Week 1
advertisement
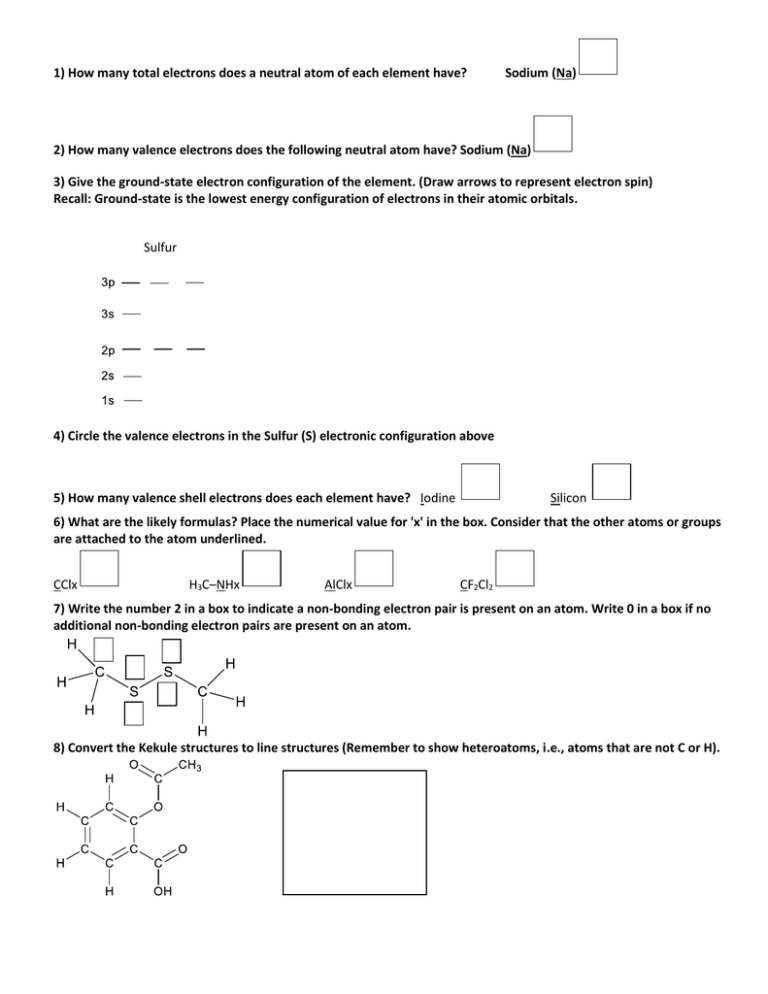
1) How many total electrons does a neutral atom of each element have? Sodium (Na) 2) How many valence electrons does the following neutral atom have? Sodium (Na) 3) Give the ground-state electron configuration of the element. (Draw arrows to represent electron spin) Recall: Ground-state is the lowest energy configuration of electrons in their atomic orbitals. Sulfur 4) Circle the valence electrons in the Sulfur (S) electronic configuration above 5) How many valence shell electrons does each element have? Iodine Silicon 6) What are the likely formulas? Place the numerical value for 'x' in the box. Consider that the other atoms or groups are attached to the atom underlined. CClx H3C–NHx AlClx CF2Cl2 7) Write the number 2 in a box to indicate a non-bonding electron pair is present on an atom. Write 0 in a box if no additional non-bonding electron pairs are present on an atom. 8) Convert the Kekule structures to line structures (Remember to show heteroatoms, i.e., atoms that are not C or H). 9) What is the molecular orbital hybridization, ie, s1px, of the indicated atom? 10) How many hydrogens (hidden in a skeletal drawing) are bonded to the atom indicated? If none, write 0 in the box. REMEMBER: Heteroatoms (i.e., non-carbon atoms are draw with their attached (H)ydrogens). 11) Lewis (electron-dot) structure: Fill in the covalent electron in the boxes between the atoms pairs and any nonbonding electrons around atoms of tetrachloroethylene. CLEARLY DRAW ELECTRONS AS DOTS ( ) 12) The compound below has...? π-bonds σ-bonds 13) Complete the partial Lewis structure of C3H9N below by filling in the missing hydrogens and electrons (as dots ( )).






