Calorimetry
advertisement

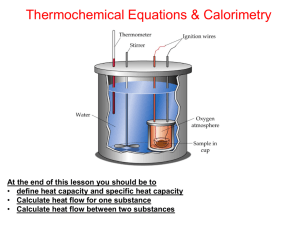
Calorimetry Calculating Heat Q can be found experimentally by measuring the heat flow accompanying a reaction This is done by measuring temperature This process is called calorimetry Specific Heat (c) This is amount of heat required to raise the temperature of 1g of a substance 1ºC Specific heat is different for different substances A lower specific heat means that it takes a small amount of heat to change the substance’s temperature Calculating the Heat Transfer Heat transfer can be calculated using Q = (c)(m)(Δt) The specific heat of iron(III) oxide is 0.75 J/g ºC. What is the heat required to increase the temperature of a 1.75 g sample from 25ºC to 380ºC? Enthalpy (ΔH) ΔH is Q at a constant pressure ΔH is always the J given off per mole of reactant (J/mol) Example When 50 ml of a 1.0 M HCl and 50 ml of a 1.0 M NaOH solution are mixed, the temp in a calorimeter increases from 21.0 ºC to 27.5ºC. The total volume of the end solution is 100 ml, its density is 1.0 g/ml, and its specific heat is 4.18 J/gºC. Find the enthalpy change (heat of reaction)? Water Chamber Calorimetry Heat lost by a reaction is gained by a quantity of water in a calorimeter Heat lost = Heat gained C(water) = 4.184 J/gºC Example 30.0 g of water at 1000C is mixed with 50.0g of water at 150C and allowed to come to thermal equilibrium. What is the final temperature of the mixture? Example In a calorimeter containing 100 g of water at 21ºC, a reaction is carried out in which 3.5 g of NH4NO3 decomposes into N2O and H2O. The temperature raises to 25ºC. Find the heat of reaction.