-
Zeroth law of thermodynamics
The zeroth law of thermodynamics states that objects are in thermal equilibrium when they are at the same temperature.
-
Objects in thermal equilibrium experience ...
... no net exchange of heat energy.
-
Temperature (qualitative and quantitative)
Temperature is a qualitative measure of how hot or cold an object is; quantitatively, it is related to the average kinetic energy of the particles that make up a substance.
-
Thermal expansion
Thermal expansion describes how a substance changes in length or volume as a function of the change in temperature.
-
System and Surroundings
A thermodynamic system is the portion of the universe that we are interested in observing, whereas the surroundings include everything that is not part of the system.
-
Isolated systems
Isolated systems do not exchange matter or energy with the surroundings.
-
Closed systems
Closed systems exchange energy but not matter with their surroundings.
-
Open systems
Open systems exchange both energy and matter with their surroundings.
-
State functions
State functions are pathway independent and are not themselves defined by a process. Pressure, density, temperature, volume, enthalpy, internal energy, Gibbs free energy, and entropy are all state functions.
-
Process functions
Process functions describe the pathway from one equilibrium state to another. Work and heat are process functions.
-
First law of thermodynamics
The first law of thermodynamics is a statement of conservation of energy: the total energy in the universe can never decrease or increase.
-
For a closed system, the total internal energy is equal to ...
... the heat flow into the system minus the work done by the system.
-
Heat
Heat is the process of energy transfer between two objects at different temperatures that occurs until the two objects come into thermal equilibrium (reach the same temperature).
-
Specific heat
Specific heat is the amount of energy necessary to raise one gram of a substance by one degree Celsius or one kelvin.
-
The specific heat of water is _____.
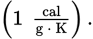
-
Heat of transformation
During a phase change, heat energy causes changes in the particles’ potential energy and energy distribution (entropy), but not kinetic energy. Therefore, there is no change in temperature. This is the heat of transformation.
-
Isothermal processes
For isothermal processes, the temperature is constant, and the change in internal energy is therefore 0.
-
Adiabatic processes
For adiabatic processes, no heat is exchanged.
-
Isobaric processes
For isobaric processes, the pressure is held constant.
-
Isovolumetric (isochoric) processes
For isovolumetric (isochoric) processes, the volume is held constant and the work done by or on the system is 0.
-
Second law of thermodynamics
The second law of thermodynamics states that in a closed system (up to and including the entire universe), energy will spontaneously and irreversibly go from being localized to being spread out (dispersed).
-
Entropy
Entropy is a measure of how much energy has spread out or how spread out energy has become.
-
Microstates as they relate to Entropy
On a statistical level, as the number of available microstates increases, the potential energy of a molecule is distributed over that larger number of microstates, increasing entropy.
-
Every natural process is ultimately _____.
irreversible
-
Under highly controlled conditions, certain equilibrium processes such as phase changes can be treated as _____.
essentially reversible