Neurotransmitters Systems I: Glutamate (Physiology 2)
Neurotransmitters Systems I: Glutamate - Dr Dean Semmens Lecture Outline Welcome to the Glutamate lecture. Glutamate is the major excitatory neurotransmitter in the central nervous system, accounting for a majority of the brain’s synaptic connections. Glutamate therefore mediates the majority of functions within the brain, and achieves this by action at a number of ionotropic and metabotropic receptors. In this session, you will be introduced to these different receptors, learning about their synaptic localisations and mechanisms of action, and their involvement in health and disease. Desired Learning Outcomes By the end of this lecture unit, students should be able to: Describe how glutamate is synthesised, stored and degraded in the brain Detail the location and signalling pathways of glutamate receptors Describe the consequences of glutamate signalling in the brain Describe synaptic plasticity and long-term potentiation (LTP) Session Resources You can find the full set of PowerPoint slides for the lecture HERE Download HERE. You can find a Kahoot! quiz for the lecture HERELinks to an external site.. Session Activities Activity 1. Glutamate Synthesis, Storage and Degradation In this video, we will discuss the synthesis, storage and degradation of the neurotransmitter glutamate. At the end of the video, you will find a short "test your knowledge" activity. Activity 2. Glutamate Receptors In this video, we will discuss the different classes of glutamate receptor. At the end of the video, you will find a short "test your knowledge" activity. Activity 3. Glutamate Signalling in the Brain In this video, we will discuss the consequences of glutamate signalling in the brain and will also discuss glutamates role in the process of long-term potentiation (LTP): Activity 4. Kahoot Quiz In order to test your knowledge at the end of the lecture, you can find a Kahoot! quiz for the lecture HERELinks to an external site.. Further Reading If you would like to read further or consolidate your knowledge on the topic, I would recommend the following further reading: Neuroscience 5th Edition (Purves) Chapter 6 (Glutamate) and Chapter 8 (LTP). Neuroscience: Exploring the Brain 3rd or 4th Edition (Bear, Connors, Paradiso) Chapter 6. Any Questions? If you have any questions on the lecture, please feel free to add it to the Padlet discussion board below. Alternatively, please do me email me (dsemmens@sgul.ac.uk) and I'll be very happy to help. I have left some student questions from last year on the Padelt discussion board, just incase this may help with any similar questions that you may have: Made with Padlet Glossary Glutamate – the major excitatory neurotransmitter in the CNS Glutamine – Amino acid precursor of glutamate Phosphate-activated glutaminase – Enzyme that converts glutamine to glutamate Vesicular glutamate transporter (VGLUT) – Vesicular membrane transporter that mediates glutamate accumulation in presynaptic vesicles (counter-transported with H+) AMPA receptor – A ligand-gated ionotropic glutamate receptor subtype selective to the synthetic molecule AMPA NMDA receptor – A ligand- and voltage-gated ionotropic glutamate receptor subtype selective to the synthetic molecule NMDA Kainate receptor – A ligand-gated ionotropic glutamate receptor subtype selective to the synthetic molecule kainite Long-term potentiation (LTP) - A persistent increase in synaptic strength following high-frequency stimulation of a chemical synapse. Studies of LTP are often carried out in slices of the hippocampus, an important organ for learning and memory. Excitatory amino acid transporter (EAAT) – A membrane transporter that mediates glutamate accumulation in the presynaptic bouton from the presynaptic cleft Excitotoxicity - The pathological process by which neurons are damaged and killed by the overactivations of the NMDA and AMPA receptors.
-
What is glutamate and its role in neurotransmission? (2)
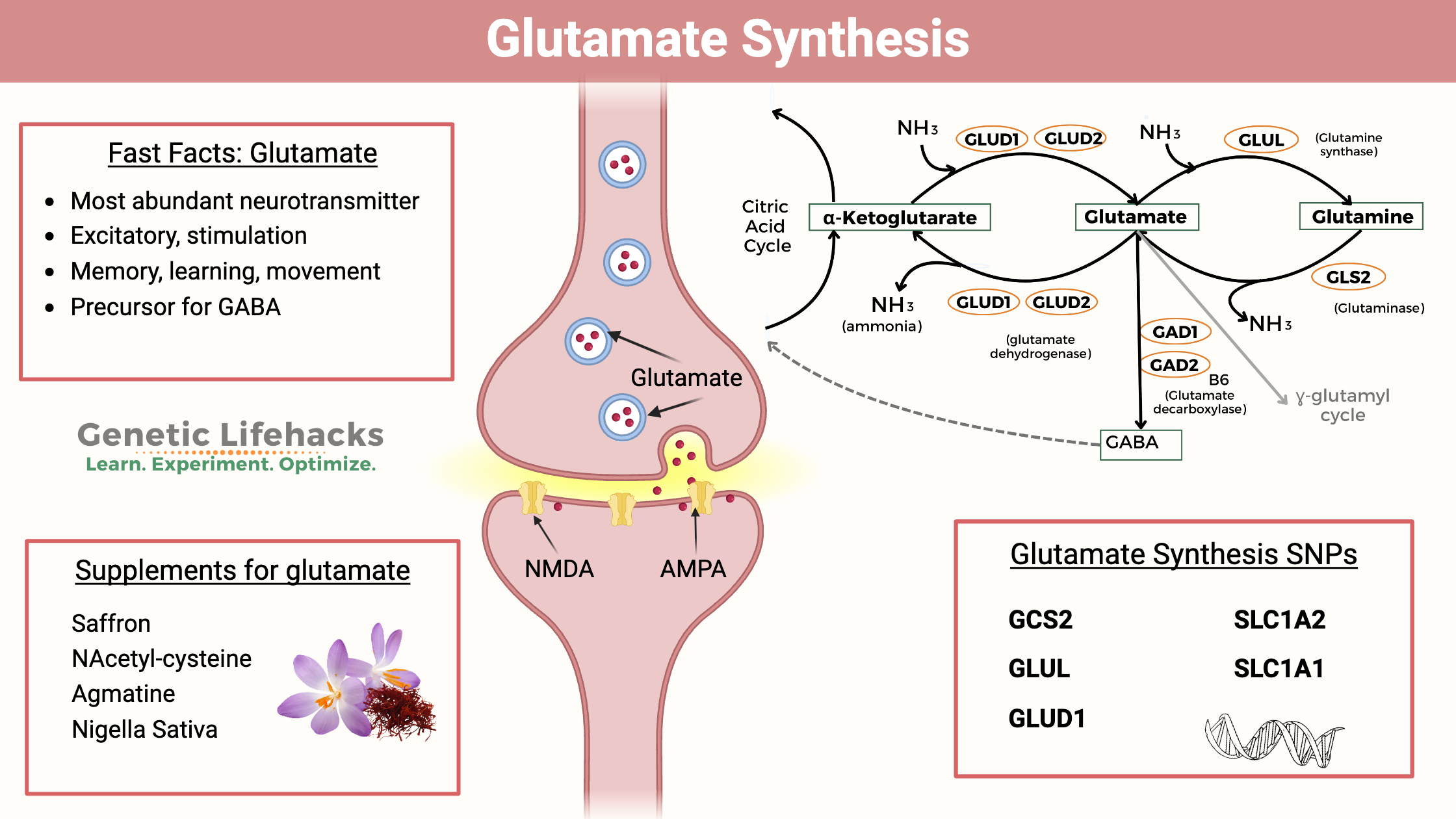
Glutamate is an amino acid and the primary excitatory neurotransmitter in the brain.
It plays a central role in neurotransmission, driving information transfer between neurons and their targets.
-
Describe the synthesis of glutamate. (2)
Glutamate is synthesized primarily from glutamine by the enzyme glutaminase in the presynaptic terminal.
It can also be synthesized from α-ketoglutarate, an intermediate of the citric acid cycle.
-
What is the process of glutamate storage? (2)
Once synthesized, glutamate is stored in synaptic vesicles within the presynaptic neuron.
Vesicular glutamate transporters (VGLUTs) transport glutamate into the vesicles for later release.
-
How is glutamate released during neurotransmission? (2)
Glutamate is released from the presynaptic neuron into the synaptic cleft in response to an action potential.
This release occurs via exocytosis when synaptic vesicles fuse with the presynaptic membrane.
-
How is glutamate removed from the synaptic cleft? (2)
After its release, glutamate is cleared from the synaptic cleft by excitatory amino acid transporters (EAATs) located on both the presynaptic neuron and surrounding glial cells.
In glial cells, glutamate is converted into glutamine by glutamine synthetase and then transported back to the presynaptic neuron for reuse.
-
What is the process of glutamate degradation? (2)
Glutamate is primarily degraded by glutamine synthetase in glial cells, converting it into glutamine.
Glutamine is then transported back to neurons, where it can be converted back into glutamate
-
What is neurotransmission and its role in the nervous system? (2)
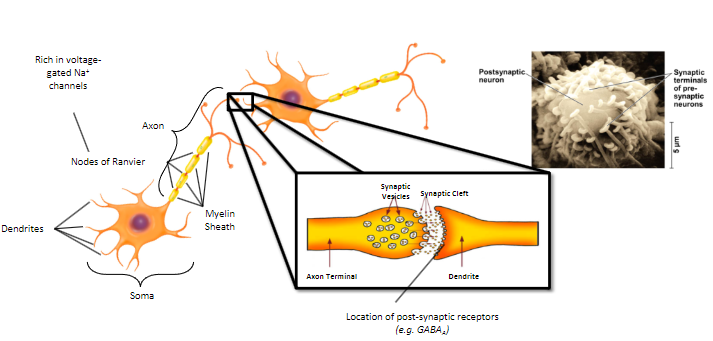
Neurotransmission is the fundamental process that enables the transfer of information between neurons and their targets, such as other neurons, muscles, or glands.
It involves the release, reception, and signaling of neurotransmitters, which allow communication within the nervous system.
-
What are the criteria for a neurotransmitter? (4)
Synthesis and storage: The molecule must be synthesised and stored in the pre-synaptic neuron.
Release: The molecule must be released by the pre-synaptic axon terminal upon stimulation.
Post-synaptic response: The molecule must produce a response in the post-synaptic cell.
Packaging and binding: The neurotransmitter is packaged into vesicles, released when the vesicles fuse, and binds to and activates post-synaptic receptors.
-
How can neurons be classified based on neurotransmitter? (6)
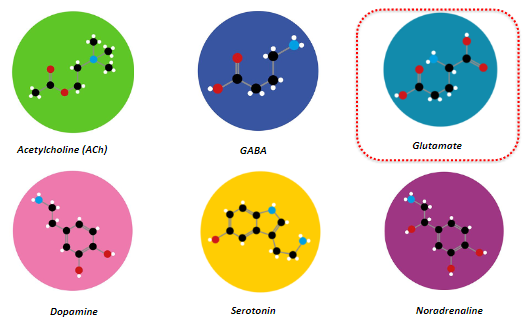
Neurons can be classified by the neurotransmitter they use, which arises from the differential expression of proteins involved in neurotransmitter synthesis, storage, and release.
GABA
Dopamine
Serotonin
Acetylcholine (ACh)
Noradrenaline
Glutamate
-
What is glutamate and its role in the CNS? (5)
Glutamate is the major excitatory neurotransmitter in the central nervous system (CNS).
It took a long time to realize glutamate was a neurotransmitter due to its involvement in multiple metabolic pathways.
The excitatory role of glutamate in the brain and spinal cord was discovered in the 1950s.
Nearly all excitatory neurons in the CNS are glutamatergic, and over half of all brain synapses release glutamate.
It became widely recognized as the principal excitatory neurotransmitter in the CNS by the late 1970s.
-
How is glutamate synthesized and stored? (5)
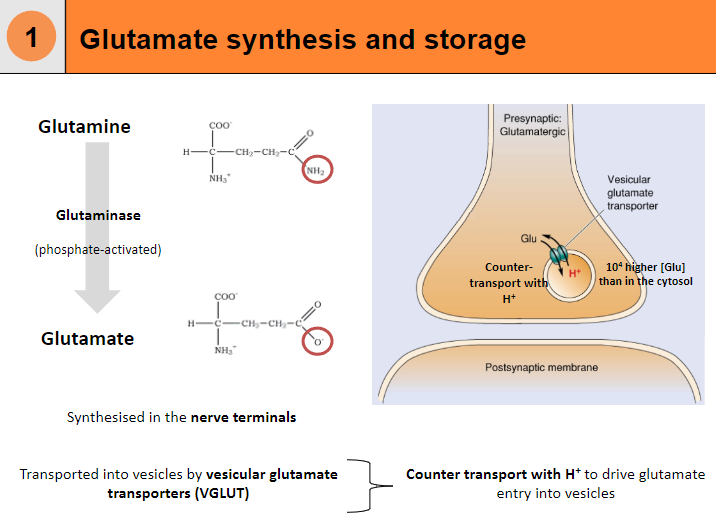
Glutamate is synthesized from glutamine in the nerve terminals.
Glutamine is converted into glutamate by the enzyme glutaminase, which is phosphate-activated.
The synthesized glutamate is then transported into vesicles by vesicular glutamate transporters (VGLUT).
Vesicular transport of glutamate involves counter-transport with protons (H+).
The process results in a higher concentration of glutamate in the vesicles than in the cytosol.
-
State the processes of Glutamate Re-uptake and Degradation (5)
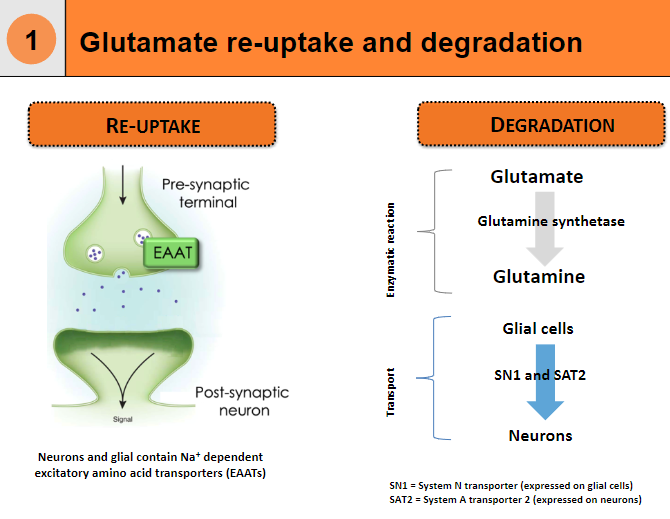
Re-uptake:
Neurons and glial cells contain Na+ dependent excitatory amino acid transporters (EAATs) to re-uptake glutamate.
Glutamate is converted back into glutamine by the enzyme glutamine synthetase.
Degradation:
Neurons: Glutamate is transported by System N transporter (SN1) and System A transporter 2 (SAT2).
Glial Cells: Enzymatic reactions help in the degradation of glutamate, converting it into glutamine.
SN1 is expressed on glial cells, while SAT2 is expressed on neurons.
-
A picture demonstrating A summary of glutamate synthesis, storage and re-uptake:
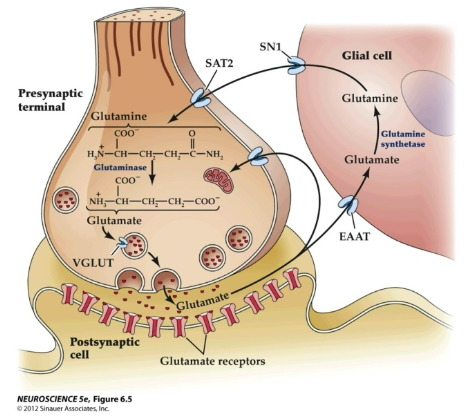
-
What are the two main types of neurotransmitter receptors, and how do they differ in their mechanism of action? (2)
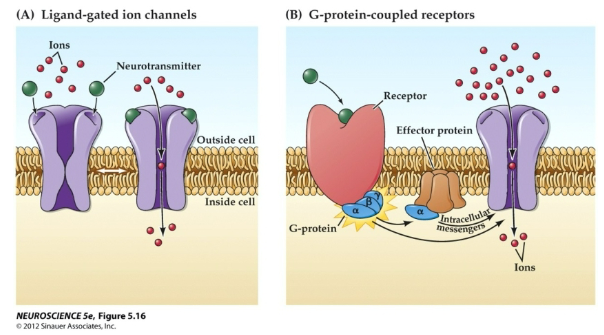
Ionotropic Receptors (Ligand-Gated Ion Channels):
These receptors directly control the opening of ion channels when a neurotransmitter binds, leading to a rapid change in membrane potential.
Metabotropic Receptors (G-Protein Coupled Receptors):
These receptors work through intracellular signaling pathways involving G-proteins, leading to slower, longer-lasting effects on the cell.
-
What are the types of glutamate receptors, and how do they differ in function and signaling mechanisms? (6)
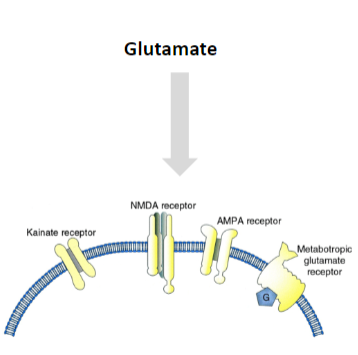
Ionotropic Receptors:
AMPA Receptors: Mediate fast excitatory synaptic transmission.
NMDA Receptors: Involved in synaptic plasticity and memory function, requiring both ligand binding and membrane depolarization.
Kainate Receptors: Involved in excitatory neurotransmission, but less understood compared to AMPA and NMDA receptors.
Metabotropic Receptors:
Group I: Typically activate phospholipase C, leading to increased intracellular calcium and activation of protein kinase C.
Group II: Inhibit adenylate cyclase, reducing cAMP levels and decreasing excitability.
Group III: Also inhibit adenylate cyclase and modulate neurotransmitter release.
-
What are AMPA receptors and their function? (4)
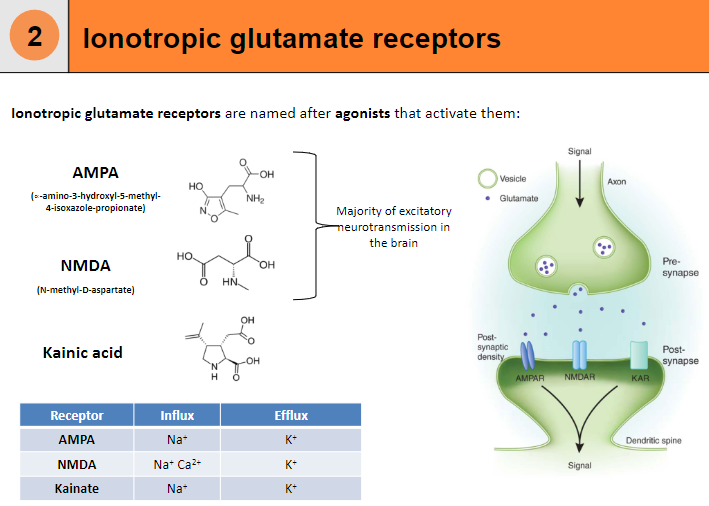
Influx: Na+
Efflux: K+
Agonist: α-amino-3-hydroxy-5-methyl-4-isoxazole-propionate (AMPA)
Function: Responsible for fast excitatory neurotransmission in the brain.
-
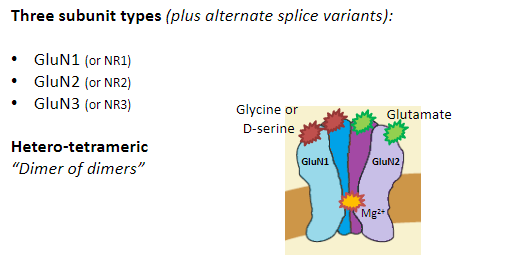
What are NMDA receptors and their function? (5)
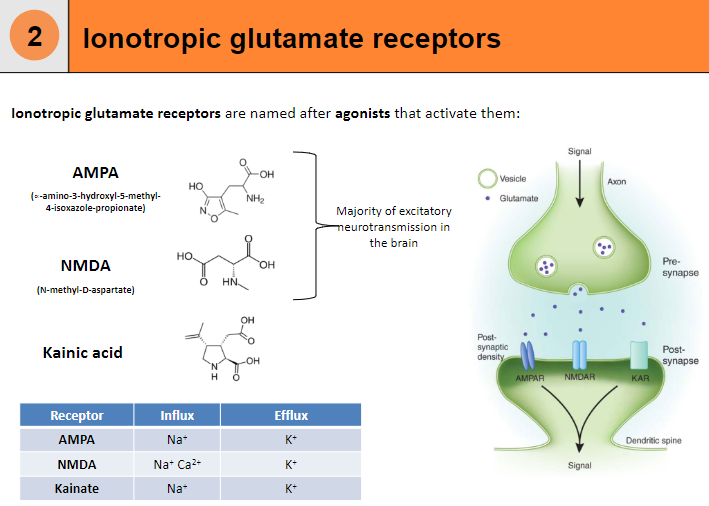
Influx: Na+, Ca²⁺
Efflux: K+
Agonist: N-methyl-D-aspartate (NMDA)
Function: Involved in synaptic plasticity, learning, and memory.
Requires: Both ligand binding and membrane depolarization to open.
-
What are Kainate receptors and their function? (4)
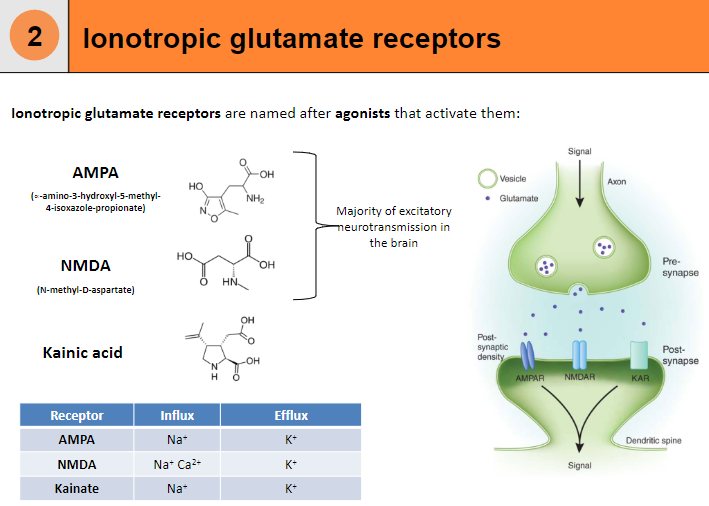
Influx: Na+
Efflux: K+
Agonist: Kainic acid
Function: Modulates excitatory neurotransmission, although less understood compared to AMPA and NMDA receptors.
-
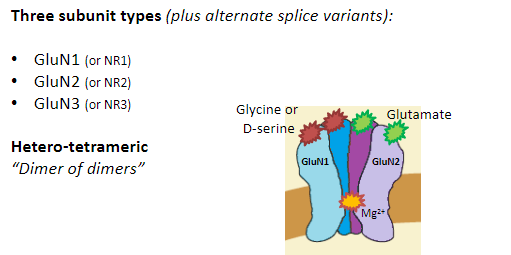
What are the four subunit types of AMPA receptors? (4)
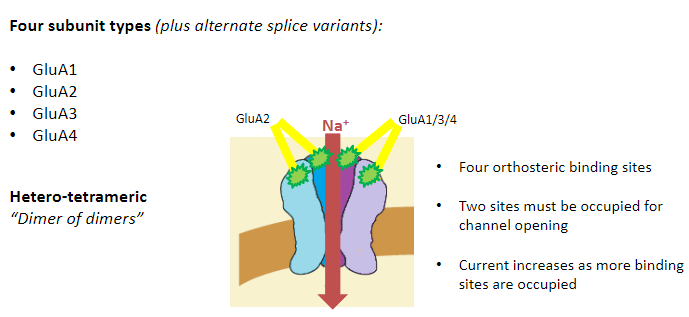
GluA1
GluA2
GluA3
GluA4
-
What is the structure of AMPA receptors? (3)
Hetero-tetrameric
“Dimer of dimers”
Most commonly:
2 GluA2 subunits
2 GluA1, 3, or 4 subunits
-
How do binding sites function in AMPA receptors? (3)
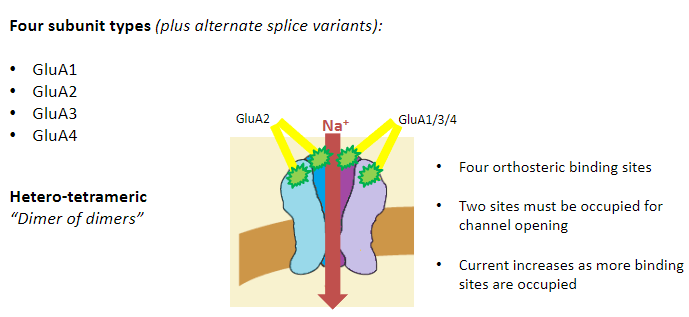
Four orthosteric binding sites
Two sites must be occupied for channel opening
Current increases as more binding sites are occupied
-
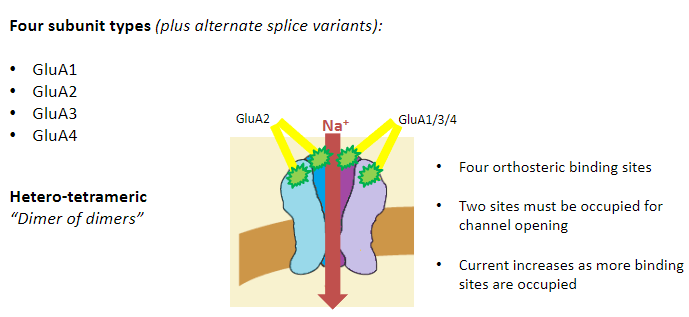
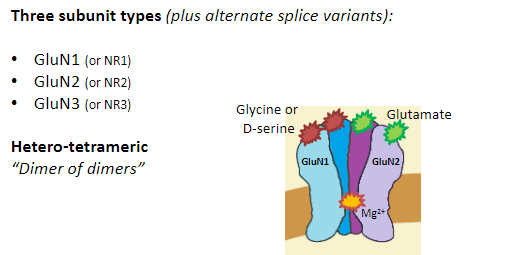
What are the four subunit types of AMPA receptors? (4)
GluA1
GluA2
GluA3
GluA4
-
What is the structure of AMPA receptors? (3)
Hetero-tetrameric
“Dimer of dimers”
Most commonly:
2 GluA2 subunits
2 GluA1, 3, or 4 subunits
-
What is the significance of GluA2 subunits in AMPA receptors? (2)
Presence of GluA2 subunits prevents Ca²⁺ flow
Protective against excitotoxicity
-
What is the general function of kainate receptors? (2)
Ligand-gated ion channel
Glutamate binding required for channel opening (not particularly well understood)
-
What is the structure of kainate receptors? (3)
Tetrameric
GluK1-3 can form homomers or heteromers
GluK4 & 5 only form heteromers with GluK1-3 subunits
-
What are the five subunit types of kainate receptors? (5)
GluK1 (GluR5)
GluK2 (GluR6)
GluK3 (GluR7)
GluK4 (KA1)
GluK5 (KA2)
-
What is the distribution of kainate receptors in the brain compared to AMPA/NMDA receptors? (1)
Limited distribution in the brain compared to AMPA/NMDA receptors
-
What are the 8 subtypes of metabotropic glutamate receptors? (8)
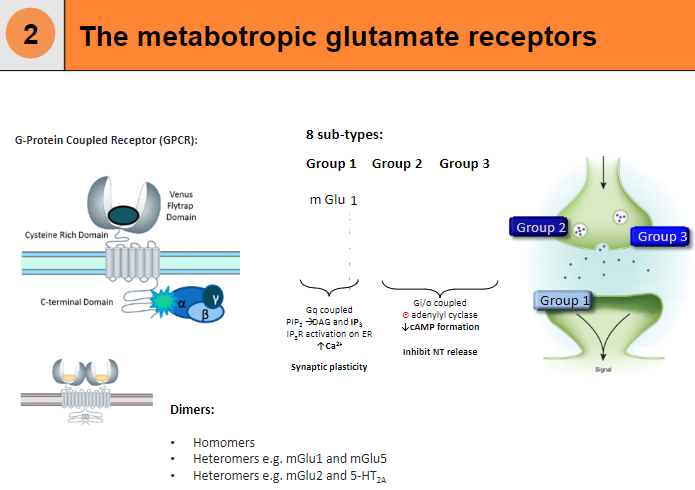
mGlu1
mGlu2
mGlu3
mGlu4
mGlu5
mGlu6
mGlu7
mGlu8
-
What is the structure and function of metabotropic glutamate receptors? (4)
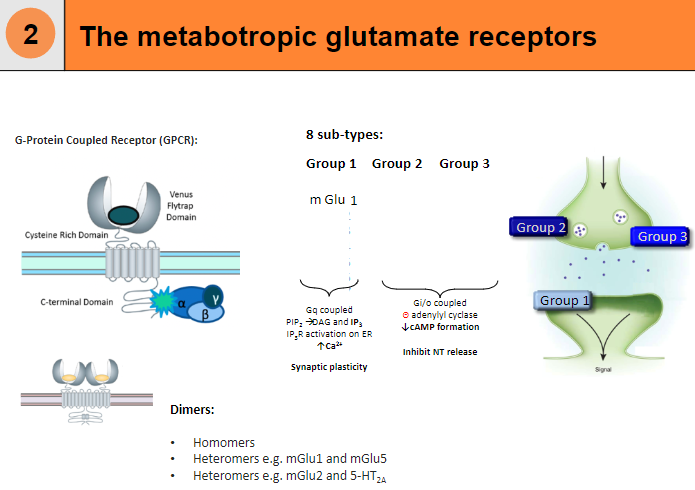
G-Protein Coupled Receptor (GPCR)
Can form dimers:
Homomers
Heteromers (e.g. mGlu1 and mGlu5)
Heteromers (e.g. mGlu2 and 5-HT2A)
Gi/o coupled, leading to a decrease in cAMP formation and inhibition of neurotransmitter release
-
What is the role of metabotropic glutamate receptor mGlu8? (5)
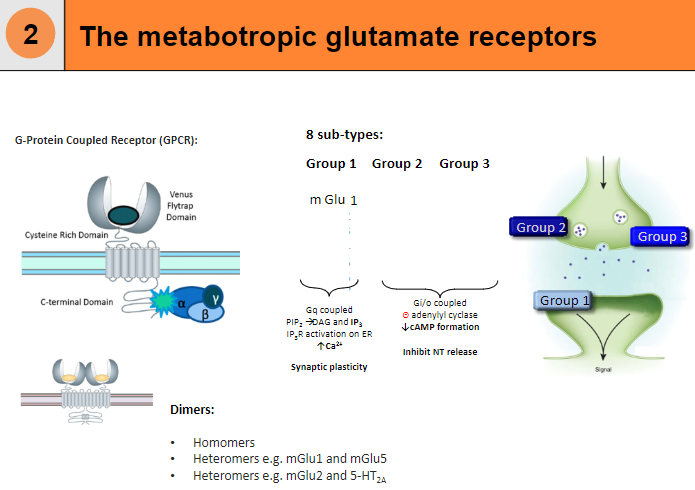
Gq-coupled receptor: Activates the phosphoinositide signaling pathway.
Key pathway steps: Stimulates the hydrolysis of PIP2 into DAG and IP3.
DAG: Activates protein kinase C (PKC).
IP3: Binds to IP3 receptors (IP3R) on the endoplasmic reticulum (ER), triggering the release of calcium ions (Ca²⁺) into the cytoplasm.
Function: Plays a role in modulating synaptic plasticity and intracellular signaling.
-
What is the role of excitatory neurotransmitters in depolarization? (3)
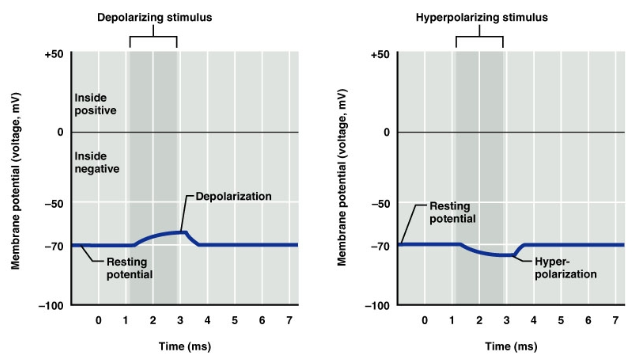
Excitatory neurotransmitters (e.g., glutamate) can lead to neuronal membrane depolarization
This involves the displacement of a membrane potential
The displacement moves the membrane potential towards a more positive value
-
What are EPSCs and their role in generating EPSPs? (3)
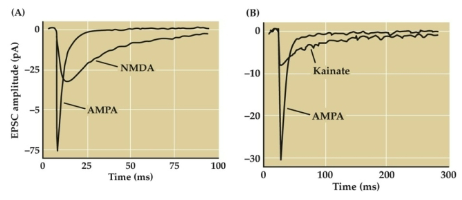
EPSCs lead to the generation of excitatory post-synaptic potentials (EPSPs)
EPSPs increase the likelihood of firing an action potential
EPSCs represent the flow of ions and the change in current across a post-synaptic membrane
-
What is the role of AMPA, NMDA, and kainate receptors in excitatory neurotransmission? (3)
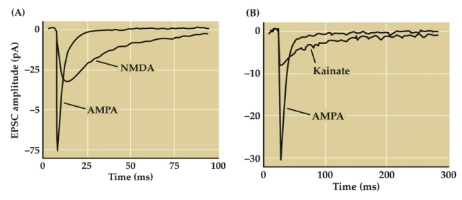
AMPA receptors are the primary mediators of excitatory neurotransmission in the brain
EPSCs (Excitatory Postsynaptic Current) produced by NMDA and kainate receptors are slower and last longer than those produced by AMPA receptors
The EPSC produced by AMPA receptors is faster and more transient
-
What is excitotoxicity and how does it lead to neuronal damage and death? (3)
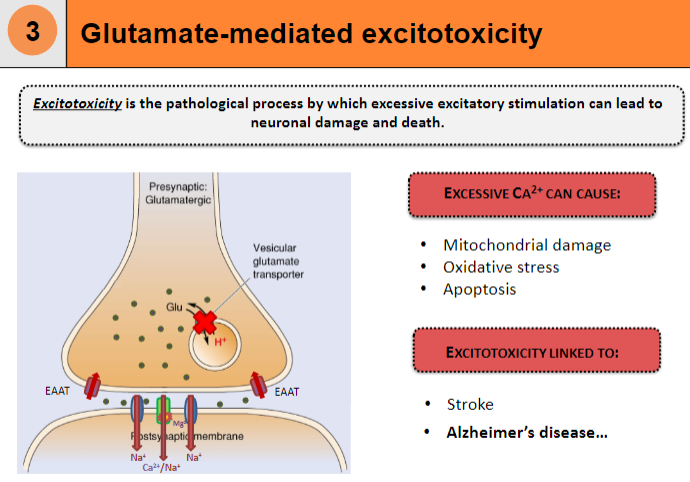
Excitotoxicity is the pathological process by which excessive excitatory stimulation leads to neuronal damage and death
Causes mitochondrial damage
Leads to oxidative stress and apoptosis
-
How does excessive Ca²⁺ contribute to excitotoxicity? (2)
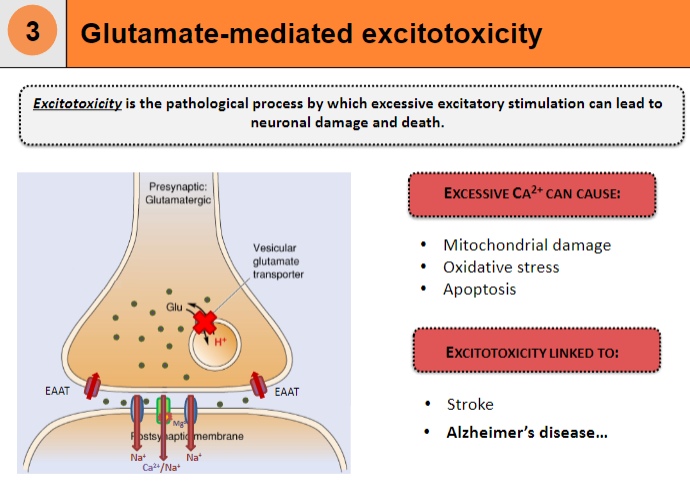
Excessive Ca²⁺ can cause neuronal damage
This is a key factor in conditions like stroke
-
What diseases are linked to excitotoxicity? (2)
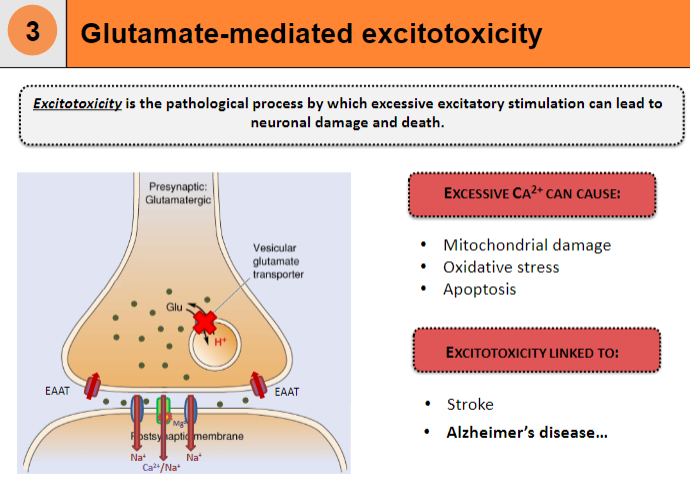
Excitotoxicity is linked to Alzheimer's disease
It is also implicated in other neurodegenerative diseases
-
What is memantine and how does it affect glutamate-mediated neurotoxicity? (2)
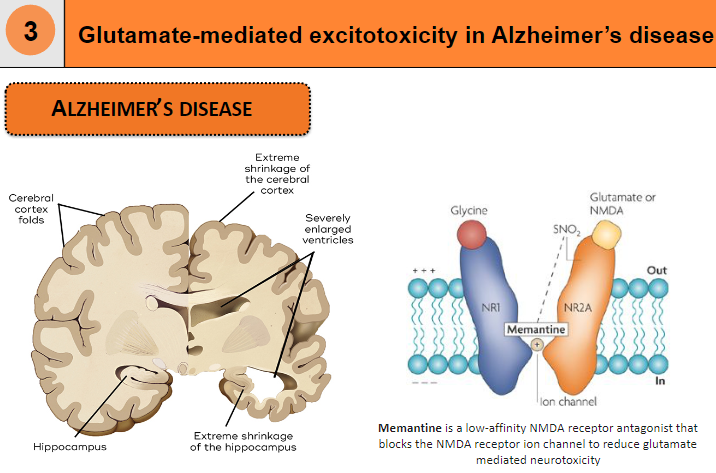
Memantine is a low-affinity NMDA receptor antagonist
It blocks the NMDA receptor ion channel to reduce glutamate-mediated neurotoxicity
-
How is glutamate-mediated excitotoxicity related to Alzheimer's disease? (1)
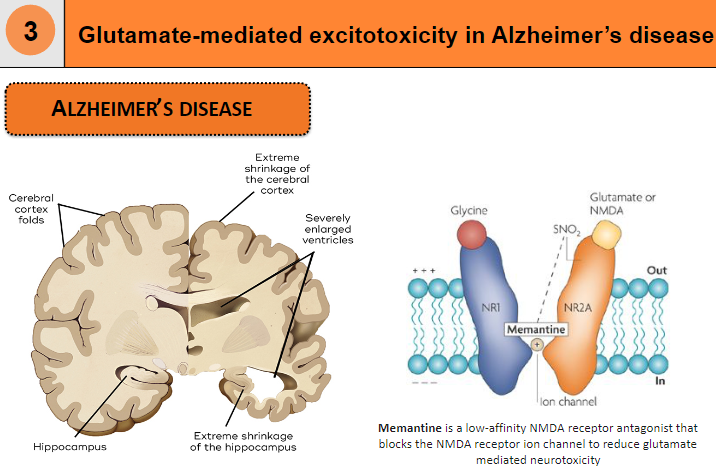
Glutamate-mediated excitotoxicity plays a role in the neurodegeneration seen in Alzheimer's disease
-
What is long-term potentiation (LTP)? (2)
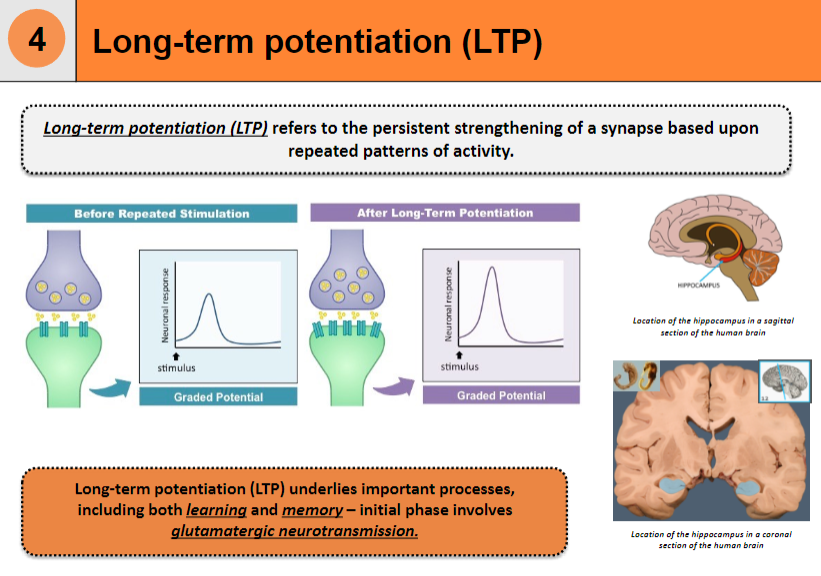
Long-term potentiation (LTP) refers to the persistent strengthening of a synapse based on repeated patterns of activity.
It is a key mechanism underlying learning and memory.
-
How does glutamatergic neurotransmission contribute to long-term potentiation (LTP)? (1)
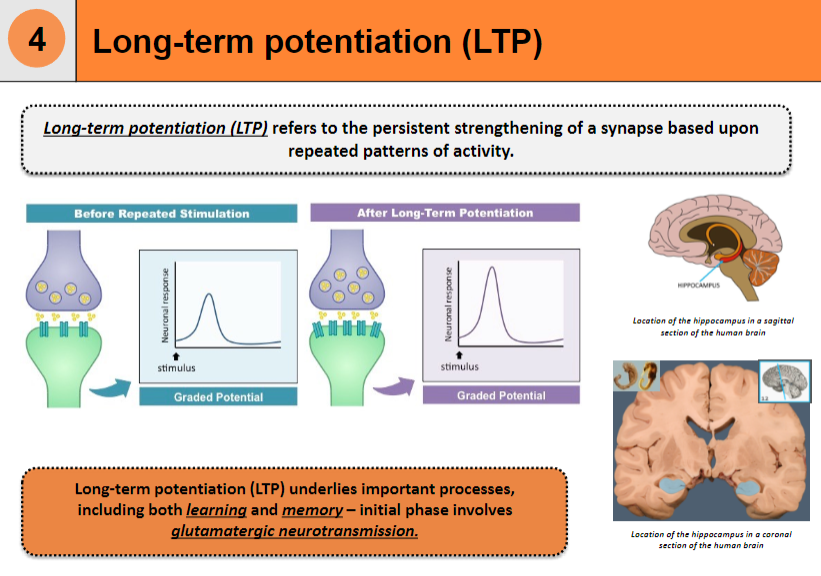
The initial phase of LTP involves glutamatergic neurotransmission, which plays a role in the synaptic strengthening.
-
Where is the hippocampus located in the human brain? (1)
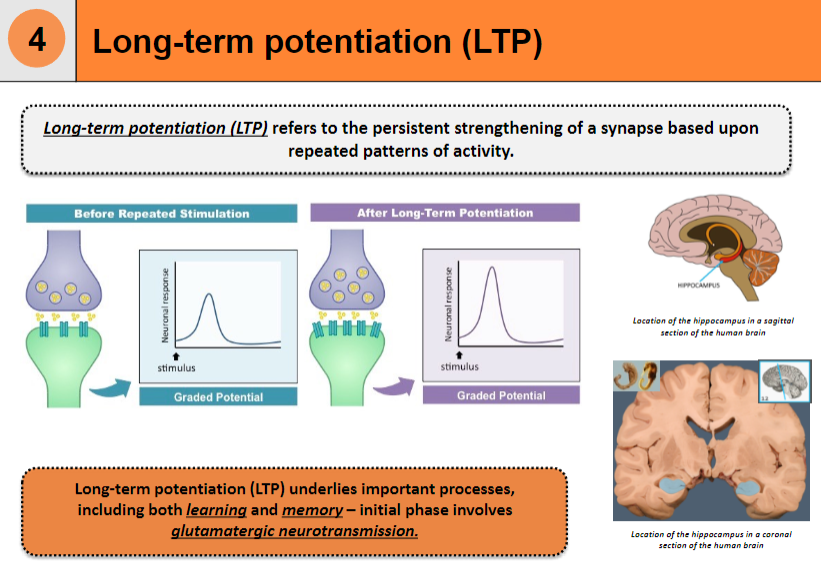
Beneath the cerebral cortex, in the medial temporal lobe of the brain.
-
What happens when glutamate activates AMPA receptors during long-term potentiation (LTP)? (2)
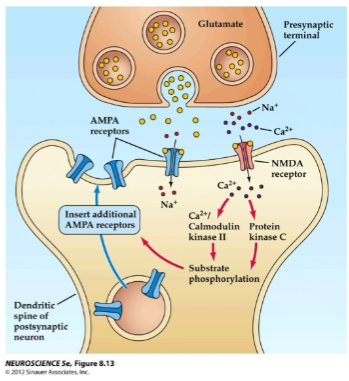
Glutamate activates AMPA receptors, allowing Na+ to flow into the post-synaptic neuron.
This causes depolarization of the post-synaptic membrane.
-
How do NMDA receptors contribute to long-term potentiation (LTP)? (2)
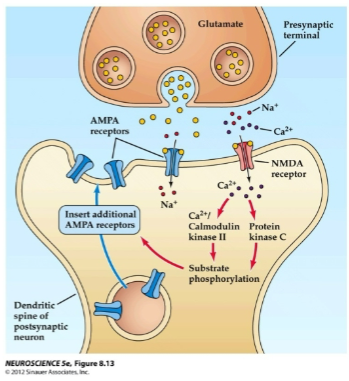
NMDA receptors open after depolarization removes the voltage-gated Mg²⁺ ion block.
This allows Ca²⁺ ions to enter the cell.
-
What happens when Ca²⁺ ions enter the post-synaptic cell during long-term potentiation (LTP)? (2)
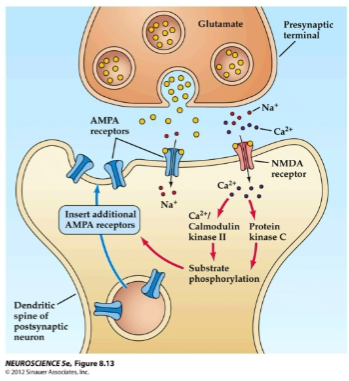
Ca²⁺ ions activate post-synaptic protein kinases such as calmodulin kinase II (CaMKII) and protein kinase C (PKC).
CaMKII and PKC trigger a series of reactions that lead to the insertion of new AMPA receptors into the post-synaptic membrane.
-
How do AMPA receptors affect the post-synaptic membrane during long-term potentiation (LTP)? (2)
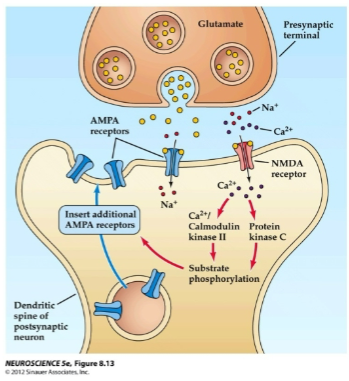
The insertion of new AMPA receptors increases the post-synaptic membrane’s sensitivity to glutamate.
This enhances ion channel conductance, contributing to the strengthening of the synapse.
-
What is glutamate and what is its role in the central nervous system (CNS)? (1)
Glutamate is the major excitatory neurotransmitter in the CNS.
-
What is glutamine and how is it related to glutamate? (2)
Glutamine is an amino acid precursor of glutamate.
It is converted into glutamate by phosphate-activated glutaminase.
-
What is the role of vesicular glutamate transporter (VGLUT)? (2)
VGLUT is a vesicular membrane transporter that mediates glutamate accumulation in presynaptic vesicles.
It operates by counter-transporting glutamate with H+.
-
What is an AMPA receptor? (2)
An AMPA receptor is a ligand-gated ionotropic glutamate receptor subtype.
It is selective to the synthetic molecule AMPA.
-
What is an NMDA receptor? (2)
An NMDA receptor is a ligand- and voltage-gated ionotropic glutamate receptor subtype.
It is selective to the synthetic molecule NMDA.
-
What is a kainate receptor? (2)
A kainate receptor is a ligand-gated ionotropic glutamate receptor subtype.
It is selective to the synthetic molecule kainate.
-
What is long-term potentiation (LTP) and its significance? (2)
LTP is a persistent increase in synaptic strength following high-frequency stimulation of a chemical synapse.
LTP studies are often carried out in slices of the hippocampus, an important organ for learning and memory.
-
What is the role of excitatory amino acid transporter (EAAT)? (2)
EAAT is a membrane transporter that mediates glutamate accumulation in the presynaptic bouton.
It helps in removing glutamate from the presynaptic cleft.
-
What is excitotoxicity? (2)
Excitotoxicity is the pathological process by which neurons are damaged and killed.
It occurs due to overactivation of the NMDA and AMPA receptors.

