-
Benzyl
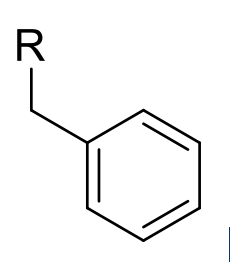
-
t-butyl

-
Isopropyl

-
low bp, low water solubility
In general, ethers have ____ & _____
-
aldehydes & ketones
Which functional groups are polarized and have permanent dipole?
-
Keto - enol tautomerism
-ketones that exist in equilibrium with the "enol" form:
-predominant form is keto
-
ester; prodrugs
Hydrolysis can be beneficial for which functional group and what can they produce?
-
soluble
Are amides soluble or insoluble?
-
neutral
Amides are ____ functional groups and cannot accept or donate protons
-
relatively stable
amides are _______ to acids and bases that will be encountered by the pharmacist.
-
Ureas & Carbamates
-have similar properties to esters and amides
-more chemically stable towards hydrolysis than the corresponding esters
-
Thioethers
-neutral (no acid-base properties)
-found in several different classes of drugs
-
Sulfoxides & Sulfones
-found in several drugs
-found in metabolites of thioether-containing drugs
-
Sulfonic acids
-strong acids
-highly soluble in water
-
sulfonamides & sulfonylureas
Which two important drug classes are sulfonic acids used to make?
-
Sulfonamides
-poor water solubility (alone!!!)
-similar chemical stability to amides
-weak acid- can form salts with strong bases ( salts are highly water soluble!!!)
-
Sulfonylureas
-considered an entire class of medicine in themselves
-weak acids
-
aniline
-its aromatic right has a delocalized cloud of electrons; serves as powerful electron withdrawing group
-decreases basicity enormously!!
-
acidic, not
Phenols are ____ whereas alcohols are

