Thermo WS
advertisement

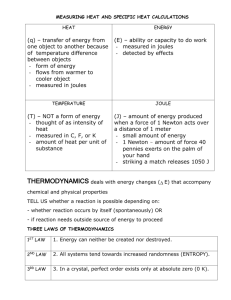
Thermochemistry Name: _____________________________ Date: _____________ Answer the following questions as completely as possible. For questions involving calculations, show all work and express your final answer with units and proper significant figures. Part 1: 1. One pound of body fat is equivalent to about 4.1 x 103 Calories. Convert this to Joules. 2. Thermal decomposition of 5.0 metric tons of limestone to lime and carbon dioxide requires 9.0 x 106 kJ of heat. Convert this energy to joules and calories. 3. Use the two diagrams on the right to answer the following questions: i. Is heat flow positive or negative in diagram a? ii. Is heat flow positive or negative in diagram b? iii. Label each diagram as either endothermic or exothermic. ________________ ________________ 4. What does a negative value for heat represent? What does a positive value for heat represent? Part 2: 1a. How many joules of heat are given off when 5.0 g of water cool from 75C to 25C? (Specific heat of water = 4.18 J/gC) b. How many calories are given off by the water in part a? 2. How many joules of heat are necessary to raise the temperature of 25 g of water from 15C to 65C? 3. What is the specific heat of a substance that absorbs 2.5 x 103 joules of heat when a sample of 1.0 x 104 g of the substance increases in temperature from 10.0C to 70.0C? 4. If 849 J of heat are required to raise the temperature of 95.4 g of copper 23.0◦C, what is the specific heat of copper? Part 3: 1. Specific heat of aluminum is 0.900 J/g ◦C. How many J of heat are needed to raise the temperature of 0.456 g of aluminum by 120.0◦C? 2. Calculate the specific heat of copper (in J/g ◦C), if 77.0 g of copper requires 16.14 kJ to raise its temperature from 22.5 ◦C to 567.0 ◦C. 3. The specific heat of silver is 0.24 J/g ◦C. It takes 3.57 kJ of energy to heat a sample of pure silver from 12.0 ◦C to 14.1 ◦C. Calculate the mass of the sample of silver (in kg). 4. The specific heat of nickel is 0.444 J/g ◦C. When 300.0 g of nickel is added to a constant-pressure calorimeter it loses 1.04 x 104 J of heat to the surroundings. What was the temperature change of the nickel in ◦C? 5. The specific heat of zinc is 0.388 J/g◦C. When 48.0 g of zinc is added to a constant-pressure calorimeter it loses 2356 J of heat to the surroundings. If the initial temperature of the zinc was 98◦C, what was the final temperature of the zinc? 6. A 225 g sample of zinc is heated to 114◦C and added to 100. g of water at 22◦C in a constant-pressure calorimeter. The temperature of both the water and the zinc become 38◦C. The specific heat of water is 4.184 J/g◦C. a. Calculate the change in temperature for water. b. Calculate the amount of heat absorbed by the water c. Calculate the amount of heat lost by the zinc sample d. Calculate the change in temperature for zinc. e. Calculate the specific heat of zinc.