lab 3 PHT313
advertisement

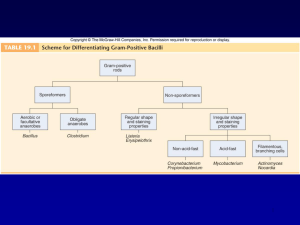
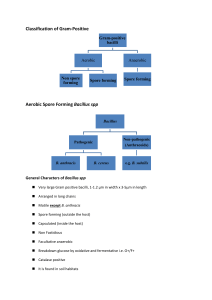
PHT 313 Lab (3) Bacteria Gram’s Stain Gram’s +ve Cocci Staphylococci Streptococci Micrococci Enterococci Gram’s -ve Bacilli Cocci Corynbacterium Clostridum Bacillus Neisseria Bacilli Enterobacteriaceae Pseudomonas. Gram +ve Bacilli Spore forming Non spore forming Anaerobic Aerobic Clostridium Bacillus Corynebacterium Listeria Lactobacilli Non-spore forming bacilli Corynebacterium C. diphtheriae causes diphtheria. Humans are the only known reservoir; carried in the oropharynx or on the skin Corynebacterium jeikeium: multiple antibiotic resistance important in opportunistic infections of immunocompromised patients Corynebacterium urealyticum: urease hydrolyzes urea; release of NH4+, increase in pH, alkaline urine, renal stones Microscopical examination Small, irregularly staining pleomorphic Gram-positive rods with club-shaped swelled ends. It may be straight or slightly curved, non-motile and non spore-forming. "Chinese letters" Palisade arrangement of cells in short chains ("V" or "Y" configurations) or in clumps resembling "Chinese letters" (Cells tend to lie parallel to one another (palisades) or at acute angles (coryneforms), due to their snapping type of division) Cultural characteristics Environment: Facultative anaerobes Temp.: 37 °C pH: 7.2 Media: Growth occurs on media containing blood or serum. On blood tellurite medium (selective & differential medium); colonies appear grey to black. The four biotypes; gravis, mitis, intermedius and belfanti are differentiated by colony morphology on blood tellurite and biochemical reactions. On Loeffler’s serum Biochemical Reactions Catalase test: All corynebacterium species are catalase +ve. Carbohydrate Fermentation Test Principle: Each species of corynebacteria has its specific carbohydrate fermentation pattaern. C.diphtheriae can be differentiated from other corynebacterium species by fermentation of glucose and maltose but not sucrose, with production of acid without gas. Procedure: 1. Inoculate three tubes of sugar medium (broth containing one type of sugar and phenol red as the pH indicator) with the test organism 2. Incubate the tubes at 35oC for 24 hrs. Glucose Maltose Sucrose Results: Sugar fermentation can be indicated by change of color of the medium from red to yellow. Glucose Maltose Sucrose C. xerosis Glucose Maltose Sucrose C. diphtheriae Toxigenicity testing of C.diphtheriae strains It is essential to demonstrate toxin production by diphtheria isolates obtained from throat swabs of cases or carriers, this is done by: 1)Elek’s test (or its modification) 2)PCR based methods are used for detection of diphtheria toxin genes 3) ELISA can be used to detect diphtheria toxin 4) Immunochromographic strip assay allows detection of diphtheria toxin in few hours 5) Tissue culture cytotoxicity assay Elek’s Toxigenicity Test Procedure: 1. Place a strip of filter paper saturated with diphtheria antitoxin on a serum agar plate. 2. Streak the test organism across the plate at right angle to the filter paper. 3. Incubate the plate at 35oC for 24 hrs. Results: Positive test: The antitoxin diffusing from the filter paper strip will form precipitation lines with the toxin diffusing from the toxigenic strain. Absence of precipitation lines indicates that the strain is non-toxigenic. Non-toxigenic strain Toxigenic strain Spore forming bacilli Aerobic Anaerobic Bacillus Clostridium B. anthracis B. cereus B. subtilis Cl. tetani Cl. perfringens Cl. difficile Cl. botulinum Vegetative cell division Sporulation Variations in endospore morphology (1, 4) central endospore; (2, 3, 5) terminal endospore; (6) lateral endospore Bacillus Saprophytic organisms prevalent in soil, water, and air. Microscopic morphology: Gram-positive non-motile rectangular large bacilli, that occur singly, in pairs, or in chains and spore forming Spore Stain: It has oval central spores. Using the Spore staining technique (Malachite green & safranin) , the spores appear green while the vegetative cells appear red. Cultural characteristics: Environment: aerobic PH: 7.2 Temperature: 37 ° C Media: • No special requirements for growth→ grow on simple nutrient media. • Bacillus species grow well on blood agar showing a double zone of hemolysis Except B. anthracis (No hemolysis). Biochemical reactions: A) Catalase test: All bacillus species are catalase +ve. B) Starch Hydrolysis Test: Principle: Starch amylase enzyme glucose I2 Blue colour I2 No colour Procedure: 1. Inoculate starch agar plate with the test organism. 2. Incubate the plate at 35oC for 24hrs. 3. Flood the plate with iodine solution. Results: Amylase activity is indicated by a clear zone around the growth while the rest of the plate gives blue color after addition of iodine solution. Key Characteristics to Distinguish between B. anthracis & Other Species of Bacillus Characteristic Bacillus anthracis Hemolysis Neg Motility Neg Gelatin hydrolysis Neg Salicin fermentation Neg Other Bacillus spp. Pos Pos (usually) Pos Pos Bacillus stearothermophilus is thermophilic, so its spores are used to test efficiency of killing in autoclaves Clostridium Habitat: Some members of the genus Clostridium are saprophytes in soil, sewage and water. Others are commensals in GIT of animals and humans. Cl. Tetani causing tetanus Cl. Perfringens causing gas gangrene Cl. Difficile causing enterocolitis Cl. Botulinum causing food poisoning Microscopic morphology: Cl. Tetani : Gram +ve bacilli, swollen at one end due to terminal spherical projecting spores (Drum stick appearance), motile with peritrichous flagella. Cl. Perfringens: Gram +ve bacilli, rod-shaped, spores are oval, subterminal and non projecting, non-motile. Cl. Botulinum: Gram +ve bacilli, motile, non-capsulated. Spores are oval, central or subterminal. Culture characteristic • • • • Environment : anaerobic Temp. 37 ° C PH: 7.2 Media: 1- Removal of O2 & replacing it with an inert gas→ Blood agar plates in Anaerobic Jar. 2- Special anaerobic media containing a reducing agent. Thioglycollate broth. Cooked meat medium. 3. Deep agar stab cultures: a straight wire is charged with inoculum and pluged into adeep column of nutrient agar Thioglycollate broth: The medium contains: 1- Sodium thioglycollate which acts as a reducing agent. 2- Small amount of agar to increase viscosity of the medium. It should be prepared in long tube aerobic bacteria anaerobic bacteria Cooked meat medium: It is anaerobic medium due to presence of: Meat particles (prepared from heart muscles) which contain hematin and glutathione that act as reducing agents. Reactions on Cooked meat medium: 1.Saccharolytic reaction: Causes fermentation of the muscle glycogen with production of acid and gas. The meat particles remain intact. 2. Proteolytic reaction: Causes digestion of the meat particles leading to formation of black, foul smelling sulphur compounds. C. difficile colonies on a blood agar plate. C. perfringens colonies on an egg yolk agar plate showing a white precipitate due to production of Lecithinase (Nagler´ reaction) Biochemical reaction Litmus milk medium: It Contains: • Skimmed milk (without fat) i.e: contains only sugar (Lactose) and protein (casine) • Litmus indicator (acid base and redox indicator). Reactions: Acidic reaction: Lactose (milk sugar) Fermentation acid Litmus indicator pink colour Stormy Clot Formation: (Lactose) milk sugar (Casine) milk protein Fermentation Coagulation acid + gas clot Stormy clot The C. Perfringens cause rapid fermentation of lactose in litmus milk and the gas produced split the clot (Stormy Clot Formation).