO2(g) + 6H2O(g) b) What volume of O2(g) at STP is required to produce 70.0 L of CO2(g) at STP? Given: V CO2=70L V O2=?L T=0 °C=273.15K P=101.3kPa R = 8.31 LkPa/(Kmol) PV=nRT n=PV/(R*T) n CO2=101.3kPa*70L/(8.31 LkPa/(Kmol)*273.15K) = 3.12mol n O2 = 3.12mol CO2*8mol O2/5 mol CO2 = 4.99mol V O2=nRT/P = 4.99mol*8.31 LkPa/(Kmol)*273.15K/101.3kPa = 111.81L Therefore 111.81L of oxygen gas is required to produce 70.0L of carbon dioxide at STP Because there is 70L CO2 and 5 moles of it are produced when 8 moles of oxygen are used it would mean that the amount of oxygen used was more as both gases are experiencing the same pressure and temperature and their particles take up the same amount of space. The answer 111.81L makes sense because 8 moles of oxygen are used up in order to produce 5 moles of carbon dioxide. c) What mass of H2O(l) is made when the combustion of C5H12(l) gives 106 L of CO2(g) at SATP? V CO2=106L mass H2O=?L T=0 °C=273.15K P=101.3kPa R = 8.31 LkPa/(Kmol) PV=nRT n=PV/(R*T) n CO2=101.3kPa*106L/(8.31 LkPa/(Kmol)*273.15K) = 4.73mol Mass H2O = 4.73mol CO2*6mol H2O /5 mol CO2*(1+1+16)g H2O/1mol H2O = 102.17g Therefore 102.17g water is produced along when 70L carbon dioxide is produced at STP when C5H12(l) is burnt 2. Given the equation C3H8(g) + O2(g) → CO2(g) + H2O(g) a) Balance the equation C3H8(g) + 5O2(g) → 3CO2(g) + 4H2O(g) b) If 50 g of C3H8 is burned in excess O2 , what volume of CO2 gas can be collected at 30∧C and 90 kPa? T=30∧C=303.15K P=90 kPa R = 8.31 LkPa/(Kmol) Mass C3H8=50g V CO2 = 3.4mol CO2 =nRT/P = 3.4mol*8.31 LkPa/(Kmol)*303.15K/90kPa = 95.17L Therefore 95.17L carbon dioxide is produced when 50 g of C3H8 is burned in excess O2 at 30∧C and 90 kPa 3. 2 ZnS(s) + 3 O2(g) → 2 ZnO(s) + 2 SO2(g) a) What volume of O2 at SATP is required for the reaction of 1.46 g of ZnS? Mass ZnS=1.46g nSO2=0.015mol SATP T= 298.15 K P=100kPa R = 8.31 LkPa/(Kmol) V O2 =nRT/P = 0.015mol*8.31 LkPa/(Kmol)*298.15K/100kPa =0.37L Therefore 0.37L oxygen is required to react with 1.46g of ZnS b) What volume of SO2 at SATP will be produced from the reaction in a)? Mass ZnS=1.46g nSO2=0.226mol SATP T= 298.15 K P=100kPa R = 8.31 LkPa/(Kmol) V SO2 =nRT/P = 0.226mol*8.31 LkPa/(Kmol)*298.15K/100kPa =5.599L Therefore 5.599L sulfur dioxide is produced from the reaction of 1.46 g of ZnS Because only 1.46g of ZnS was used in the reaction and the molar ratio is 2:2 it makes sense that the answer will be small too, and thus 5.599L seems reasonable. 4. Given the equation (NH4)2SO4(aq) + 2KOH(aq) → 2NH3(g) + K2SO4(aq) + 2H2O(l) a) Calculate the volume of ammonia gas, measured at 23∧C and 64 kPa, that could be produced from 264 g of ammonium sulfate and 280.0 g of potassium hydroxide. Mass (NH4)2SO4 =264g = 4 mol T= 296.15 K P=64kPa R = 8.31 LkPa/(Kmol) V NH3 =nRT/P = 4mol*8.31 LkPa/(Kmol)*296.15K/64kPa =153.81L Therefore 153.81L NH3 is produced from the reaction of 264g (NH4)2SO4 Mass KOH =280g n NH3=5mol T= 296.15 K P=64kPa R = 8.31 LkPa/(Kmol) V NH3 =nRT/P = 5mol*8.31 LkPa/(Kmol)*296.15K/64kPa =192.26L Therefore 192.26L NH3 is produced from the reaction of 280g KOH Because (NH4)2SO4 has a greater molar mass and a smaller quanitity but 1:2 molar ratio it makes sense that less volume of NH3 is formed than 280g of KOH which is lighter. It would make sense that the volume of ammonia gas would be high because the pressure is really low and thus it would indicate that the gas particles have a lot of space between them indicating a higher volume. 5. Given the equation 2KClO3(s) → 2KCl (s) + 3O2(g) What volume of a gas can be produced by the decomposition of 122.6 g of potassium chlorate measured under the following conditions? Mass KClO3=122.6g n O2=122.6g*1mol KClO3/122.55g KClO3*3 mol O2/2 mol KClO3 = 1.5mol O2 a) at STP T=0 °C=273.15K P=101.3kPa R = 8.31 LkPa/(Kmol) Mass KClO3=122.6g V O2=nRT/P =1.5mol*8.31 LkPa/(Kmol)*273.15K/101.3kPa = 33.61L b) at SATP T= 298.15 K P=100kPa R = 8.31 LkPa/(Kmol) V O2=nRT/P = 1.5mol*8.31 LkPa/(Kmol)*298.15K/100kPa =37.16L Therefore 33.61L of gas is produced at STP conditions while 37.16L of gas is produced at SATP conditions Because the pressure and temperature are higher at SATP than STP it would make sense that the volume of gas produced at SATP conditions would be greater as the gas particles will have greater kinetic energy as the temperature is higher.