-
Because the binding energy per nucleon is greatest for intermediate-sized atoms, _____, a great amount of energy is released.
when small atoms combine or large atoms split
-
Isotopic notation
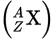
X is the element
Z is the atomic number
A is the mass number
-
Atomic number
The atomic number (Z) corresponds to the number of protons in the nucleus.
-
Mass number
The mass number (A) corresponds to the number of protons plus neutrons.
-
When balancing nuclear equations, it is important to balance the number of nucleons on both sides by _____.
balancing the atomic numbers and mass numbers
-
Fusion
Fusion occurs when small nuclei combine to form a larger nucleus.
-
Many stars (including the Sun) power themselves by fusing _____.
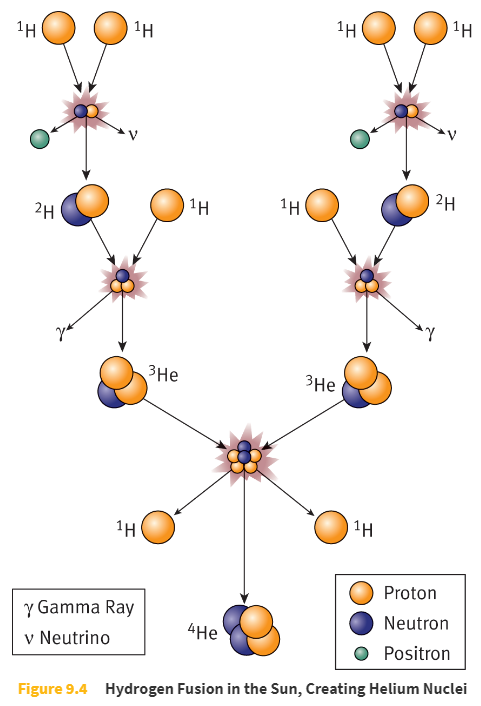
four hydrogen nuclei to make one helium nucleus
-
The Sun produces _____ joules per second.
3.85 × 1026 joules per second (385 yottawatts)
-
On Earth, fusion power plants generate energy from _____ and _____ nuclei.
deuterium and lithium
-
Deuterium
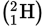
-
Positron
A positron is a particle of matter with the same mass as an electron but an opposite charge.
-
Fission
Fission is a process by which a large nucleus splits into smaller nuclei.
-
Spontaneous fission rarely occurs. However, through the _____, fission can be induced in certain nuclei.
absorption of a low-energy neutron
-
Fission chain reaction
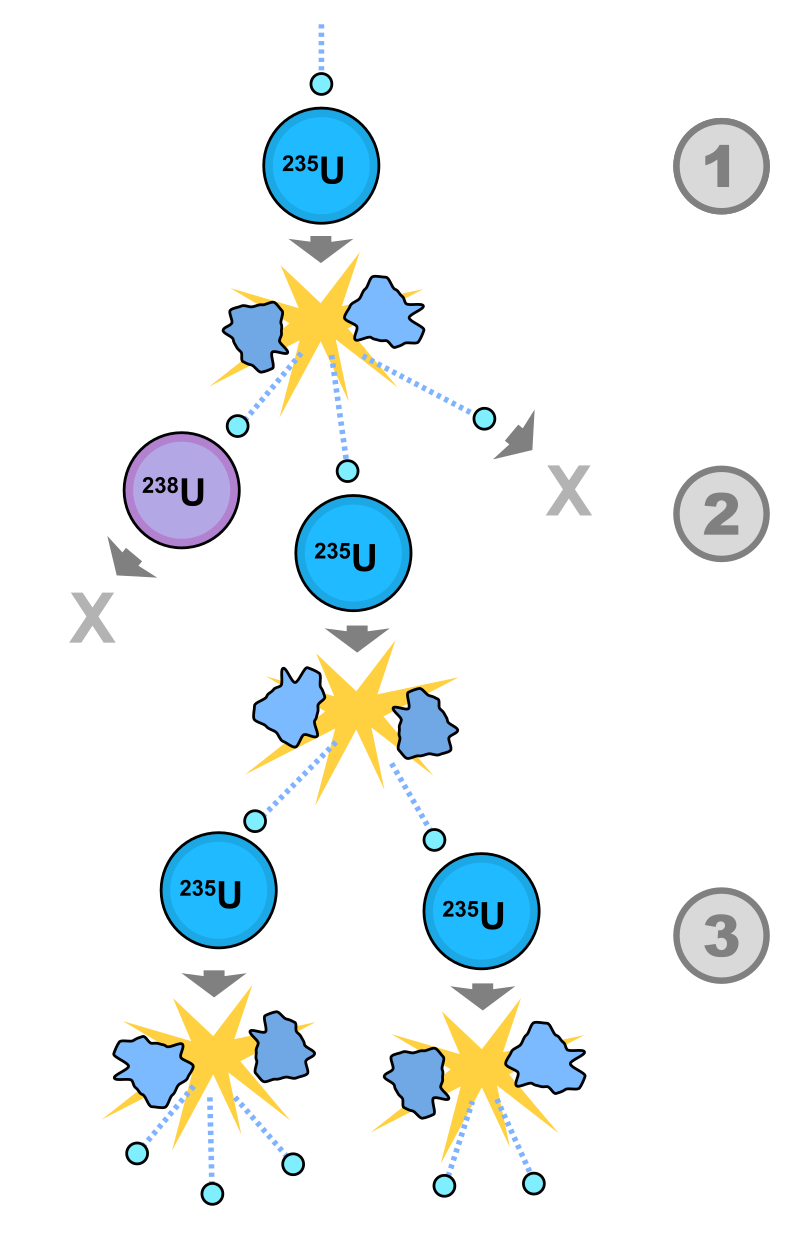
When an atom undergoes nuclear fission, a few neutrons are ejected from the reaction. These free neutrons will then interact with the surrounding medium, and if more fissile fuel is present, some may be absorbed and cause more fissions. Thus, the cycle repeats to give a reaction that is self-sustaining.
-
Neutrino
A neutrino is a fermion (an elementary particle with spin of 1/2) that interacts only via the weak interaction and gravity. It has no charge and only a very small mass (~1/500,000 that of an electron).
There are three types of neutrinos.
-
Radioactive decay
Radioactive decay is a naturally occurring spontaneous decay of certain nuclei accompanied by the emission of specific particles.
-
On the MCAT, you should be prepared to answer three general types of radioactive decay problems:
The integer arithmetic of particle and isotope species
Radioactive half-life problems
The use of exponential decay curves and decay constants
-
Parent nucleus/Daughter nucleus equation
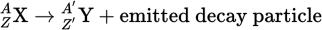
When balancing nuclear reactions, the sum of the atomic numbers must be the same on both sides of the equation, and the sum of the mass numbers must be the same on both sides as well.
-
Parent nucleus
A nuclide before disintegration
-
Daughter nucleus
A nuclide after disintegration
-
Alpha decay
Alpha decay is the emission of an α-particle, which is a helium nucleus that consists of two protons, two neutrons, and zero electrons.
-
α-particle (with 3 of electrons and charge)
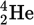
No electrons, +2 charge
-
Alpha particles interact with matter very easily; hence, they _____.
do not penetrate shielding (such as lead sheets) very extensively
-
The emission of an α-particle means that the atomic number of the daughter nucleus will be _____ than that of the parent nucleus, and the mass number will be _____.
two less
four less
-
Alpha decay equation
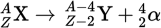
-
Beta decay
Beta decay is the emission of a β-particle, which is an electron or a positron.
-
β-particle (with symbols)
β-particles are electrons (symbol e− or β−) or positrons (symbol e+ or β+)
-
Electrons do not reside in the nucleus, but they are emitted by the nucleus in beta decay when a _____ decays into _____.
neutron
a proton, a β-particle, and an antineutrino
-
Antineutrino
The antimatter counterpart to a neutrino.
Like, neutrinos, they have no charge and a spin of 1/2. However, they have an opposite-signed lepton number and weak isospin, and right-handed instead of left-handed chirality (you don't need to know this for the MCAT)
-
The beta radiation from radioactive decay is _____ than alpha radiation.
more penetrating
-
Positron emission
Positron emission, beta plus decay, or β+ decay is a subtype of radioactive decay called beta decay, in which a proton inside a radionuclide nucleus is converted into a neutron while releasing a positron and an electron neutrino.
-
Positron
A positron (symbol e+ or β+) is the antimatter counterpart to an electron. It has the same mass as an electron but a +1 charge.
-
β− decay equation
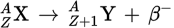
-
β+ decay equation
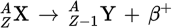
-
During β− decay, a _____ is converted into a _____ and a _____ is emitted. A(n) _____ is also produced.
neutron
proton
β−-particle
antineutrino
-
During β+ decay, a _____ is converted into a _____ and a _____ is emitted. A(n) _____ is also produced.
proton
neutron
β+-particle
neutrino
-
During β+ decay, the atomic number of the daughter nucleus will be _____ than that of the parent nucleus, and the mass number _____.
one lower
will not change
-
During β− decay, the atomic number of the daughter nucleus will be _____ than that of the parent nucleus, and the mass number _____.
one higher
will not change
-
Gamma decay
The emission of γ-rays, which simply lowers the energy of the parent nucleus without changing the mass number or the atomic number.
-
Gamma decay equation
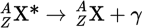
-
Electron capture
A process in which the proton-rich nucleus of an electrically neutral atom absorbs an inner atomic electron. This process thereby changes a nuclear proton to a neutron and simultaneously causes the emission of an electron neutrino.
-
Electron capture equation
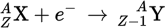
-
Electron capture is perhaps best thought of as _____.
the reverse of β− decay
-
Half-life
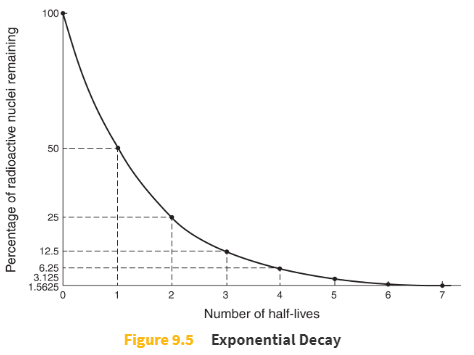
In a sample of radioactive particles, the half-life of the sample is the time it takes for half of the sample to decay.
-
Rate of nuclear decay equation
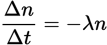
λ is known as the decay constant
-
Exponential decay equation
n = n0e−λt
n0 is the number of undecayed nuclei at time t = 0
-
Relation of the decay constant to the half-life equation
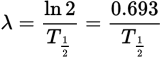
-
Which type of nuclear decay could be detected in an atomic absorption spectrum?
Gamma decay

