CHEM 111 /1 Chris Brendel Density Partners: Meghan Hudson
advertisement

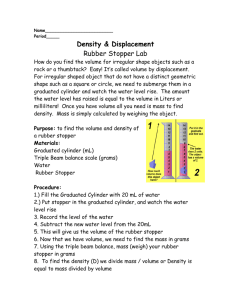
CHEM 111 Chris Brendel Partners: Meghan Hudson, Rachel Collister 1/1 Density 5/16/11 The aim of the experiment was to calculate the density of water (de-ionized and tap), to calculate the density of a rubber stopper, and to acclimate the student to basic laboratory equipment. In order to determine the density of the water samples, we used a buret to measure out amounts of both de-ionized and tap water, used a balance to determine the mass and noted the volume of each sample. We measured the mass of the rubber stopper and then calculated its volume through two separate methods: dimensional analysis (using a ruler) and water displacement (in a graduated cylinder). Using the mass and volume figures, we determined the DI water sample to have a density of .9949 g/mL and the tap water sample to have a density of .9947 g/mL—these results could be attributed to experimental error, as properly de-ionized water should be less contaminated than tap water and therefore less dense. Using the equation V = ((π*h)/3)(R^2+Rr+r^2) and the previously measured mass, we determined the rubber stopper’s density to be 1.6 g/mL, which agrees with the density 1.6 g/mL calculated by the more-accurate water displacement method.