Lectures 8-9
advertisement
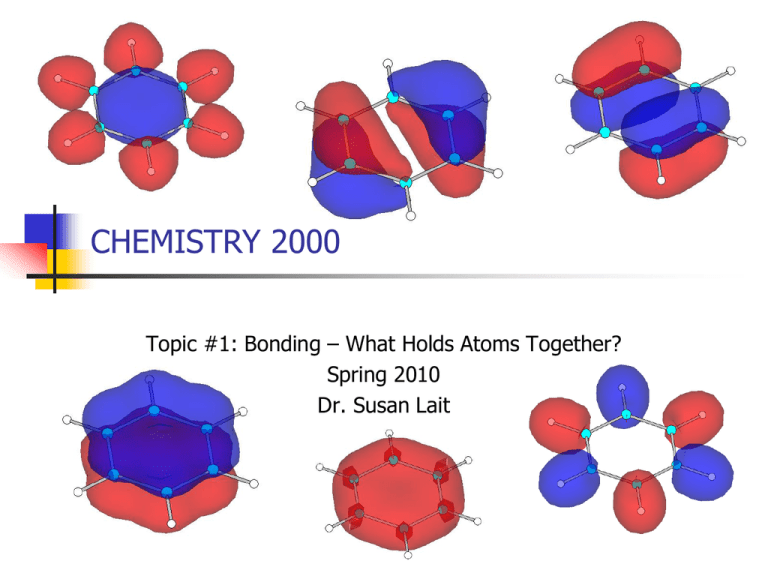
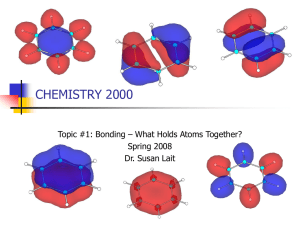
CHEMISTRY 2000 Topic #1: Bonding – What Holds Atoms Together? Spring 2010 Dr. Susan Lait Taking MO Theory Past the Diatomics Molecular orbital theory is valid for all molecules. Thus far, we’ve focused on the diatomics because it’s only convenient to manually derive complete MO diagrams for molecules that are either very small or highly symmetric. It was easier for us to predict MOs for symmetric molecules (like O2) than asymmetric molecules (like CO). For this reason, our studies of larger molecules will be confined to highly symmetric ones – like BeH2, CO2 and C6H6. Computers are used to predict MOs for less symmetric molecules (“computational chemistry”). The principles used to predict MOs and MO diagrams for the diatomics are the same as those used for larger molecules. Continue to look for valence atomic orbitals with: COMPATIBLE SYMMETRY ( vs. ) COMPATIBLE ENERGY (as close as possible; no farther apart than ~ 1 Ry) Make sure you make the same number of MOs as you had AOs! 2 Keep electron distribution in MOs symmetric if molecule is symmetric. Molecular Orbitals for BeH2 BeH2 is the simplest triatomic molecule. In the gas phase, it is linear (as we’d expect from the Lewis structure): The relative energies for the atomic orbitals of Be and H are: 1s (Be) = -9.38 Ry 2s (Be) = -0.61 Ry 2p (Be) = +0.14 Ry ▪ 1s (H) = -0.99 Ry Which atomic orbitals will combine to make MOs? Which will combine to make MOs? Which will not combine at all (leaving them as either or nonbonding MOs)? 3 Molecular Orbitals for BeH2 We are combining four -symmetry AOs to make four -MOs. To predict what these four MOs will look like, we use symmetry and an increasing number of nodes: To predict their energies, we expect the lowest energy -MO to be lower in energy than all AOs from which it’s made, and we expect the highest energy -MO to be higher in energy than all the AOs from which it’s made. We then use these two MOs to 4 estimate the energies of the middle MOs. Molecular Orbitals for BeH2 As in the MO diagrams for diatomics, the MOs are tracked in the middle of the diagram with the AOs at the sides. Equivalent atoms (such as the Hs in BeH2) are grouped at one side, making sure to include all their valence AOs (one 1s AO for each H): 2p 2s 1s Energy Be H (x2) 5 MO Diagram for BeH2 4 3 1 2p 2s 1s 2 Energy 1 Be BeH2 H (x2) 6 Molecular Orbitals for CO2 CO2 may not appear much more complicated than BeH2 – both of them being linear triatomic molecules – but it has rather more valence orbitals to consider. As usual, we will split them into -symmetric AOs and -symmetric AOs: 7 Valence Molecular Orbitals for CO2 The six -symmetric AOs can be subdivided into two sets: the 2px AOs and the 2py AOs. Each of those sets can be combined to make three MOs. We use symmetry and nodes to predict the shapes of these MOs and their relative energies: 8 Valence Molecular Orbitals for CO2 The six -symmetric AOs must be treated as a group. They are combined to make six -symmetric MOs. Again, we use symmetry and nodes to predict the shapes of these MOs and their relative energies: 9 Valence MO Diagram for CO2 A computer helps us find the relative energies of the MOs (particularly vs. ): 3 2p 2 Energy 2p 2s 4 3 1 2 1 C CO2 2s O (x2) 10 Valence MO Diagram for CO2 Use the MO diagrams on the previous pages to: Write the valence orbital occupancy for CO2 Sort the MOs into bonding, antibonding and nonbonding MOs. Is the number of each type of MO consistent with CO2’s Lewis structure? What is the main difference between the picture of CO2 provided by MO theory and the picture of CO2 provided by the Lewis structure? 11 MO Theory and Delocalized Electrons Once we move beyond the linear triatomics, the -MOs quickly become relatively complicated and difficult to predict. e.g. the valence -MOs of BH3: The -MOs, on the other hand, are still relatively straightforward to predict. When we can draw multiple resonance structures for a molecule, we are often interested in the electrons that “move” between resonance structures. MO theory treats these electrons as being in delocalized -MOs. Since -MOs and MOs are always treated separately (a phenomenon called sigma-pi separation), we often ignore the -MOs and discuss just the -MOs for large molecules with multiple resonance structures. This is similar to our practice of ignoring core MOs 12 and discussing bonding using only the valence MOs. MO Theory and Delocalized Electrons Important points to remember: We are approximating that, for every bond (single, double or triple), there are two electrons in bonding MOs. This does not mean that there is a -MO focussed on the bonding region. It may, for example, mean that there are three bonding -MOs holding together three different bonds (as shown for BH3 on the previous page – the three leftmost MOs are -bonding MOs, each containing 2 electrons) When discussing bond order, only bond order can be determined from a -MO diagram. Total bond order will typically be 1 greater. Total bond order = bond order + bond order Recall that: A orbital with lower energy than p orbitals is net bonding. A orbital with the same energy as p orbitals is net nonbonding. A orbital with higher energy than p orbitals is net antibonding. Looking at the nodes tells us which atoms are pulled together and which atoms are pulled apart by electrons in a particular MO. 13 MO Theory and Delocalized Electrons Before we look at delocalized electrons, let’s construct a couple of -MO diagrams for localized electrons: Ethyne (CHCH) has two perpendicular systems: Ethene (CH2CH2) has only one system: 14 Resonance and MO Theory: Benzene (C6H6) The classic example of delocalized electrons and MO theory is benzene. The Lewis structure of benzene is: H H H C C H C C H H C C H H C C H H C C C C H H If we assign molecular geometries to each carbon atom, we see that benzene is a planar molecule: = As usual, we divide the valence atomic orbitals into -symmetric and -symmetric. Since -symmetric orbitals are perpendicular to the plane/line of the molecule, only one set of 2p orbitals is -symmetric in benzene (usually designated 2pz). 15 Resonance and MO Theory: Benzene (C6H6) This gives us ____ -MOs and ____ -MOs. To divide the electrons between these MOs, we recall that: There are 2 electrons in -MOs for every bonded pair of electrons in the molecule. We also count 2 electrons for every lone pair of electrons that doesn’t move * between resonance structures. Benzene contains ____ C-C bonds, ____ C-H bonds and no lone pairs, so it has ____ electrons in its -MOs. Any electrons which move between resonance structures are in MOs. In benzene, ____ electrons are in different places in the two resonance structures, so benzene has ____ electrons in its -MOs. H H H C C C C H H H C C C C H * H C C H H C C H H There are rare cases where lone pairs that remain in place for all reasonable resonance structures are, in fact, 16 in -MOs. These exceptions can be explained by MO theory, but will not be addressed in CHEM 2000. Resonance and MO Theory: Benzene (C6H6) So, we know that benzene has ____ -MOs containing ____ electrons. We also know that these -MOs were made by combining six 2pz orbitals on the six carbon atoms. Cyclic molecules tend to have degenerate energy levels. A “quick and dirty” trick to predict the relative energy levels for this kind of molecule is to draw a circle. Inside the circle, draw a polygon the same shape as your molecule. Make sure the tip of the polygon is at the bottom of the circle. The corners of the polygon are at the same height as the relative energy levels. If you draw a horizontal line across the middle of the circle (representing the energy of the p atomic orbitals), all orbitals below it are net bonding and all orbitals below it are net antibonding. This method is a huge oversimplification but it works. H H C C C H 4 3 H C C C H H E 2 1 17 Resonance and MO Theory: Benzene (C6H6) We’ve worked out the energy levels, but what do the MOs look like? To answer that, we go back to our old standby method – symmetry and nodes! The lowest energy -MO (1) has one nodal plane – the one cutting through the plane of the molecule. It has no nodes perpendicular to the plane of the molecule. Every time you go up to the next energy level, add a node perpendicular to the plane of the molecule, keeping them as evenly spaced as possible. If there are two degenerate -MOs, keep their nodes as askew as possible (perpendicular if possible, at a 45º angle as next choice, etc.) The highest energy -MO should be “as antibonding as possible” – i.e. nodes between every pair of neighbouring atoms Once you’ve determined the energy levels and the shapes of the -MOs, the last step is to fill in the electrons. Recall that we have ____ electrons to add to the -MO diagram. 18 Resonance and MO Theory: Benzene (C6H6) H H C C C H H C E 4 C C H 3 H Energy of 2p AOs 2 1 This MO diagram confirms that benzene can’t be considered to have “three double bonds and three single bonds”. It really does have three bonds with bond order _____. This has been proven experimentally: all six C-C bonds in benzene are 140 pm (whereas pure C-C bonds are 154 pm and pure C=C bonds are 134 pm). 19 Resonance and MO Theory: Ozone (O3) Another molecule whose resonance structures we looked at in CHEM 1000 was ozone. The resonance structures for ozone are: The molecular geometry of ozone is _____________________. Ozone has: ____ -symmetric valence AOs therefore ____ valence -MOs. ____ -symmetric valence AOs therefore ____ valence -MOs. Ozone has: ____ electrons in valence -MOs. ____ electrons in valence -MOs. 20 Resonance and MO Theory: Ozone (O3) Use symmetry and nodes to work out what the ____ -MOs of ozone look like, ranking them from lowest to highest energy. Then, fill in the electrons to give a complete -MO diagram. How does this MO diagram relate to the Lewis structures you drew? Can it justify the formal charges of the atoms? E 21 Resonance and MO Theory: Nitrate (NO3-) The resonance structures for nitrate are: The molecular geometry of nitrate is _____________________. Nitrate has: ____ -symmetric valence AOs therefore ____ valence -MOs. ____ -symmetric valence AOs therefore ____ valence -MOs. Nitrate has: ____ electrons in valence -MOs. ____ electrons in valence -MOs. 22 Resonance and MO Theory: Nitrate (NO3-) Use symmetry and nodes to work out what the ____ -MOs of nitrate look like, ranking them from lowest to highest energy. Then, fill in the electrons to give a complete -MO diagram. How does this MO diagram relate to the Lewis structures you drew? Can it justify the formal charges of the atoms? E 23