CH 20.2 Power Point
advertisement
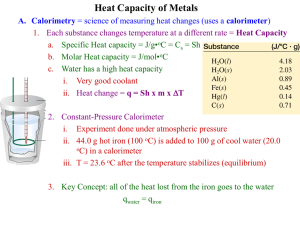
Measuring and Using Energy Changes Section 20.2 Main Idea Energy stored in chemical bonds can be converted to other forms and used to meet the needs of individuals and of societies. Calorimetry The heat generated in chemical reactions can be measured by a technique called calorimetry and a device called a calorimeter. Calorimetry The amount of heat energy of a reaction is calculated using the following equation: q = m x c x ΔT q= heat lost or gained by sample m= mass of the sample c= specific heat of sample ΔT = temperature change of the water (ΔT = Tfinal – Tinitial) (ΔT negative endothermic) Calorimetry You will be using a calorimeter where a hot substance and cold substance are brought together. Example problem How much heat is absorbed by a reaction that lowers the temperature of 500.0g of water in a calorimeter by 1.10°C? Use q = m x c x ΔT m= 500.0 g ΔT = 1.10°C What is c? Specific heat. Look up for water… c= 4.18 J/g•°C Cont… q= 500.0g x 4.18 J/g•°C x 1.10°C q= 2,299J Another problem… Aluminum reacts with iron (III) oxide to yield aluminum oxide and iron. Calculate the heat given off in the reaction if the temperature of the 100.00 g of water in the calorimeter increases by 3.00°C. Use q = m x c x ΔT Another problem… Burning a small quantity of hexane raises the temperature of 424g of water in a calorimeter from 18.4°C to 32.7°C. How much heat was released in the reaction? q= (m)(c)(ΔT) q= 25,400J or ?kJ Next… In this experiment you will determine the specific heat of an unknown metal. The metal will be heated to a known temperature then placed in a styrofoam cup that contains a known amount of water a room temperature. Since Styrofoam is an insulator, it acts as a calorimeter. Heat will be transferred from the hot metal to the water, but heat should not be lost to the surroundings. You will observe what happens to the temperature of the metal and the water. Heat will be transferred from a hot substance to a cold substance until the temperatures are equal. Sound familiar… equilibrium At the end of the experiment, the metal and water will be the same temperature. The heat lost by the metal will be gained by the water. You can then calculate the specific heat of the metal by knowing the mass of the metal, mass of the water and the change of temperature. Energy Value of Food A calorie if the heat required to raise the temperature of 1 g of liquid water by 1°C. A kilocalorie is a unit = 1000 calories One calorie = 4.184 J One Joule = 0.239 calorie The calories on a nutrition label for the food you eat is actually a Calorie (1 kcal) Cont. Different macromolecules provide different amounts of energy per gram: 1 g fat = 9 Calories 1 g carbohydrate OR protein = 4 Calories CALORIMETRY can be used to measure the energy content in food. Practice Problems, p. 718 #s 8-10