-
What is internal energy?The sum of the potential and kinetic energies of a system.
-
How can you increase the thermal energy of a system?In crease it by heating it up or doing work on the object.
-
Explain the energy changes that occur during a change of state.During change of state the potential energy of the particles change but the kinetic energies doesn't change.
-
What equation can be used to determine the energy required to change the temperature of a substance?
 Q - J m - kg c - JK-1 T - K
Q - J m - kg c - JK-1 T - K -
Give the equation to work of the energy for change of state?Q = ml m - kg I - Jkg-1
-
What is the fist law of thermodynamics?
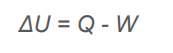 Where Q = the heat added to a system And W is the work done by the system
Where Q = the heat added to a system And W is the work done by the system -
What is absolute zero?-273 degrees celsius This is where objct have no/minimum kinetic energy
-
What is Brownian motion?Brownian motion is the idea that very small objects have random motion in a liquid or gas due to random bombardment by the molecules in this substance. This movement will be fractionally more on one side than the other so a force will push it for an instant as the net forces shifts directions. This random motion is Brownian Motion and gives evidence for the existence of atoms.
-
What is heat?Heat is the energy that is transferred from one body to another as a result of difference in temperature.
-
Thermal equilibriumSubstances in contact with each other will transfer heat energy until both bodies are at the same temperature The object with highest energy (the hotter object) will always transfer energy to the object with lower energy (the cooler object) Two substances in thermal equilibrium are said to be at the same temperatre, there will be no exchange of heat between them The total energy of the system remains constant
-
What does the rate of transfer depend on in thermal equilibrium?The conductivity of the objects
-
How can temperature be measured?Temperature can be measured by any device where one property changes in a predicatble way as temperature chhanges, thernionic property
-
What is thermal equilibrium?no net flow of thermal energy between two r more bodies at the same temperature
-
Explain thermal equilibrium by reference to the behaviour of the molecules when a sample of hot gas is mixed with a sample of cooler gas and thermal equilibrium is reachedkinetic energy is echanged in molecular collisions until average kinetic energy of all molecules is the same
-
The equation to work out the energy for a change of state.Q = ml Q - J m - kg l - J/kg
-
What is the specific heat capacity of substance?The energy required to raise the temperature of 1kg of a substance by 1K
-
What is the specific latent heat of a substance?The energy required to change the state per unit mass of a substance, while keeping the temperature constant.
-
Explain the energy changes that occur during a change of state.During change of state the potential energy of the particles change but the kinetic energies doesn't change.
-
Graph of temperature against time for a substance explained
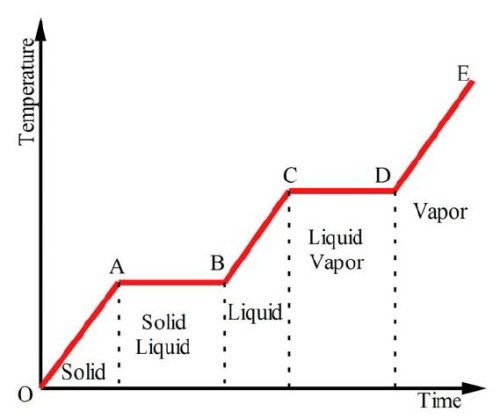 NOTE CD should be longer AB and CD are flat because energy is going to break/weaken bonds CD should be longer as it takes more energy to break the bonds of liquid to gas than weaken solid to liquid - energy goes into breaking the bonds between particles - potential energy increases not kinetic energy
NOTE CD should be longer AB and CD are flat because energy is going to break/weaken bonds CD should be longer as it takes more energy to break the bonds of liquid to gas than weaken solid to liquid - energy goes into breaking the bonds between particles - potential energy increases not kinetic energy -
Specific latent heat of fusionThe heat/energy required to change 1kg of substance from a solid to a liquid without a change in temperature (l - J/kg)
-
Specific latent heat of vaporisationThe heat required to change 1 kg of a substance from liquid to gas, without change in temperature (l - J/kg)
-
What does Q=ml mean?The amount of heat/energy absorbed or released to change the state of a subject from a solid to liquid/liquid to gas or vice versa
-
What is the Ideal gas equation?pV = nRT p - Pa R - molar gas constant T - K V - m^3 n - no. of moles
-
What is the first law of thermodynamics?
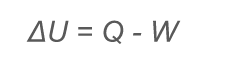 Change in internal energy - change in U Q - the heat added to a system W - the work done by the system
Change in internal energy - change in U Q - the heat added to a system W - the work done by the system -
What is an ideal gas?A gas that: The gas molecules don't interact with each other The molecules are tought to be perfectly spheres
-
What is the internal energy of a gas equal to?It is equal to the internal energy of an ideal gas
-
What is Boyle's law?Pressure is inversely proportional to volume, providing temperature is constant.
-
In an ideal gas, how would increasing the volume change the temperatuure is pressure remains constant?As you increase the volume, you also increase the temperature.
-
How does increasing the temperature of a balloon, while keeping the volume the same will increase the pressure?As temperature increases the average kinetic energy increases And so the particles sare travelling at a higher speed on average There are also more frequent collisions And so the particles would exert a greater force Which would cause a increased rate of change in momentum And so increasing pressure
-
What is avogadro's constant?The number of atoms there are in one mole of a substance
-
What is an assumption of the collisions between particles and the wall in an ideal gas equation?Elastic
-
An assumption relating to time in an ideal gas equation?Time for each collision is negligible compared to the time taken between collisions
-
Describe 3 other assumption of the ideal gas equationThe particles move randomly They follow Newton's laws of motion No intermolecular forces act between particles Volume of the particles is negligible compared to the volume of conatiner they are in
-
What is root mean square speed?The square root of the mean of the squares of the speeds of the molecules
-
Charles' lawThe volume of an ideal gas is directly proportioinal to the temperature when pressured is constant
-
Conversion between Kelvin and Celsius
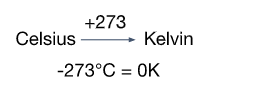
-
Assumptions in kinetic theorymotion of molecules is random collisions between molecules are elastic time taken for a collsion is negligible campared to the time between collisions molecules move in straight lines at a constant speed between collisions all particles are identical and have same massand volume intermolecular forces are negligible except during collisions
-
derivatrion of kinetic theory diagram
-
Derivation of force/kinetic theory

-
derivation of pressure, kinetic theory

-
boyle's law equationpV = constant
-
Boyle's law explainationBy considering the particles in a fixed quatity of a substance: As the volume decreases the particles come closer together Causes the rate of collisions to increase An increase in the rate of collisions leads to an increased rate of change of momentum Consequently the force on the constainer falls increases so pressure increases
-
Charle's law equationV/T = constant
-
Charles' law explainationBy considering the particles in a fixed quantity: As temperature increases the average kinetic energy of the particles increases Since the pressure is constant, the rate of change of momentum must remain constant To achieve this the rate of collisions should remain constant, but since particles are travelling faster, for this to happen they must become more seperated This results as increase in volume
-
Pressure lawAs temperature increases the presure of a gas at constant volume increases
-
Pressure law equationp/t = constant
-
Pressure law explainationConsidering the particles in a fixed quantity: As temperature increases, the average kinetic energy of the particles increases This means the particles are moving faster and so the particles are moving faster and so there is a higher rate of collisions Since the particles are moving faster and so there is a higher rate of change of momentum of the particles is increased Consequently the force on the container and so the pressure is increased
-
What does the are under a p-V graph equal to?The work done by the gas
-
ideal gas equation in terms of kk = R/n pV = NkT N - no atoms in the gas
-
Thermal equilibrium equationm1c1(T-T1)=m2c2(T2-T)
-
conductionheat transferred through vibrations
-
convectionhot substance rises cold substance falls
-
radiationinfared em waves absorbed
-
When T is constantp1V1=p2V2
-
When P is constantV1/T1 = V2/T2
-
When V is constantp1/T1 = p2/T2
-
2nd law of thermodynamicsHeat cannot be converted into work unless it flows from a hot space to a cold one
-
to find absolute zero from a graph
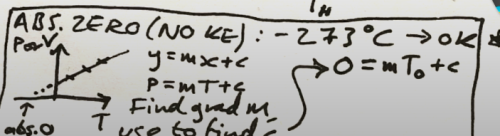 P = mT +c P = 0 at absolute 0
P = mT +c P = 0 at absolute 0 -
work done by gas
Pressure x change in volume
-
How the energy transferred to the sample changed the arrangement of atoms when changing state
Energy transferred reduces the number of atomic particles near by breaking atomic bonds

