
Protein breakdown and Urea Formation (The Living Cell)
The aim of this lecture is to describe the relationship between protein breakdown in the body (particularly in skeletal muscle) and the formation of urea in the liver. We will discuss the involvement of the urea cycle in the elimination of nitrogen from the body and the role of amino acids in the production of energy. The terms glucogenic and ketogenic amino acids will be defined. The regulation of cellular and whole body nitrogen balance by nutritional status, hormones and cytokines will be discussed in both health and disease. Why it is important: Clinical significance: effects of injury, burns, physiological stress, cancer cachexia, chronic inflammation, diabetes and starvation on protein metabolism. Learning Objectives Outline the sources of amino acids found in the circulation Describe the metabolic fate of amino acids Outline the importance of the urea cycle Describe the major steps in the metabolism of amino acids Discuss what is meant be nitrogen balance
-
A picture summary of all the cycles:
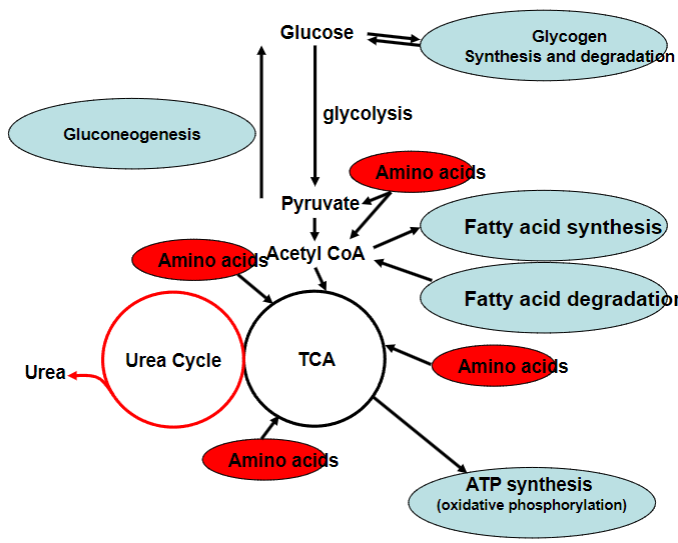
-
Picture demonstrating the various amino acid uses:
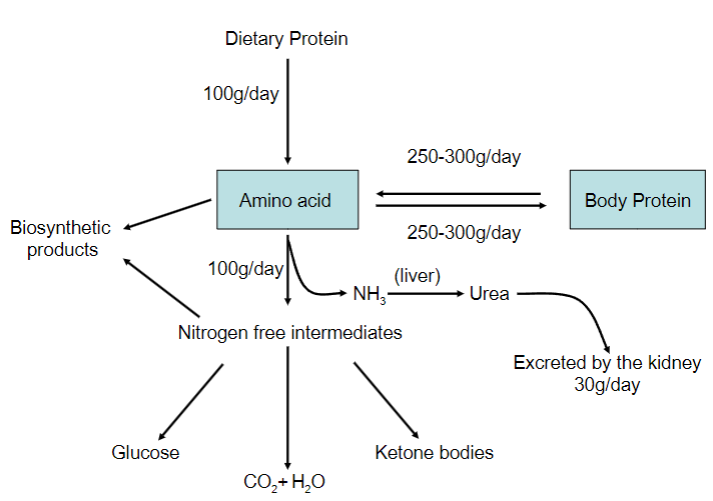
-
Picture demonstrating nitrogen balance:
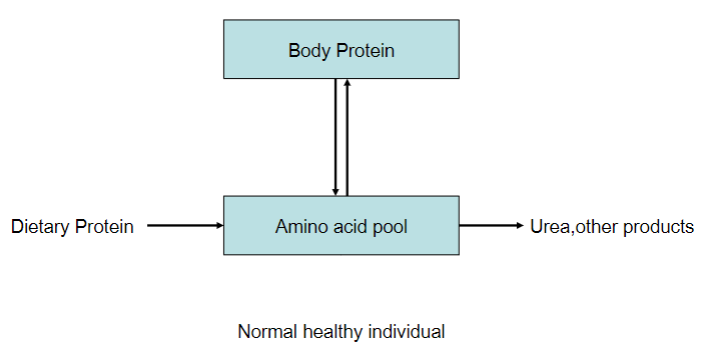
Balance of body protein
-
Picture demonstrating positive nitrogen balance:
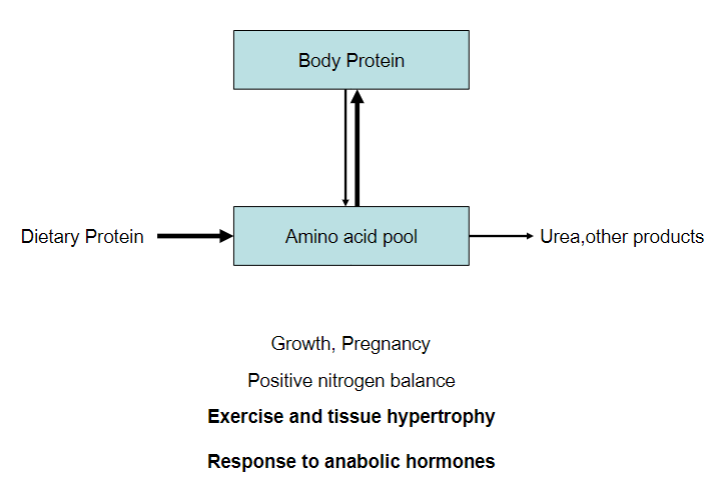
Increase of body protein
-
Picture demonstrating negative nitrogen balance:
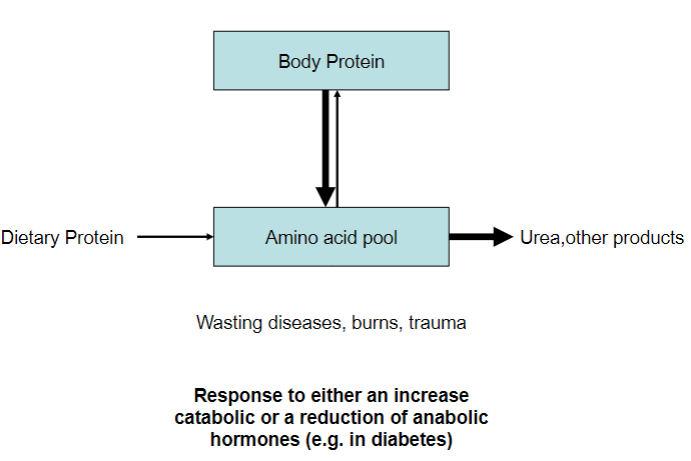
Decrease of body protein
-
Can amino acids be stored?
No
They are either used or broken down
-
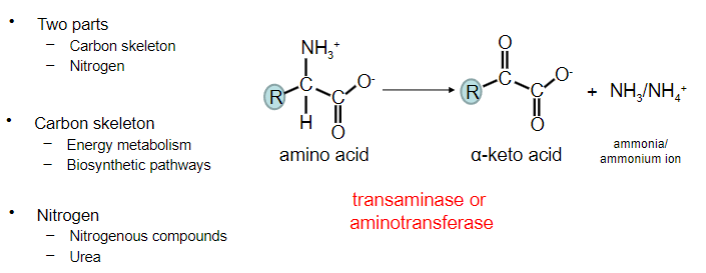
What is transamination?
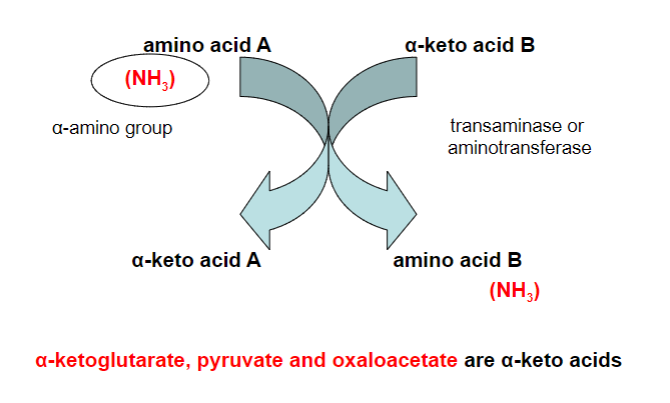
A metabolic process where an amino group (NH2) is transferred from one molecule, usually an amino acid, to another molecule, typically a keto acid. This exchange creates a new amino acid and a new keto acid
-
What is the enzyme that carries out transamination?
-Transaminase/aminotransferase
-
What are the TWO most important transaminase enzymes?
Alanine (ALT) and aspartate (AST) transaminase
-
Outline the enzyme action of Alanine (ALT) transaminase:
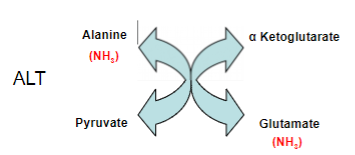
Alanine reacts with alpha Ketoglutarate to produce Pyruvate and glutamate
-
Outline the enzyme action of Aspartate (AST) transaminase:
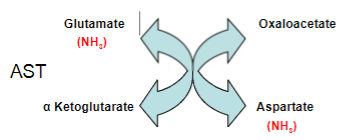
Aspartate reacts with alpha Ketoglutarate to produce oxaloacetate and Glutamate
-
In the liver OAA (Oxaloacetate) and α-ketoglutarate can be used to make what?
Glucose
-
In the muscle pyruvate can be used in the TCA cycle and ETC to make what?
ATP
-
High levels of AST and ALT in the blood are indicative of tissue damage, particularly what?
Liver and cardiac muscle
-
Picture outlining the formation of ammonia
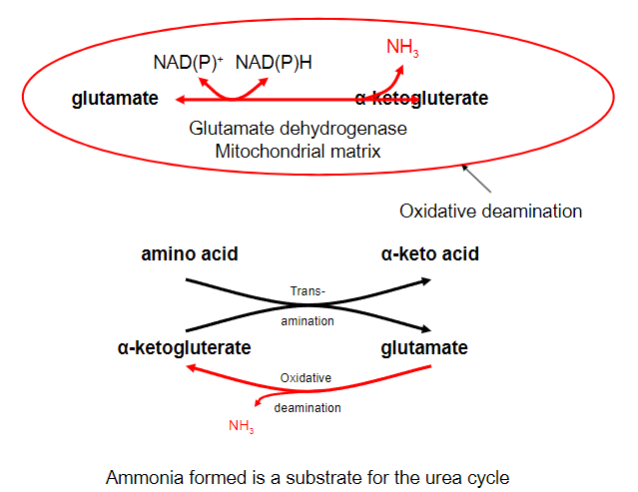
-
Free ammonia generated in non-hepatic tissue combines with glutamate to give what?
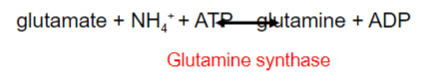
Glutamine
-Which is soluble and readily transported in the blood
-
How is nitrogen mainly transported?
By the amino acids alanine and glutamine
-
In mammals the ammonia is converted to what?
To the non-toxic neutral compound urea and excreted in the urine
-
Amino acid nitrogen is transferred to urea in three steps; What are these steps?
-Transamination
-Formation of ammonia
-Synthesis of urea
-
State some information about the Urea cycle: (4)
• The means of excreting nitrogen• Enzymes are present in the liver but not muscle• Takes place in the mitochondria and the cytoplasm• Substrates are bicarbonate, aspartate and ammonium ions (released from glutamine or glutamate)
-
How many amino groups does the formation of urea require?
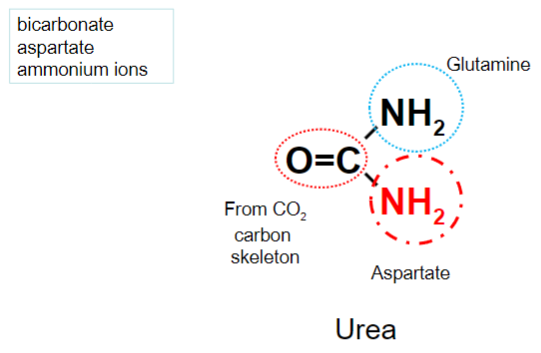
Two
-
Outline the steps in the Urea cycle:
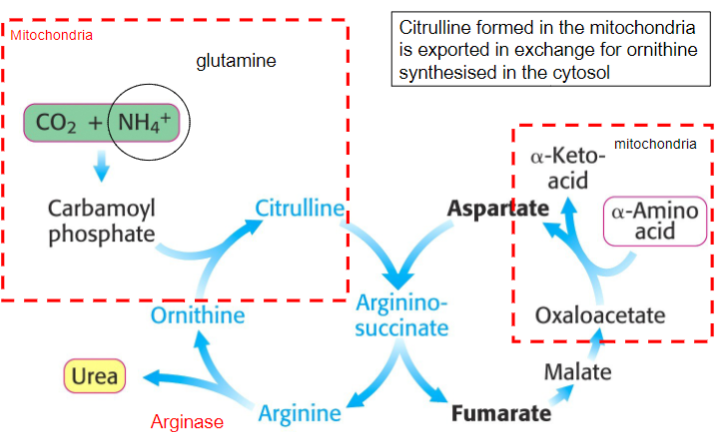
-
State some information about the muscle in this context:
• Enzymes of the urea cycle not present• In prolonged exercise or starvation, branched aminoacids are used for energy (leucine, isoleucine and valine)• Two routes are used to transport nitrogen to the liver– Alanine and glutamine
-
State the steps in the glucose alanine cycle:
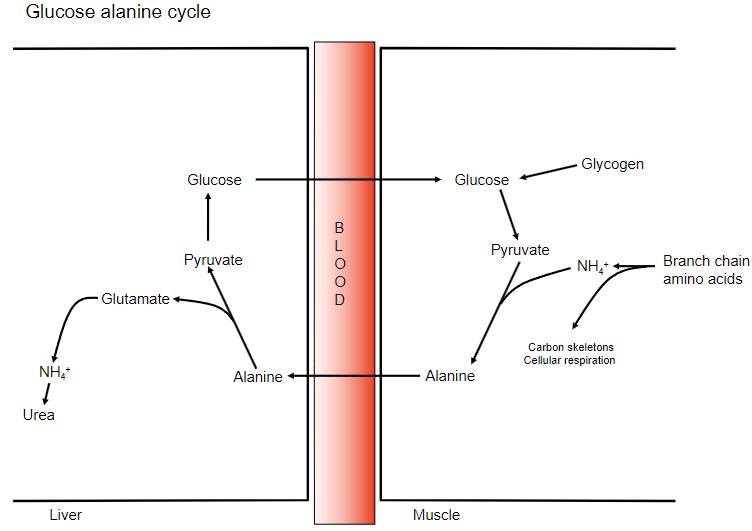
-
State the steps of ammonia transport from the peripheral tissue to the Liver:
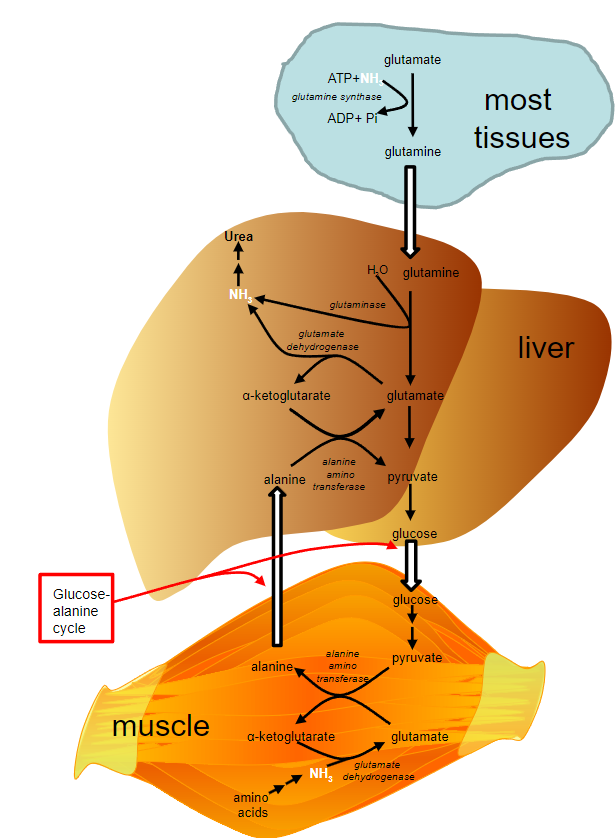
-
Picture outlining the fate of the carbon skeleton:
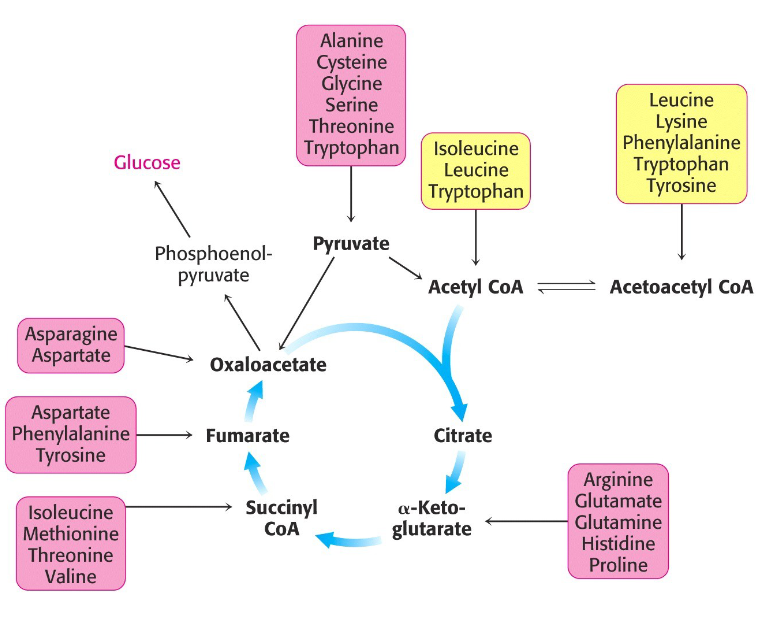
-
A general summary:
• Proteins/Amino acids are not stored• Excess proteins/amino acids are broken down to ammonia and a carbon skeleton• Ammonia is toxic so has to be removed as urea• Removal requires transamination and the urea cycle• Carbon skeletons can be used for the production of glucose, ketone bodies and/or energy

