Skeletal Muscle Contraction & Dysfunction (Physiology 2)
Skeletal muscle contraction & dysfunction - Prof Iain Greenwood Lecture outline This session is designed to build on your Year 1 knowledge from the Fundamental in Pharmacology and Physiology module. In addition to a recap of some key concepts learned previously in the Year 1 lecture units, you will be introduced to pathologies associated with skeletal muscle disorders. Outcomes At the end of this session students will be able to: -describe the molecular mechanisms behind skeletal muscle contraction. -understand how muscle structure leads to a rise in calcium -understand that role of ATP hydrolysis in the powerstoke. -describe the major types of muscle pathophysiology with particular focus on dysfunctional ion channels, nicotinic acetylcholine receptors and molecular stablisers. -appreciate the role of the myelin sheath in neuromuscular contraction. Lecture Slides
-
What are the main ion channels involved in nerve excitation and propagation? (2)
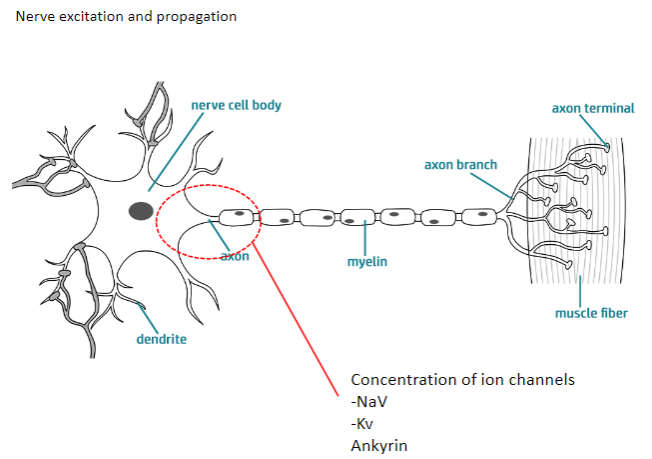
NaV (Sodium Voltage-gated channels)
Kv (Potassium Voltage-gated channels)
-
How do NaV and Kv channels contribute to nerve excitation and propagation? (4)
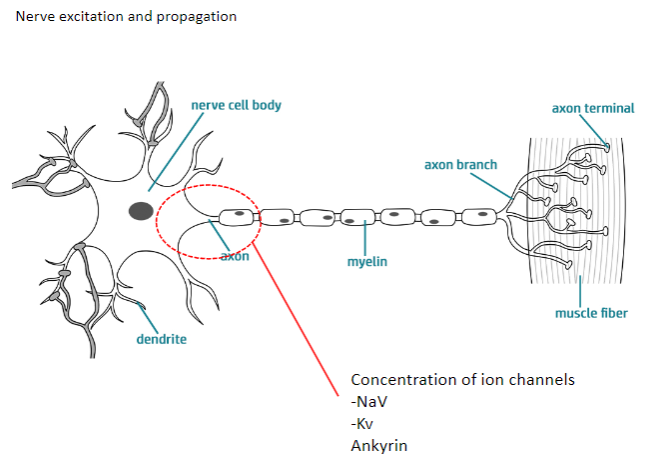
NaV channels: Allow sodium ions to flow into the neuron, causing depolarization.
Kv channels: Allow potassium ions to flow out, causing repolarization.
Both channels work together to generate action potentials and propagate them along the axon.
Ankyrin: A protein that anchors ion channels to the cell membrane, ensuring proper function.
-
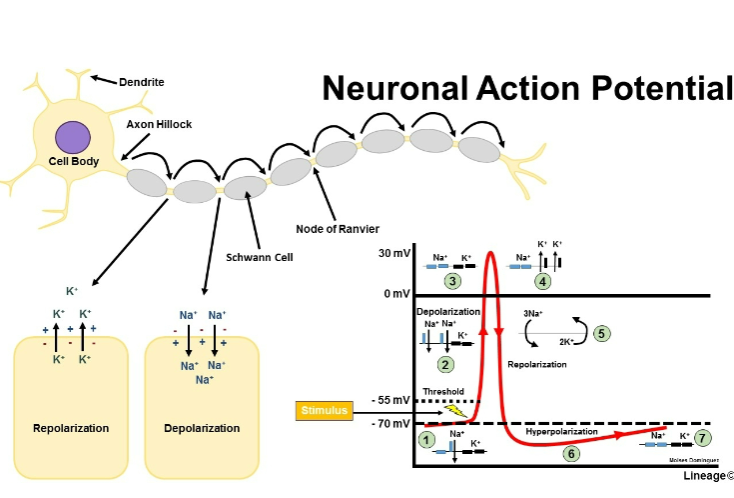
Picture demonstrating neuronal action potential:
-
What is the distinctive feature of voltage-gated sodium channels? (1)
They exhibit distinctive inactivation after opening.
-
What family of genes encodes voltage-gated sodium channels? (1)
The SCNA1-9 gene family encodes voltage-gated sodium channels.
-
What happens to voltage-gated sodium channels upon depolarization? (1)
They exhibit rapid opening upon depolarization.
-
Picture demonstrating voltage gated sodium channels:
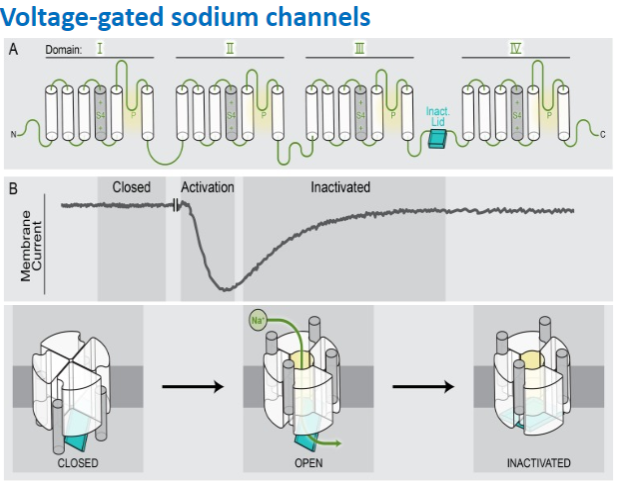
-
What happens when the action potential reaches the nerve terminal? (2)
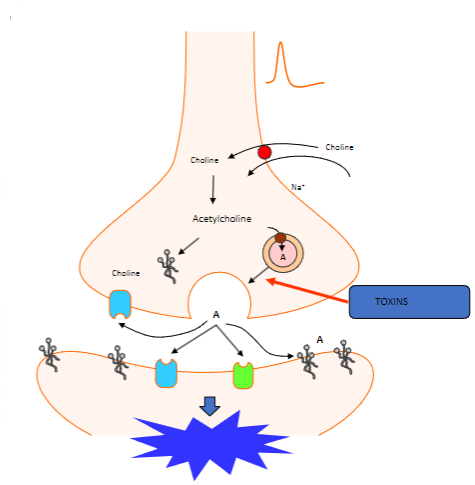
Vesicles containing acetylcholine collide with the cell membrane.
This process is calcium-dependent.
-
Which proteins are involved in the fusion of synaptic vesicles at the motor nerve terminal? (3)
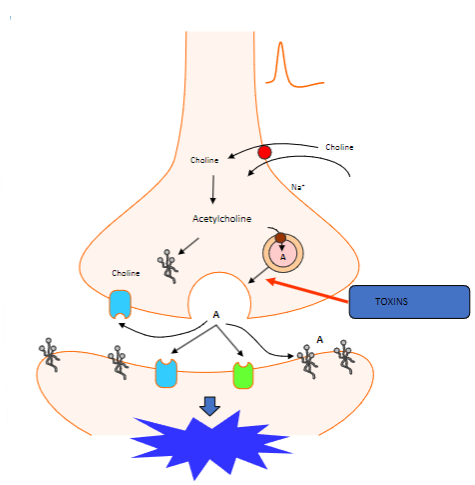
Synaptotagmin (Calcium sensor) causes synaptobrevin to bind with syntaxin 1 or SNAP 25.
-
What happens to acetylcholine after vesicle fusion at the motor nerve terminal? (1)
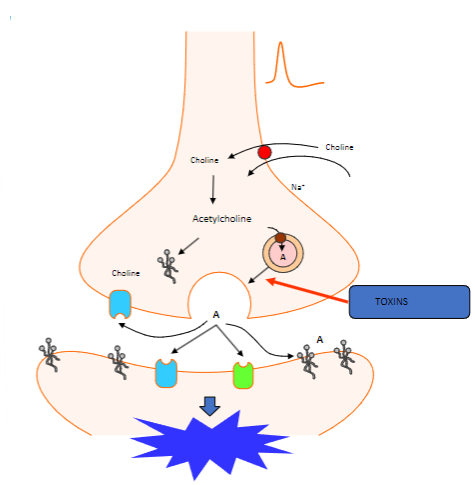
Acetylcholine diffuses to interact with target receptors in the muscle cell.
-
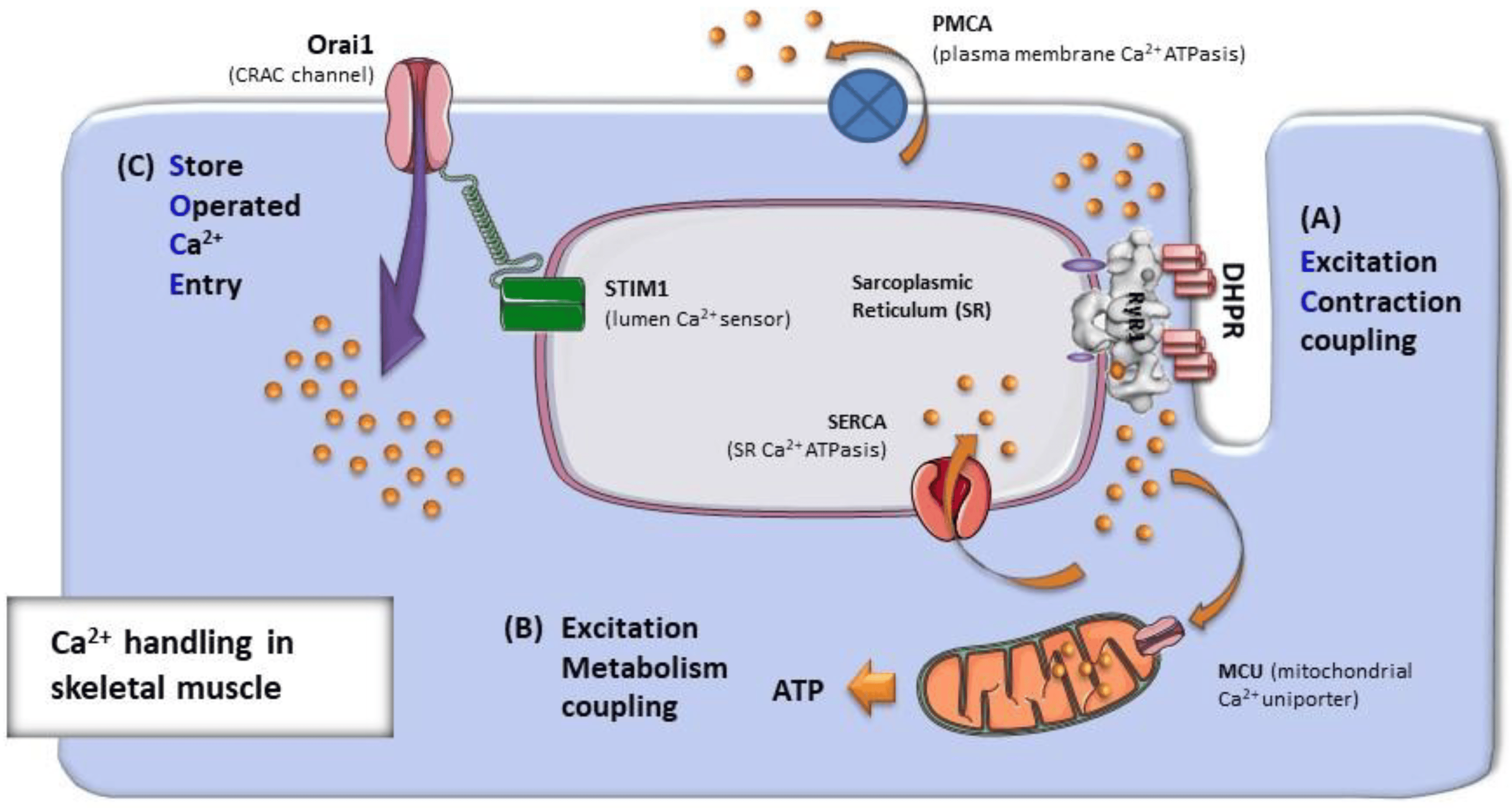
What is the basic process of muscle contraction? (2)
The interaction of actin and myosin.
ATP fuels the contraction, and the process is driven by a rise in [Ca2+]
-
What are the key membrane events that lead to muscle contraction? (3)
A rise in calcium concentration.
Calcium binds to the sensor (troponin on actin).
This leads to the activation of the contractile mechanism.
-
What is the role of ATP hydrolysis in muscle contraction? (1)
ATP hydrolysis by myosin provides the energy needed for the muscle contraction.
-
How do actin and myosin contribute to muscle contraction? (2)
The interaction between actin and myosin forms cross-bridges.
These interactions cause the muscle cell to shorten.
-
What are the components of the thick and thin filaments involved in muscle contraction? (2)
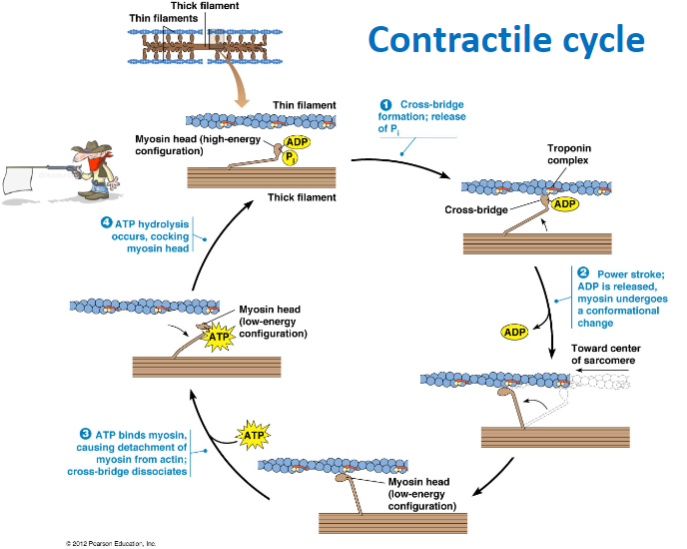
Thick filament: Contains myosin heads in a high-energy configuration.
Thin filament: Contains actin and the troponin complex, which regulate contraction.
-
What are the main steps of the contractile cycle in muscle contraction? (4)
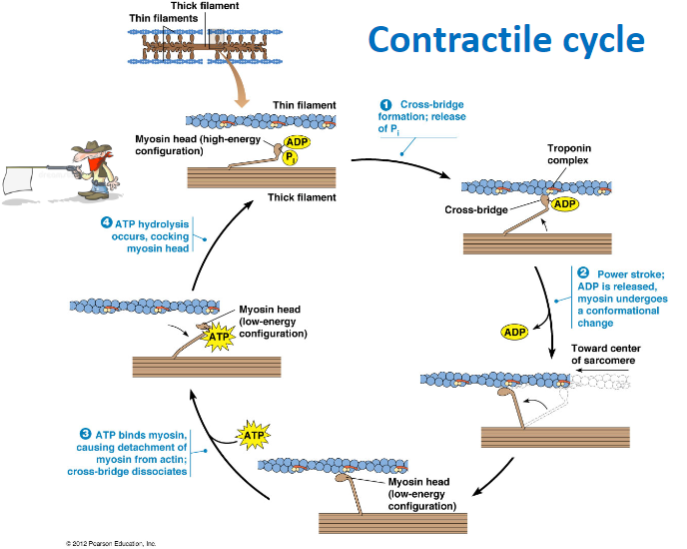
Cross-bridge formation: Myosin head binds to actin.
Power stroke: ADP is released, and myosin undergoes a conformational change, moving toward the center of the sarcomere.
ATP binding: ATP binds to myosin, causing detachment from actin and dissociation of the cross-bridge.
ATP hydrolysis: ATP is hydrolyzed, cocking the myosin head back into its high-energy configuration.
-
What happens during cross-bridge formation in muscle contraction? (2)
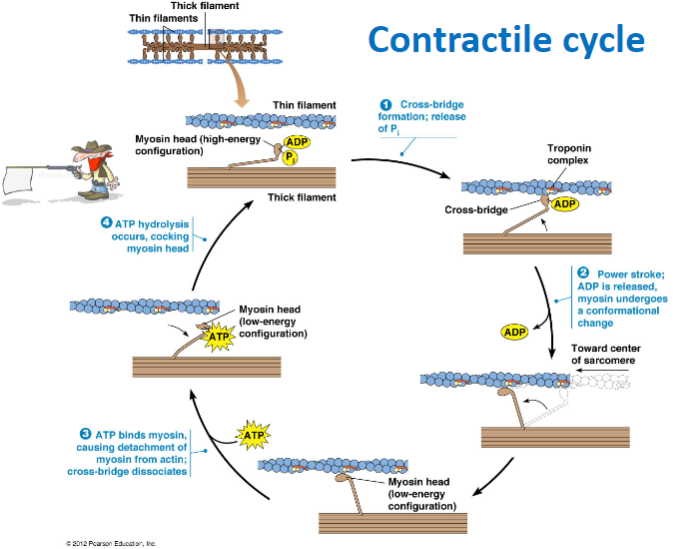
The myosin head binds to actin forming a cross-bridge due to the release of phosphate (P) .
-
What is the role of ATP in the muscle contraction cycle? (3)
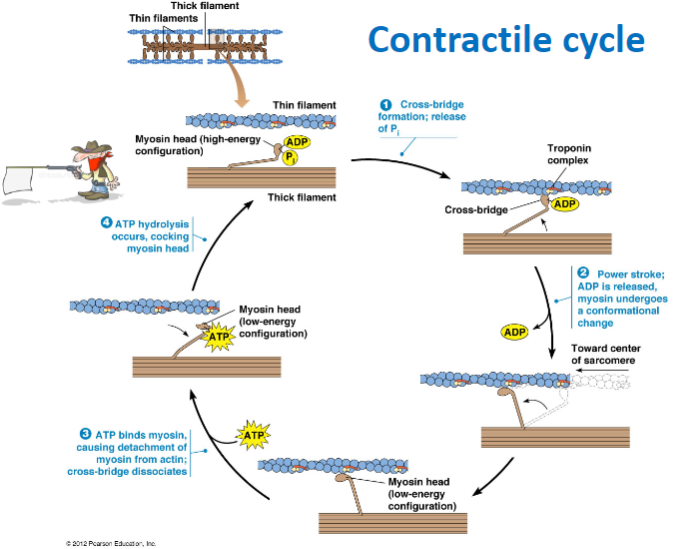
ATP hydrolysis: Powers the cocking of the myosin head (high-energy configuration).
ATP binding to myosin: Causes the detachment of myosin from actin, breaking the cross-bridge.
ATP hydrolysis: Prepares the myosin head for the next cycle by re-cocking it.
-
What happens when an action potential arrives at the neuromuscular junction? (2)
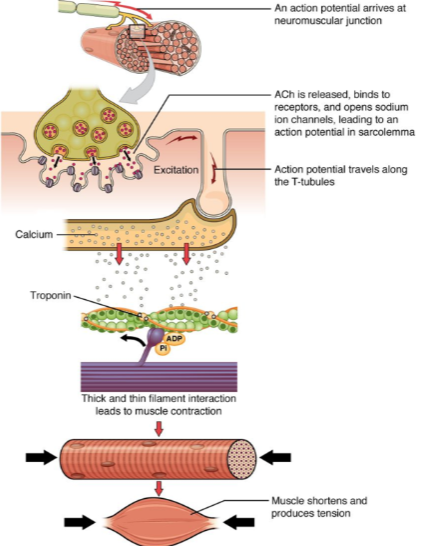
ACh (acetylcholine) is released.
ACh binds to receptors on the muscle cell, opening sodium ion channels, which leads to an action potential in the sarcolemma.
-
How does the action potential propagate through the muscle cell after it is generated at the neuromuscular junction? (2)
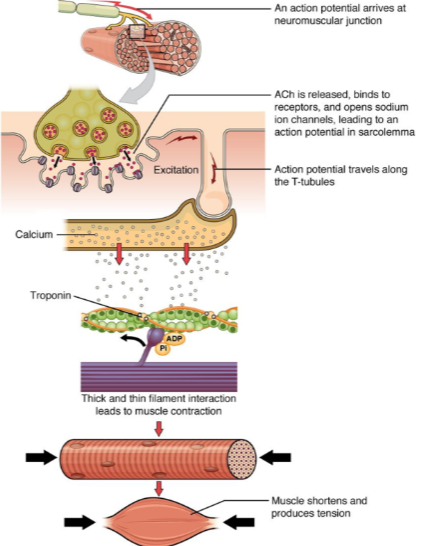
The action potential travels along the sarcolemma (muscle cell membrane).
It moves into the muscle fiber via the T-tubules (transverse tubules).
-
What is the role of calcium in muscle contraction? (2)
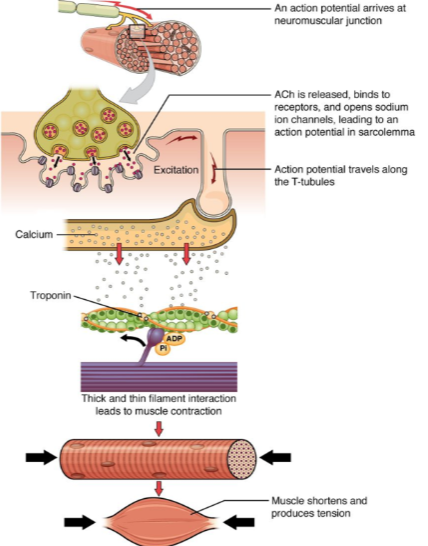
Calcium ions bind to the troponin complex on the thin filaments.
This binding leads to a conformational change that allows for the interaction between thick and thin filaments, initiating muscle contraction.
-
What happens when calcium interacts with troponin in muscle contraction? (1)
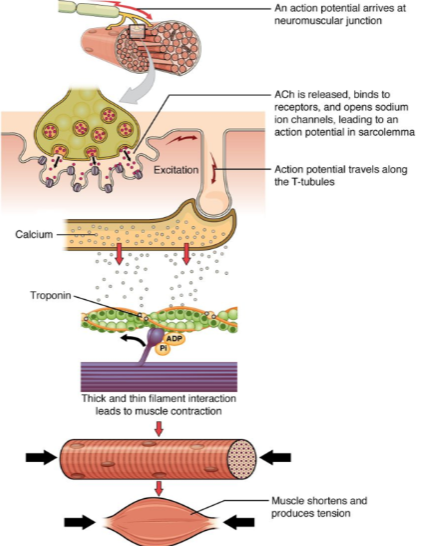
The interaction between troponin and calcium leads to the thick and thin filament interaction, which generates muscle contraction.
-
What is the result of muscle contraction? (2)
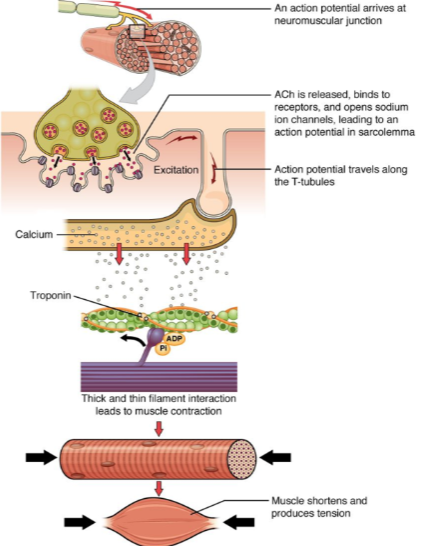
Muscle fibers shorten, producing tension.
This leads to overall muscle contraction and force generation.
-
What is Multiple Sclerosis and how does it affect the neuromuscular system? (2)
Multiple Sclerosis (MS) is an autoimmune disease that causes demyelination of the central nervous system (CNS).
This disrupts the normal transmission of nerve impulses, leading to muscle weakness, loss of coordination, and other neurological symptoms.
-
What is Myasthenia Gravis and what causes muscle weakness in this condition? (2)
Myasthenia Gravis is an autoimmune disorder in which antibodies attack and block or destroy nicotinic acetylcholine receptors at the neuromuscular junction.
This results in reduced transmission of nerve impulses to muscles, causing muscle weakness, particularly in voluntary muscles.
-
What are Non-Dystrophic Myotonias and how do they affect muscle function? (2)
Non-dystrophic myotonias are genetic disorders that cause muscle stiffness (myotonia) without muscle wasting.
Mutations in ion channels, particularly sodium channels, lead to abnormal muscle relaxation after contraction, causing persistent muscle stiffness.
-
What is Muscular Dystrophy and how does it affect skeletal muscle? (2)
Muscular Dystrophy refers to a group of genetic disorders that cause progressive muscle weakness and degeneration.
These conditions result from mutations in genes encoding proteins that are essential for muscle structure and function, leading to muscle fibers breaking down over time.
-
How is myelin produced in the CNS and PNS, and what is the role of these cells in Multiple Sclerosis? (4)
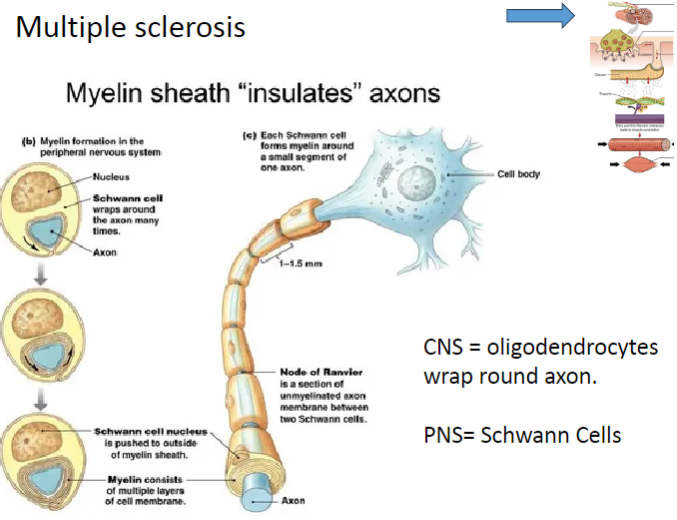
In the CNS, oligodendrocytes produce myelin and wrap it around the axons of neurons.
In the PNS, Schwann cells are responsible for myelin production around axons.
In Multiple Sclerosis (MS), demyelination occurs when the immune system attacks the oligodendrocytes in the CNS, impairing nerve function.
Schwann cells are not typically affected by MS as much as oligodendrocytes, but their role in myelin repair in the PNS is crucial in other demyelinating conditions.
-
Why does myelin not allow the movement of ions, and how does this relate to nerve conduction? (3)
Myelin is a fatty substance that insulates axons, preventing ion movement across the membrane.
This insulation ensures that action potentials travel more efficiently, without significant ion leakage.
Myelination speeds up nerve conduction by allowing action potentials to jump between the nodes of Ranvier rather than traveling along the entire length of the axon.
-
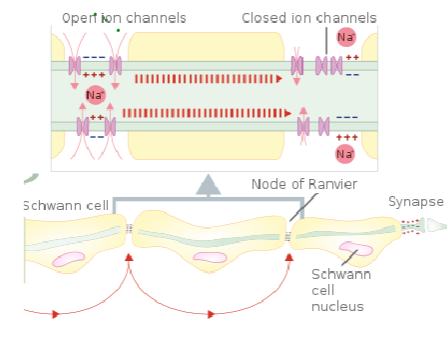
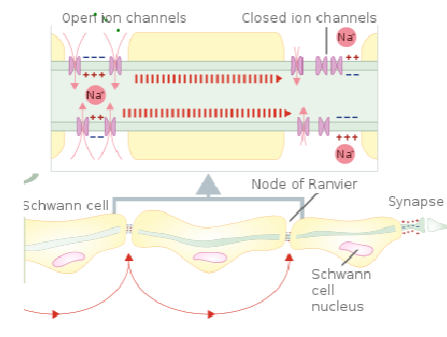
What is the role of the nodes of Ranvier in myelinated axons? (3)
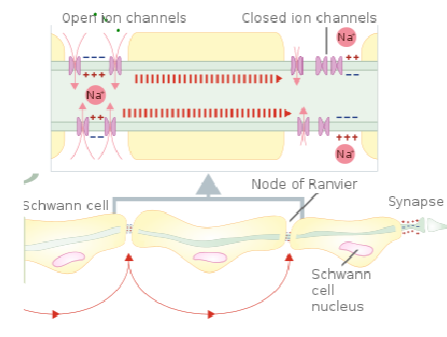
Nodes of Ranvier are small gaps in the myelin sheath where NaV (voltage-gated sodium channels) and Kv (voltage-gated potassium channels) are concentrated.
These channels allow ion exchange, enabling the action potential to regenerate and jump from node to node.
This process is called saltatory conduction, which significantly increases the speed of nerve signal transmission.
-
How does myelination minimize the number of Na+/K+ ATPases required for ion balance? (2)
Myelin reduces the need for Na+/K+ ATPases by limiting ion exchange to the nodes of Ranvier (through saltatory conduction).
Since ion flow is restricted to these nodes, fewer ATP molecules are required to maintain the ion gradients across the membrane.
-
What is Multiple Sclerosis (MS), and how does it affect the nervous system? (2)
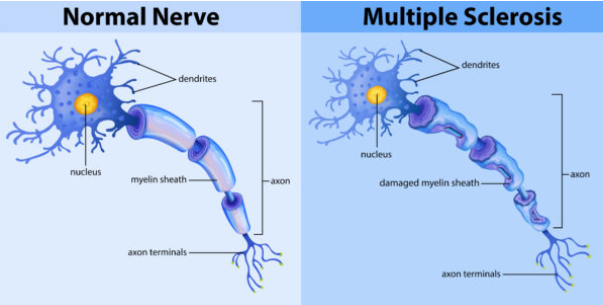
Multiple Sclerosis (MS) is an autoimmune disorder where the body's immune system attacks the myelin that insulates nerve fibers.
This leads to demyelination, disrupting the normal flow of electrical impulses along nerves and causing various neurological symptoms.
-
What is the role of the blood-brain barrier and how does it contribute to MS? (2)
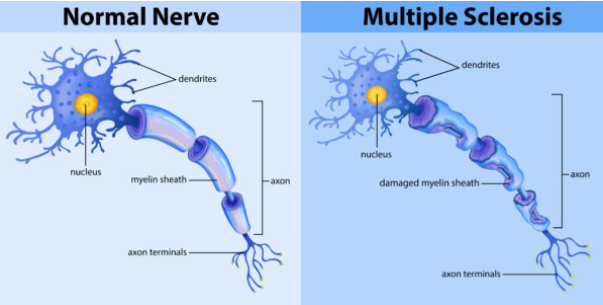
In MS, a leaky blood-brain barrier allows immune cells to cross into the central nervous system (CNS) and attack the myelin.
This results in inflammation and the formation of sclerotic lesions (scars) on nerve fibers.
-
What are the common symptoms of Multiple Sclerosis? (5)
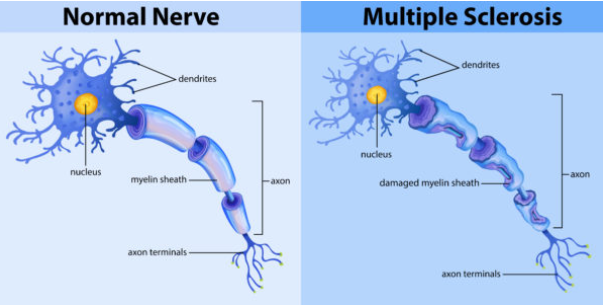
Numbness or tingling sensations in parts of the body.
Speech problems, such as difficulty with articulation.
Visual problems, including blurred or double vision.
Urinary incontinence due to bladder dysfunction.
Debilitating muscle weakness, affecting movement and coordination.
-
What is Myasthenia Gravis and how does it affect skeletal muscle function? (2)
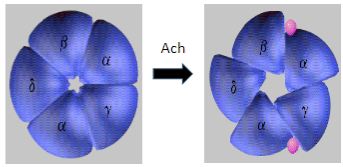
Myasthenia Gravis (MG) is an autoimmune disease where antibodies target and degrade the muscle nicotinic acetylcholine receptors (AChRs), particularly the α1 subunit.
This leads to impaired neuromuscular transmission and causes muscle weakness and fatigue.
-
What is the mechanism behind Myasthenia Gravis in terms of the AChR? (2)
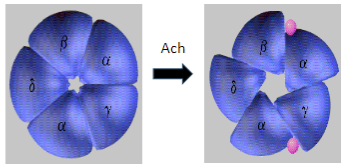
In Myasthenia Gravis, antibodies target and degrade the muscle nicotinic acetylcholine receptors (AChRs), specifically the α1 subunit.
This results in decreased receptor function and disrupted neuromuscular signaling, leading to muscle weakness.
-
What are the common symptoms of Myasthenia Gravis? (5)
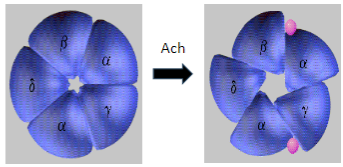
Muscle weakness, especially in proximal muscles.
Drooping eyelids (ptosis) due to weakness of the eye muscles.
Fatigue that worsens with exertion.
Difficulty swallowing (dysphagia) or talking (dysarthria).
Exertion is difficult, and muscle weakness worsens with activity.
-
How does Myasthenia Gravis affect muscle and neuronal nicotinic AChRs differently? (2)
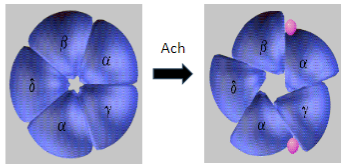
Muscle nicotinic AChRs are targeted and degraded in Myasthenia Gravis, leading to muscle weakness.
Neuronal nicotinic AChRs are unaffected, allowing normal signaling in the nervous system.
-
Which three proteins involved in receptor clustering in muscle folds can be affected in Myasthenia Gravis? (3)
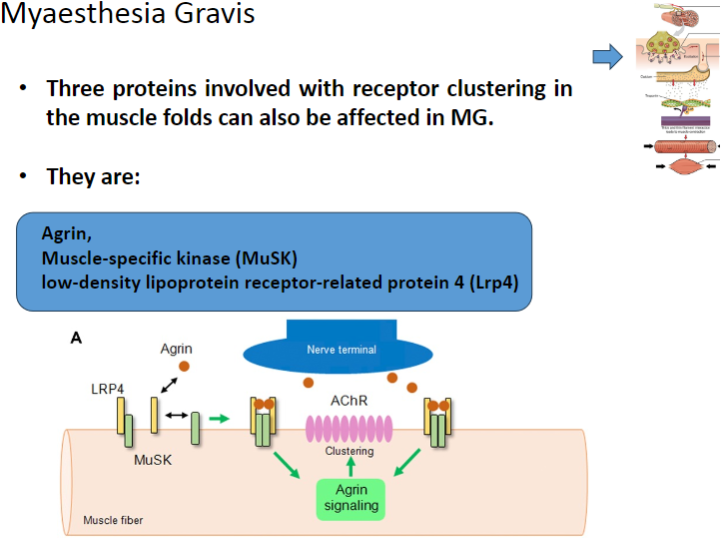
Agrin
Muscle-specific kinase (MuSK)
Low-density lipoprotein receptor-related protein 4 (Lrp4)
-
What is the role of Agrin in muscle function and how is it affected in Myasthenia Gravis? (2)
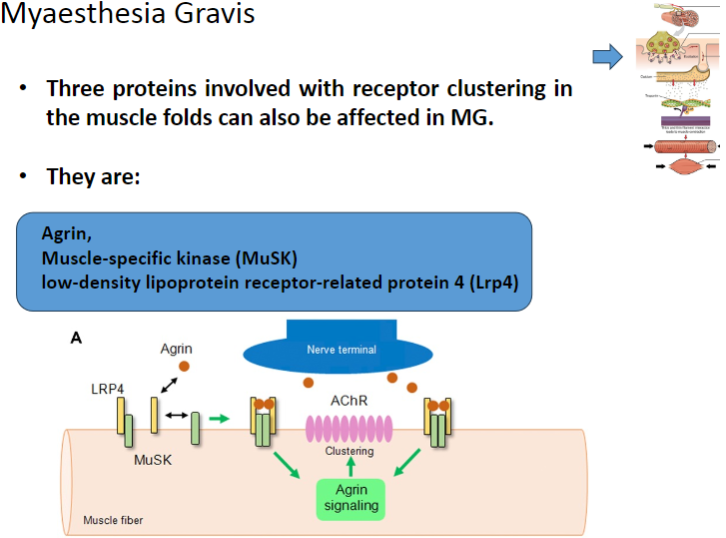
Agrin is a protein that helps to cluster nicotinic acetylcholine receptors (AChRs) at the neuromuscular junction.
In Myasthenia Gravis, Agrin function may be disrupted, impairing proper receptor clustering and neuromuscular transmission.
-
What is the role of Muscle-specific kinase (MuSK) in the neuromuscular junction and how does it relate to Myasthenia Gravis? (2)
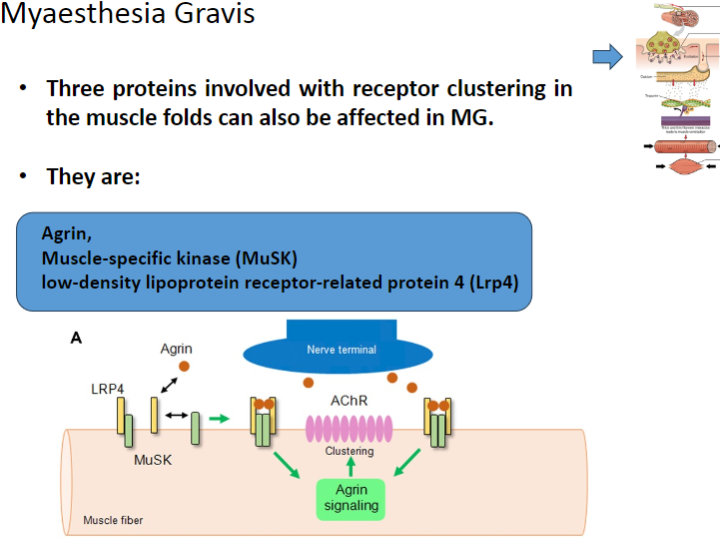
MuSK is involved in the clustering of nicotinic acetylcholine receptors (AChRs) at the neuromuscular junction.
In Myasthenia Gravis, MuSK can be affected, leading to improper receptor clustering and compromised neuromuscular signaling.
-
What is the function of Lrp4 in muscle cells, and how is it involved in Myasthenia Gravis? (2)
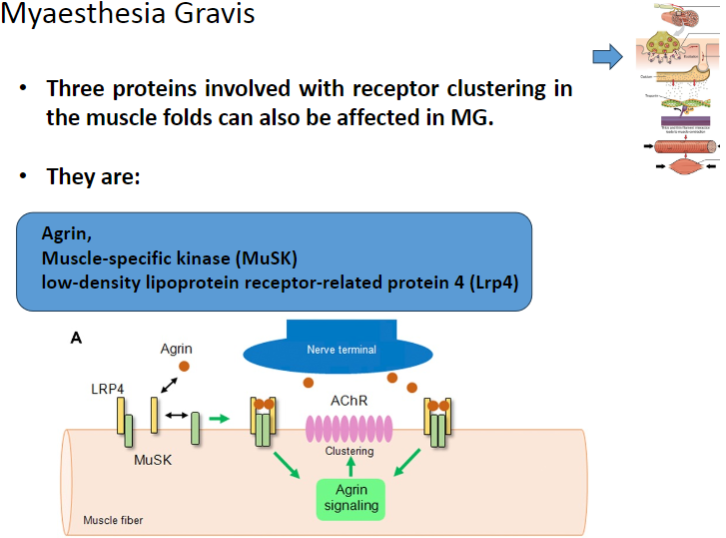
Lrp4 (Low-density lipoprotein receptor-related protein 4) is involved in the activation and clustering of nicotinic acetylcholine receptors (AChRs) at the neuromuscular junction.
In Myasthenia Gravis, Lrp4 can be affected, impairing the clustering of AChRs and disrupting neuromuscular communication.
-
What are the new diagnostic features of Myasthenia Gravis related to antibodies? (2)
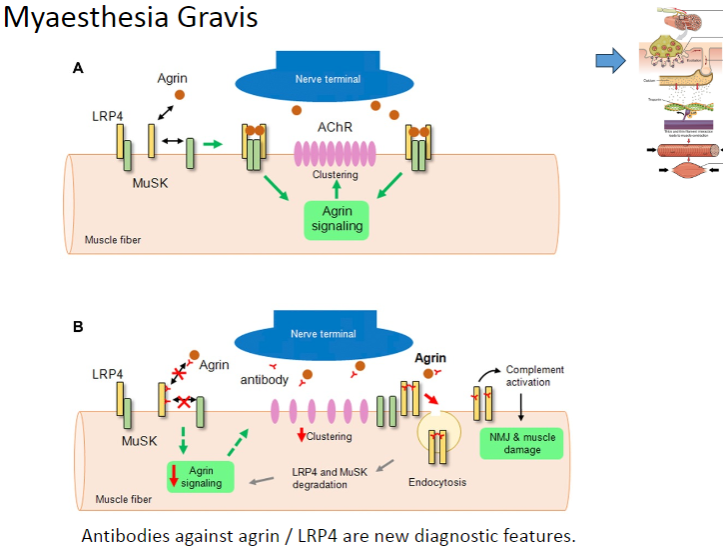
Antibodies against Agrin
Antibodies against LRP4
These antibodies are now used as diagnostic markers for Myasthenia Gravis.
-
What are the features and electrophysiological characteristics of Non-Dystrophic Myotonias? (3)
Delayed relaxation of the muscle after voluntary contraction or mechanical stimulation.
Electrophysiologically characterized by highly organized repetitive electrical activity of the muscle fibers.
Muscle stiffness due to the inability to relax after contraction.
-
Which skeletal muscle disorders are caused by mutations in the SCN4A gene that encodes a voltage-gated sodium channel, and what are their characteristics? (5)
Potassium-aggravated myotonia (PAM) – Muscle stiffness exacerbated by elevated potassium levels.
Paramyotonia congenita (PMC) – Muscle weakness and stiffness that worsens with cold and improves with heat.
Hyperkalemic periodic paralysis (HyperPP) – Episodes of muscle weakness triggered by high potassium levels.
Hypokalemic periodic paralysis (HypoPP) – Episodes of muscle weakness caused by low potassium levels.
Congenital myasthenic syndrome (CMS) – A form of neuromuscular junction disorder leading to weakness and fatigue.
-
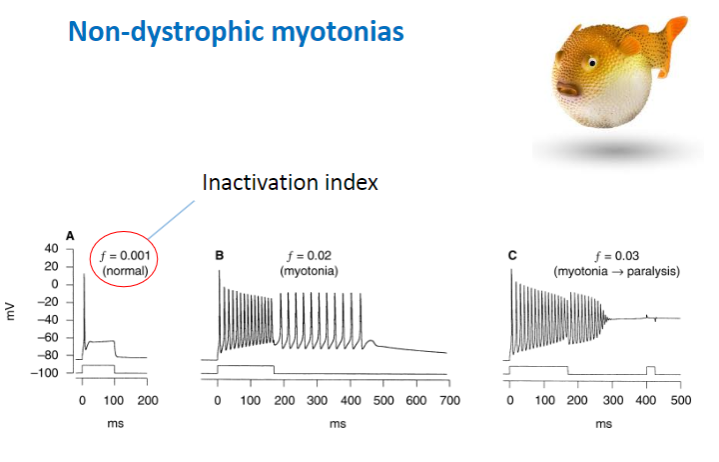
What are the typical effects of mutations in non-dystrophic myotonias, and how do they affect sodium channels? (3)
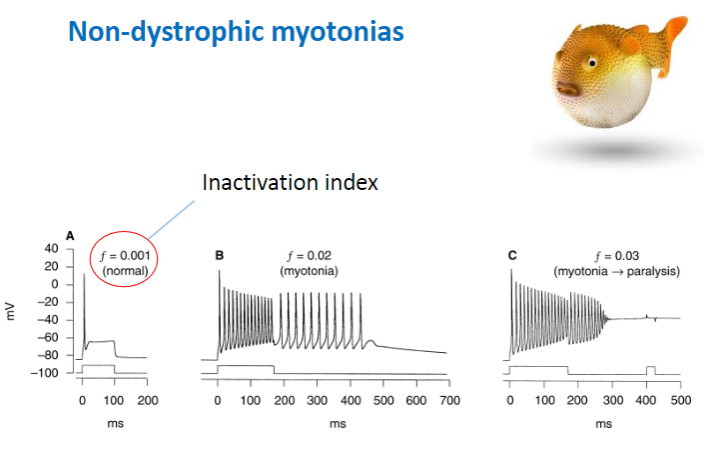
Decreased rate of channel inactivation – Sodium channels remain open longer, leading to prolonged contraction.
Increased rate of recovery from inactivation – Channels recover and reopen too quickly, causing continuous muscle activity.
Slower channel deactivation – The sodium channels close more slowly, maintaining muscle contraction for a longer period.
Result: More sodium channel activity and prolonged muscle contraction
-
How does the loss of Cl channel activity contribute to non-dystrophic myotonias? (2)
Loss of CLC-1 Cl (Chloride Channel-1 Chloride Ion) channel activity – Decreases chloride ion flow across the muscle membrane.
Result: This causes a prolonged muscle contraction due to the imbalance in ion flow, affecting muscle relaxation.
-
What is the role of the dystrophin gene in dystrophic myopathy? (3)
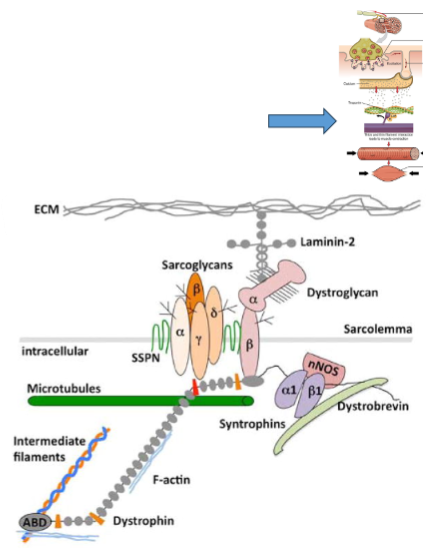
Largest gene in humans: The dystrophin gene contains 79 exons and spans over 2,200 kb, accounting for about 0.1% of the human genome.
Location: Found on the short arm of the X chromosome, which explains why the disease predominantly affects males.
Mutations: Mutations lead to premature stop codons, resulting in truncated dystrophin proteins that are functionally ineffective in muscle cells.
-
What is the difference between Duchenne muscular dystrophy and Becker muscular dystrophy in terms of dystrophin? (2)
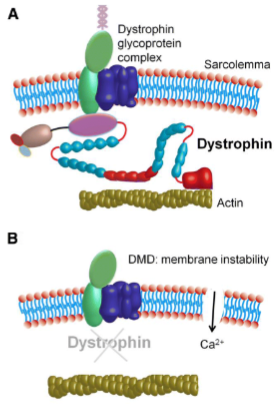
Duchenne muscular dystrophy (DMD): Characterized by total loss of dystrophin.
Becker muscular dystrophy (BMD): Involves a reduced or dysfunctional dystrophin. Dystrophin links the internal cytoskeleton of the muscle cell to the extracellular matrix, which helps protect muscle fibers from damage during contraction and relaxation.
-
What happens to the muscle fiber membrane permeability in dystrophic myopathy? (3)
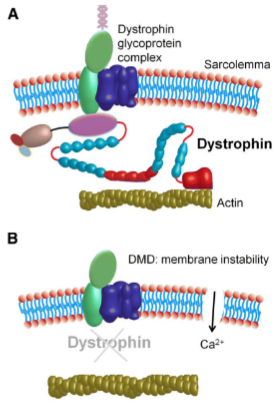
There is increased permeability to macromolecules due to the loss or dysfunction of dystrophin.
The abnormal permeability is exacerbated by mechanical stress.
This leads to muscle fiber necrosis, fibrosis, and fat infiltration.
-
What is affected in Multiple Sclerosis and how does it impact the nervous system? (2)
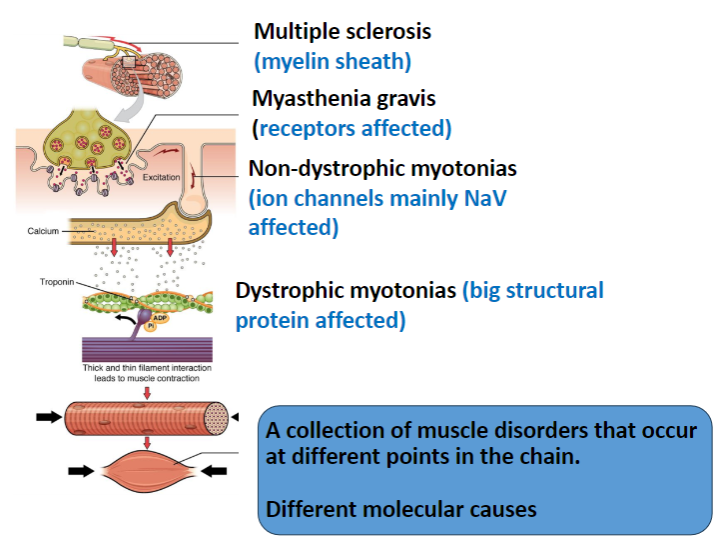
The myelin sheath in the central nervous system (CNS) is targeted.
This results in immune attacks on myelin, leading to impaired nerve conduction.
-
In Myasthenia Gravis, which receptors are affected and how? (2)
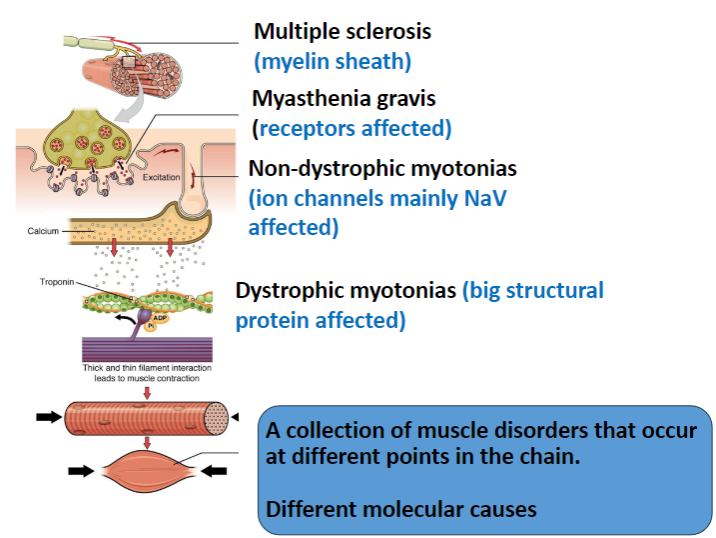
The nicotinic acetylcholine receptors (AChRs) in muscles are targeted.
The disease is autoimmune, where antibodies degrade these receptors, leading to muscle weakness.
-
Which ion channels are primarily affected in Non-Dystrophic Myotonias? (1)
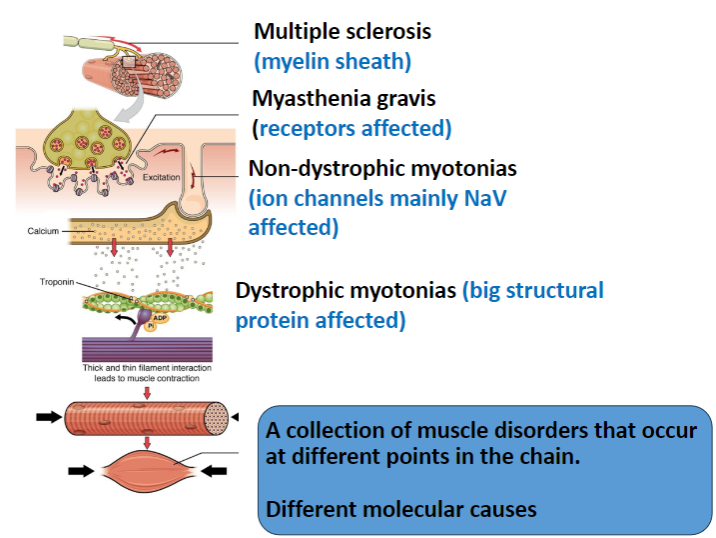
Voltage-gated sodium channels (NaV) are mainly affected, leading to delayed muscle relaxation.
-
What is affected in Dystrophic Myotonias and how does it impact muscle function? (2)
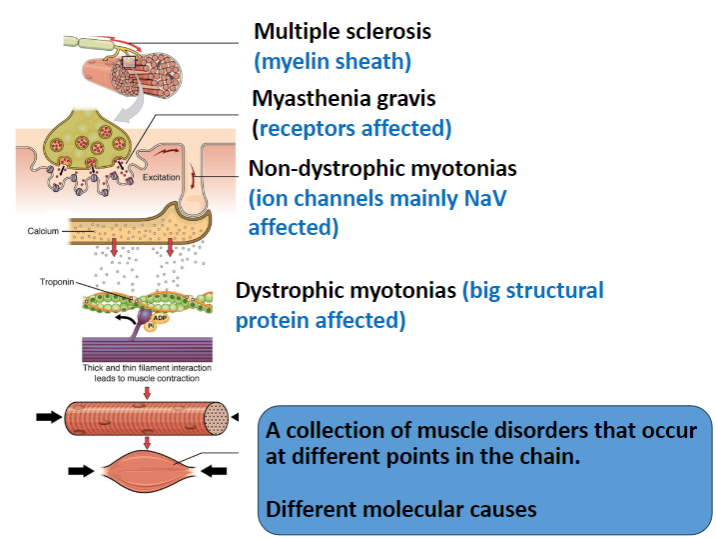
A large structural protein, dystrophin, is affected.
Dystrophin mutations lead to muscle weakness, and the structural integrity of muscle fibers is compromised.
-
How do the different muscle disorders (Multiple Sclerosis, Myasthenia Gravis, Non-Dystrophic Myotonias, Dystrophic Myotonias) differ in terms of their molecular causes? (4)
Multiple Sclerosis: Involves myelin sheath damage in the CNS.
Myasthenia Gravis: Involves autoimmune degradation of muscle nicotinic ACh receptors.
Non-Dystrophic Myotonias: Caused by mutations in ion channels (mainly NaV).
Dystrophic Myotonias: Caused by mutations in dystrophin, a structural protein.

