-
One of the major factor that determines what properties molecules have is their
polarity
-
the two important practical manifestations of polarity
solubility and miscibility.
-
refers to the ability of a solute to dissolve in a certain amount of solvent.
Solubility
-
is the ability of two liquids to mix in all proportions.
Miscibility
-
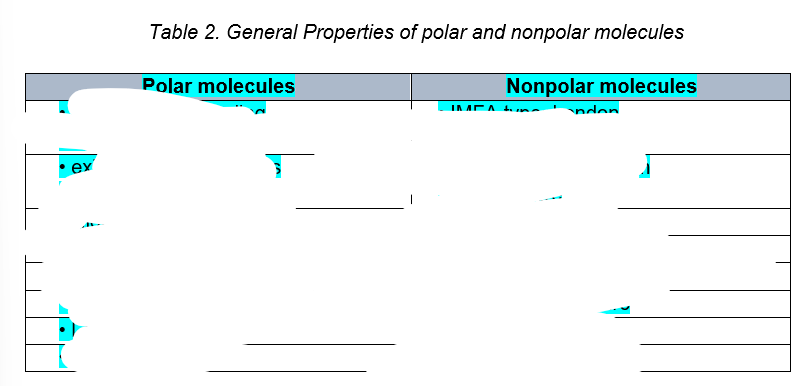
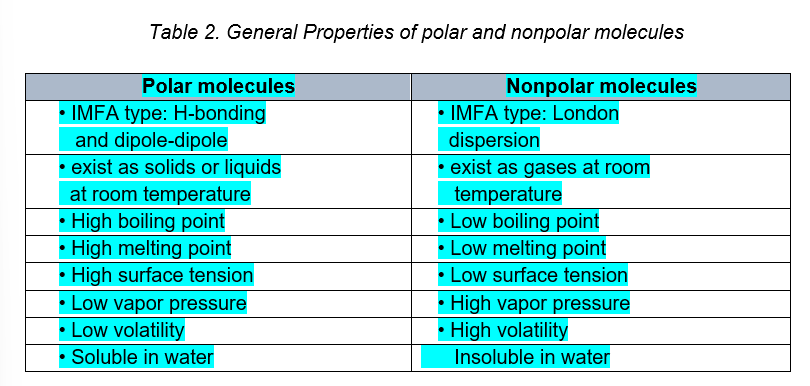
It is possible to determine the polar nature of various substances knowing that
like dissolves like
-
WILL DISSOLVE in WATER (a polar solvent),
Polar substances
-
WILL not DISSOLVE in WATER (a polar solvent),
non-polar solutes dissolve only in other non-polar materials.
-
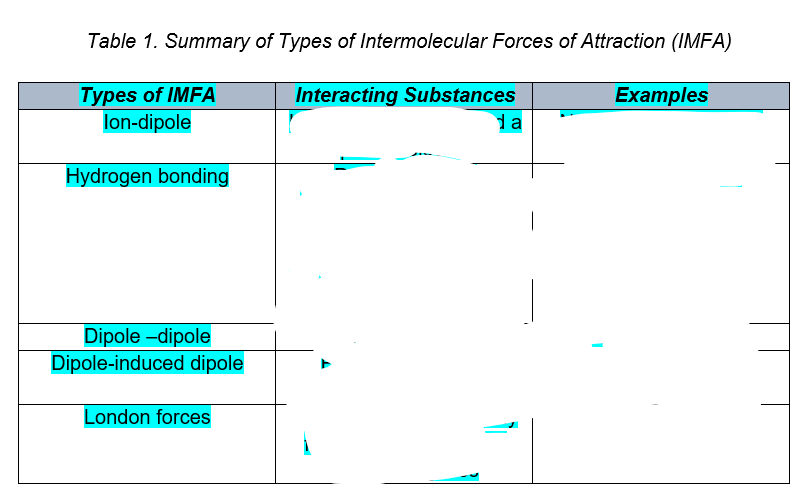
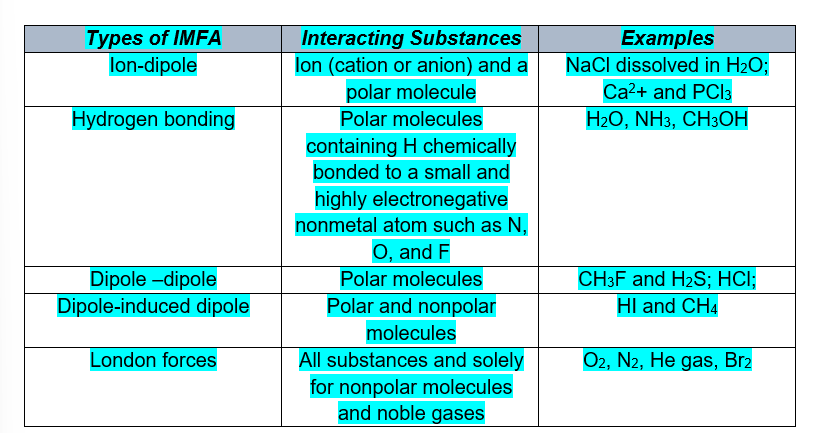
of IMFA and below they are arranged from STRONGEST to WEAKEST.
Ion-dipole → H-bonding → Dipole-dipole → Dipole-induced dipole → London forces of attraction
-
-
-


-
Ion-dipole interaction
Ion-dipole interaction happens when an ion meets a polar molecule. In this case, the ionic charge determines which part of the molecule attracts and which part repels. A cation or positive ion would be attracted to the negative part of a molecule and repelled by the positive part. An anion or negative ion would be attracted to the positive part of a molecule and repelled by the negative part.
Example: An example of the ion-dipole interaction is the interaction between a Na+ ion and water (H2O) where the sodium ion and oxygen atom are attracted to each other, while the sodium and hydrogen are repelled by each other.
-
Dipole-Dipole Interaction
Dipole-dipole interaction occurs whenever two polar molecules get near each other. The positively charged portion of one molecule is attracted to the negatively charged portion of another molecule. Since many molecules are polar, this is a common intermolecular force.
Example: An example of dipole-dipole interaction is the interaction between two sulfur dioxide (SO2) molecules, in which the sulfur atom of one molecule is attracted to the oxygen atoms of the other molecule.
-
Hydrogen Bond
The bond that exists between water molecules is Hydrogen bond (Fig.3). It is a special kind of dipole-dipole interaction between Hydrogen which is a polar molecule and a highly electronegative elements Fluorine, Oxygen and Nitrogen. In Hydrogen bond, the highly electronegative element F, O, N causes the hydrogen to become strongly positive.
The ability of water to form H-bond relates to its ability as a universal solvent. H-bond prevents the water from evaporating quickly into the atmosphere. It also causes ice to float in water since at freezing temperature, water molecules tend to form a crystal lattice as it expands.
-
London Dispersion Forces
It is present in all molecules. It is the weakest intermolecular force which is formed due to temporary dipoles of a non-polar molecule. The strength of the dispersion forces increases as the molecular weight of the substance increases. Think about an atom like argon. It’s an inert gas, right? But if you cool it to –186 °C, you can actually condense it into liquid argon. The fact that it forms a liquid it means that something is holding it together. That “something” is dispersion forces.

