-
Temperature conversions
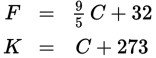
-
Thermal expansion equation
ΔL = αLΔT
-
Volume expansion equation
ΔV = βVΔT
-
First law of thermodynamics
ΔU = Q – W
-
Heat gained or lost (with temperature change)
q = mcΔT
-
Heat gained or lost (phase change)
q = mL
-
Entropy and heat
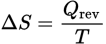
-
Second law of thermodynamics
ΔSuniverse = ΔSsystem + ΔSsurroundings > 0

