please click here
advertisement

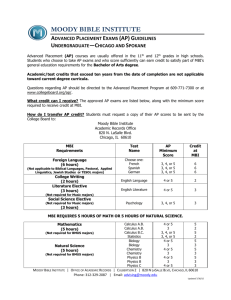
Design of Experiments for Bioprocess Optimisation – MBI® Short Courses When: 7-9 March 2016 Where: Biochemical Engineering, University College London This course shows laboratory scientists and engineers how experimental design techniques can be used to rapidly understand and optimise bioprocess performance. It provides managers with insights on how experimental design and small scale experimental models can underpin Quality by Design (QbD) approaches. Training is focused on the hands on use of DoE software rather than underlying statistics. Bioprocess case studies, supported by a series of introductory and expert lectures will enable you to: Generate appropriate experimental designs within fixed research budgets and timescales. Ensure analytical methods can be validated and provide reliable data for process evaluation. Rapidly screen and optimise different fermentation media and operating conditions. Obtain the highest yield of active product throughout your downstream process sequence. Understand the relationship between DoE and QbD Who should attend The course is designed for all those involved in bioprocess development and optimisation including: Bioprocess applications specialists Hands-on bioprocess scientists and engineers Research managers and consultants Further course information and confirmed speakers here For bookings, please email mbi-training@ucl.ac.uk or phone +44 (0) 20 7679 9619. Key benefits of MBI® Program Knowledge transfer and upskilling Deeper understanding of process options and engineering constraints Acquire the tools to optimised process performance and economics Updates on latest technical and regulatory developments Access to cutting edge research methodologies Network with sector leaders and subject matter experts Flexible professional development and options for qualifications Modular Training Program, MBI® course list here. …………………………………………………………………………………………………………………………… Industrial Biotechnology: Biocatalysis through to Synthetic Biology – MBI® Short Courses When: 18-20 April 2016 Where: Biochemical Engineering, University College London This unique course will introduce options for integrating biocatalysis into chemical manufacturing processes, and also explore successful examples of biocatalyst design, process operation and biorefining for sustainable manufacturing. In this module a series of lectures, problem solving case studies, and a practical workshop will provide close interaction with key academic and industrial speakers, enabling you to: Strengthen your knowledge in biocatalysis, and whole biotransformation processes, from fermentation to product. Understand the range of chemical conversions accessible by biocatalysis, and examples of successful industrial applications. Discover the industrial potential of synthetic biology. Explore the challenges and options for large scale biocatalysis. Who should attend This course is designed for all those individuals working in any of the following industries where biocatalysis and synthetic biology is important: fine and specialty chemicals, pharmaceuticals, personal care products, dagnostics as well as sustainable processing in the biorefining, biofuels and bulk polymer sectors Further course information and confirmed speakers here For bookings, please email mbi-training@ucl.ac.uk or phone +44 (0) 20 7679 9619. Key benefits of MBI® Program Knowledge transfer and upskilling Deeper understanding of process options and engineering constraints Acquire the tools to optimised process performance and economics Updates on latest technical and regulatory developments Access to cutting edge research methodologies Network with sector leaders and subject matter experts Flexible professional development and options for qualifications Modular Training Program, MBI® course list here. …………………………………………………………………………………………………………………………… Analytical Data Analysis for the Bioprocessing Industry – MBI® Short Courses When: 25-28 April 2016 Where: CPI, Darlington The module explores risk based decision making for bioprocess analytics by considering why a measurement is being made and what it conveys with regard to product critical quality attributes. Expert lectures supported by a series of workshops and case studies will enable you to: Gain deeper understanding of how and why measurements are made. Use statistical methods to assess the validity of data and its impact on critical product quality attributes. Empower you to make a greater impact in the practical aspects and to be a more effective member of an interdisciplinary development or manufacturing team. Explore best practice in team working exercises and in numerical analysis of case studies Who should attend This course is intended for engineers and scientists who are involved in the generation and use of analytical data for bioprocesses, including: R&D scientists, engineers or managers who wish to learn more about the analytics lifecycle and be more actively involved in creating understanding from analytical data. Further course information and confirmed speakers here For bookings, please email mbi-training@ucl.ac.uk or phone +44 (0) 20 7679 9619. Key benefits of MBI® Program Knowledge transfer and upskilling Deeper understanding of process options and engineering constraints Acquire the tools to optimised process performance and economics Updates on latest technical and regulatory developments Access to cutting edge research methodologies Network with sector leaders and subject matter experts Flexible professional development and options for qualifications Modular Training Program, MBI® course list here. …………………………………………………………………………………………………………………… Vaccines Bioprocess Development and Commercialisation – MBI® Short Courses When: 17th - 19th May 2016 Where: Biochemical Engineering, University College London This three-day course will explore the critical issues at the various stages of vaccine development. International experts with over 10 years experience in their fields will lead delegates in developing their understanding in the research, operational and regulatory challenges of the vaccine market. Key topics covered will include: Scale-up from lab to pilot scale Quality by Design (QbD) as part of vaccine development Expression systems Single use platforms Regulation and its impact on development Final formulation and adjuvants Developing World’s needs Who should attend This course is of interest to research scientists, programme managers, process engineers and policy makers. The course is accessible to those new to vaccine development or those working in the field from business and academia. Further course information and confirmed speakers here For bookings, please email mbi-training@ucl.ac.uk or phone +44 (0) 20 7679 9619. Key benefits of MBI® Program Knowledge transfer and upskilling Deeper understanding of process options and engineering constraints Acquire the tools to optimised process performance and economics Updates on latest technical and regulatory developments Access to cutting edge research methodologies Network with sector leaders and subject matter experts Flexible professional development and options for qualifications Modular Training Program, MBI® course list here. …………………………………………………………………………………………………………………………… Bioprocess Design & Economic Evaluation – MBI® Short Courses When: 6-9 June 2016 Where: Biochemical Engineering, University College London The module focuses on how to specify a complex bioprocess and determine its economic feasibility. It will: Specify a major item of process equipment. Interpret engineering drawings used in equipment procurement. Perform economic analyses of the process and determine sensitivities to process changes. Achieve value for money in complex engineering projects. Recommend a process that is technically feasible and economically viable. Determine the types of data needed to specify unit operations and processes. Develop process flow sheets and mass balances. Who should attend This course is intended for engineers, chemists, biologists, biochemists and biotechnologists who are interested in process design for the manufacture of biological products. Each concept and topic covered will be explained for the beginner - an example case study is followed through the bioprocess design procedure to allow an economic appraisal of the design to be performed. This learning is carried forward to the following “Bioprocess Facility Design” course which examines the next stage of a bioprocess design. Typically, delegates are: R&D scientists, engineers or managers who need to learn more about the typical steps which constitute the preliminary design process in conjunction with the “Bioprocess Facility Design” course. Further course information and confirmed speakers here For bookings, please email mbi-training@ucl.ac.uk or phone +44 (0) 20 7679 9619. Key benefits of MBI® Program Knowledge transfer and upskilling Deeper understanding of process options and engineering constraints Acquire the tools to optimised process performance and economics Updates on latest technical and regulatory developments Access to cutting edge research methodologies Network with sector leaders and subject matter experts Flexible professional development and options for qualifications Modular Training Program, MBI® course list here. …………………………………………………………………………………………………………………………… Bioprocess Facility Design – MBI® Short Courses When: 27-30 June 2016 Where: Biochemical Engineering, University College London This course focuses on how to take a process from a paper design to fully-operational facility, and will enable you to: Design a plant layout considering the relevant regulatory requirements. Understand the impact of facility design requirements on costs. Determine a project schedule including phases, installation, commissioning and validation. Accurately interpret engineering drawings used in design. Prepare a safety and environmental review to define risks, hazards and safety strategy. Understand the role of contractors, work with them more effectively and analyse their proposals. Who should attend This course is intended for engineers, chemists, biologists, biochemists and biotechnologists who are interested in facility design for bioprocesses. Each concept and topic covered will be explained for the beginner - an example case study is followed through the sequence of steps followed when performing facility design, the example selected is drawn from the previous “Bioprocess Design & Economic Evaluation” course. Typically, delegates are: R&D scientists, engineers or managers who need to learn more about the typical steps which constitute the preliminary design process in conjunction with the “Bioprocess Design & Economic Evaluation” course. Further course information and confirmed speakers here For bookings, please email mbi-training@ucl.ac.uk or phone +44 (0) 20 7679 9619. Key benefits of MBI® Program Knowledge transfer and upskilling Deeper understanding of process options and engineering constraints Acquire the tools to optimised process performance and economics Updates on latest technical and regulatory developments Access to cutting edge research methodologies Network with sector leaders and subject matter experts Flexible professional development and options for qualifications Modular Training Program, MBI® course list here. ………………………………………………………………………………………………………………………… Single-Use Technologies for Rapid Manufacturing – MBI® Short Courses When: 11-13 July 2016 Where: Biochemical Engineering, University College London The three-day course aims to deliver new training material specific to single-use technologies encompassing different process steps, scales of operation and cell/product systems. It will: Consider the whole process and provide tools useful for the evaluation and adoption of single use systems for a range of process steps Build knowledge of engineering fundamentals in single-use systems and provide confidence in scale translation and in the use of these as scale-down models Focus on the different stages of the development pathway, from initial discovery and screening studies to manufacturing Discuss adoption of these technologies for different cell systems and products (antibody producing) mammalian cells used as base case study, adherent cells and high cell density organisms among others) Provide training on single-use technologies specific characteristics and requirements (sterile connections and disconnections, standardization, sensor technology, toxicological profiles and regulatory approval). Who should attend The course is suitable for engineers, biotechnologists and those with a life science background who are or plan to work with single-use technologies for different processes and products. In particular, the module will suit those responsible for the uptake and selection of the technology, for process development, technology transfer and scale translation. Practical sessions will help building confidence in the use of the technology for those planning to use it for the first time. Delegates will also learn from industrial experts experiences on the challenges and opportunities around single-use systems. Further course information and confirmed speakers here For bookings, please email mbi-training@ucl.ac.uk or phone +44 (0) 20 7679 9619. Key benefits of MBI® Program Knowledge transfer and upskilling Deeper understanding of process options and engineering constraints Acquire the tools to optimised process performance and economics Updates on latest technical and regulatory developments Access to cutting edge research methodologies Network with sector leaders and subject matter experts Flexible professional development and options for qualifications Modular Training Program, MBI® course list here. ………………………………………………………………………………………………………………………… Principles of Fermentation Processes – MBI® Short Courses When: October 2016, dates to be confirmed Where: Biochemical Engineering, University College London ‘Principles of Fermentation Processes’ will strengthen your knowledge of fermentation processes, and through a series of lectures, case studies and a pilot plant visit, will enable you to: Define different modes of fermentation and know their limitations. Develop a suitable medium and perform a material balance. Interpret fermentation data and use it effectively. Characterise the kinetics of cell growth and how they apply to different cell systems. Determine fermentation productivity and yields. Learn about new developments in fermentation technology (single use bioreactors) Develop a strategy for fermentation process development. The module consists of lectures from industrial and academic experts, and interactive problem-solving case studies performed in small groups for effective learning. Who should attend This module is suitable for scientists and engineers who wish to familiarise themselves with fermentation processes and those who wish to build underlying principles into their operational expertise in areas of: research, process development and manufacturing Further course information and confirmed speakers here For bookings, please email mbi-training@ucl.ac.uk or phone +44 (0) 20 7679 9619. Key benefits of MBI® Program Knowledge transfer and upskilling Deeper understanding of process options and engineering constraints Acquire the tools to optimised process performance and economics Updates on latest technical and regulatory developments Access to cutting edge research methodologies Network with sector leaders and subject matter experts Flexible professional development and options for qualifications Modular Training Program, MBI® course list here. ………………………………………………………………………………………………………………………… Rapid Fermentation Process Design – MBI® Short Courses When: October 2016, dates to be confirmed Where: Biochemical Engineering, University College London This course focuses on rapid fermentation process development and scale-up, through a series of lectures and case studies enabling you to: Understand engineering principles of fermentation. Determine power consumption and oxygen mass transfer. Evaluate different strategies for scale-up and scale-down. Know about miniaturised bioreactors and their role in fermentation process development. Understand how to design a bioreactor and use data from microscale experiments for scaleup. Who should attend Fermentation scientists and engineers in biotechnology and biopharmaceutical companies’ or similar who are involved in process transfer to manufacturers and also those in established fermentation development groups wishing to be kept up to date with the latest research in this area. Further course information and confirmed speakers here For bookings, please email mbi-training@ucl.ac.uk or phone +44 (0) 20 7679 9619. Key benefits of MBI® Program Knowledge transfer and upskilling Deeper understanding of process options and engineering constraints Acquire the tools to optimised process performance and economics Updates on latest technical and regulatory developments Access to cutting edge research methodologies Network with sector leaders and subject matter experts Flexible professional development and options for qualifications Modular Training Program, MBI® course list here. ………………………………………………………………………………………………………………………… Downstream Processing: From Cell to Column, Integrated Solutions for Rapid Bioprocessing of Biologics – MBI® Short Courses When: November 2016, dates to be confirmed Where: Biochemical Engineering, University College London This course focuses on the biological product recovery stage and, through a series of lectures, case studies and pilot plant practical sessions, will enable you to: Understand the principles of all the major recovery operations: Centrifugation, Membrane Systems, Filtration, Cell Disruption, Precipitation, Flocculation Compare these unit operations for suitability within the process and study them in our unique pilot plant Determine the most appropriate equipment for separations and specify the right operating conditions Learn to use Ultra Scale Down tool for prediction of large scale separation performance Understand the interaction among fermentation, recovery and purification and learn the integrated solutions for bioprocess development. Who should attend This course is designed for scientists and engineers involved in design, development and operation of primary recovery steps as well as associated steps in upstream and purification processes, including bioprocess developers, R&D scientists, manufacturing technologists, manufacturing operational managers. Further course information and confirmed speakers here For bookings, please email mbi-training@ucl.ac.uk or phone +44 (0) 20 7679 9619. Key benefits of MBI® Program Knowledge transfer and upskilling Deeper understanding of process options and engineering constraints Acquire the tools to optimised process performance and economics Updates on latest technical and regulatory developments Access to cutting edge research methodologies Network with sector leaders and subject matter experts Flexible professional development and options for qualifications Modular Training Program, MBI® course list here. ………………………………………………………………………………………………………………………… Downstream Processing: Chromatography – MBI® Short Courses When: November 2016, dates to be confirmed Where: Biochemical Engineering, University College London This module focuses on product purification and, through a series of lectures, case studies and a pilot plant session, will enable you to: Understand the principles of different methods of process chromatography, including Packed Bed, Monoliths, Expanded Bed. Select the appropriate media for, and predict the performance of, chromatography separations upon scale-up. Assess the suitability of affinity separation as a product purification operation and gain awareness of advances in rational ligand design for difficult separations. Determine appropriate strategies for the maintenance of column and process hygiene. Consider how chromatography is changing at industrial scale, and which new technologies will have an impact in the future. Who should attend This course is intended for engineers, chemists, biologists, biochemists and biotechnologists who are interested in the biochemical engineering aspects of chromatography. Each concept and topic covered will be explained for the beginner - without assuming detailed prior knowledge. Typically, delegates are: R&D scientists, engineers or managers who need to learn more about scale-up, scale down and operation of chromatographic methods in a production environment. Further course information and confirmed speakers here For bookings, please email mbi-training@ucl.ac.uk or phone +44 (0) 20 7679 9619. Key benefits of MBI® Program Knowledge transfer and upskilling Deeper understanding of process options and engineering constraints Acquire the tools to optimised process performance and economics Updates on latest technical and regulatory developments Access to cutting edge research methodologies Network with sector leaders and subject matter experts Flexible professional development and options for qualifications Modular Training Program, MBI® course list here. …………………………………………………………………………………………………………………………